Abstract
Thirty-six phytohormone-affected mutants of Arabidopsis thaliana (L.) Heynh. and their parental ecotypes were tested for resistance/susceptibility to Botrytis cinerea Pers.; Fr. and ability to develop Trichoderma-mediated induced systemic resistance (ISR). Ecotype Colombia-0 (Col-0) was relatively resistant to B. cinerea, and Trichoderma harzianum Rifai T39 application at sites spatially separated (roots) from the B. cinerea inoculation (leaves) resulted in reduction of grey mold symptoms. Ecotypes Wassilewskija-4, Nossen-0 and Landsberg-0 had low levels of basal resistance to B. cinerea and were unable to express ISR. Mutants derived from ISR-non-inducible ecotypes displayed ISR-non-inducible phenotypes, whereas the ISR inducibility of mutants derived from the ISR-inducible genotype Col-0 varied according to the type of mutant. Thus, salicylic acid (SA)-impaired mutants derived from Col-0 were ISR-inducible, while ethylene/jasmonic acid (ethylene/JA)-impaired mutants of the same origin were ISR-non-inducible. SA-impaired mutants retained basal level of resistance to B. cinerea, while most ethylene/JA-impaired mutants were highly susceptible. Abscisic acid- and gibberellin-impaired mutants were highly susceptible to B. cinerea and showed ISR-non-inducible phenotypes irrespective of their lines of origin. Auxin-resistant mutants derived from Col-0 were ISR-inducible; mutant originating from Landsberg-0 and mutants which were resistant to both auxin and ethylene were ISR-non-inducible. Most of the arabidopsis genotypes which were unable to express Trichoderma-mediated ISR against B. cinerea exhibited enhanced susceptibility to this pathogen. T. harzianum treatments enhanced the growth of arabidopsis plants regardless of genotype or ISR inducibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Induced resistance to pathogens can be subdivided into two broad categories. The first of these is systemic acquired resistance (SAR), associated with the production of pathogenesis-related (PR) proteins and mediated via a salicylic acid (SA)-dependent process. This type of resistance develops either locally or systemically in response to a pathogen that causes a necrotic lesion, as a result of a successful infection or a hypersensitive response (HR). The second type of induced resistance develops systemically in response to colonization of plant roots by certain rhizosphere bacteria, known as plant growth promoting rhizobacteria (PGPR). This type of resistance, known as induced systemic resistance (ISR), does not involve expression of PR proteins and is mediated by a signaling pathway in which the phytohormones jasmonic acid (JA) and ethylene (E) play key roles (Pieterse et al. 1998; Van Loon et al. 1998; Hammerschmidt 1999). In the past decade, the model plant species Arabidopsis thaliana has been extensively explored to study the molecular basis of systemically induced resistance. It was shown, in general, that pathogens which are controlled by SA-dependent defense responses colonize the apoplast and multiply within host tissue before causing plant cell death and tissue damage (Peronospora parasitica (Fr.) Tul., Erisyphe sp., Pseudomonas syringae v. Hall), whereas pathogens that are controlled by JA-dependent defense responses employ a virulence strategy that involves rapidly killing plant cells to obtain nutrients. These pathogens are often referred to as “necrotrophs” (Alternaria brassicicola Wilst., Botrytis cinerea Pers.; Fr., Pythium sp., and Erwinia carotovora L. R. Jones) (Kunkel and Brooks 2002). It should be noted that the correlation between necrotrophs and JA-dependent defense responses versus biotrophs and SA-dependent defense responses can be made in Arabidopsis, whereas it is not so clear-cut in other plants. The classification of the pathogens to necrotrophs and biotrophs also should be done with precaution, because numerous of fungi that were once assumed to be necrotrophs have been reclassified as hemi-biotrophs since colonization involves a brief or extended period before dead cells appear (Thaler et al. 2004). Infection of Arabidopsis thaliana (L.) Heynh. by B. cinerea does not induce SAR and SA does not accumulate; B. cinerea actively kills the host to obtain nutrients, and even utilizes plant HR for rapid colonization (Govrin and Levine 2000; Govrin and Levine 2002).
In addition to Pseudomonas spp., there are other soil microorganisms that can provide protection against disease and promote plant growth, such as the fungus Trichoderma harzianum Rifai (De Meyer et al. 1998; Elad 2000; Harman et al. 2004). Following Kloepper et al. (2004), we will use the term ISR for the process whereby treatment of plants with plant growth promoting microorganisms elicits a host defense response, as indicated by reduction in the severity or incidence of disease caused by pathogens that are spatially separated from the inducing agent. The general goal of this work was to better understand the role of signaling molecules in the Trichoderma-mediated ISR. Using A. thaliana mutants with altered susceptibilities to or production of phytohormones, the specific purposes of this work were to: (i) study the basal susceptibility of different ecotypes and their phytohormone-related mutants to B. cinerea; (ii) study the ability of ecotypes and mutants to develop Trichoderma-mediated ISR against B. cinerea; and (iii) study the potential of T. harzianum to protect arabidopsis against B. cinerea and enhance plant growth. Some of these results have been presented previously in preliminary reports (Okon Levy et al. 2003; Korolev et al. 2004).
Materials and methods
Plants
Seeds of the wild-type backgrounds (ecotypes) and mutants derived from them were provided by the Nottingham Arabidopsis Stock Center (UK) and the Arabidopsis Biological Resource Center (Columbus, OH). The examined mutants belong to five groups according to the phytohormone involved in their respective mutations: abscisic acid (ABA)-, auxin-, ethylene or JA-, gibberellin (GA)-impaired mutants, and a group of pathogenesis-related SA-impaired mutants. The ecotypes Colombia (Col-0), Landsberg (Ler-0), Nossen (No-0) and Wassilewskija (Ws-4) were included in experiments as non-manipulated hosts. Stock numbers, alleles, background lines and phenotypes of all mutants are listed in Table 1. Seeds were sown in autoclaved soil mix containing 70:30% volcanic gravel (tuff): peat in 7 × 5 × 5 cm pots at a rate of three seeds per pot. Following germination, seedlings were culled so that one plant was left in each pot. Following sowing, seeds were vernalized at 4°C for 2 days prior to germination, after which the pots were transferred to a growth chamber which was kept at 20–22°C with 8 h of light per 24 h, because short days delay flowering and encourage rosette growth (Weigel and Glazenbrook 2002; Rusterucci et al. 2005). Any floral buds that developed, despite the short day, were removed. Plants were watered on alternate days and fertilized once a week (0.0125:0.0075:0.0200% NPK). Four- to six-week-old plants were inoculated. Wild-types and mutants were grown under the same conditions, except for the ABA-impaired mutants which required high relative humidity and the GA-deficient mutants which required supplemental gibberellin. Pots with ABA-impaired mutants were placed in plastic troughs which were covered with transparent polypropylene to maintain high humidity. According to the instructions of collection holders, GA-deficient mutants were treated with an aqueous solution of gibberellic acid: germination in the presence of 0.10 mM and spray of plants once per week with 0.01 mM. Spraying was ceased two weeks before inoculation.
B. cinerea
Strain B4 was isolated from grapes (France, Bordeaux) and stored in glass tubes with potato dextrose agar (PDA) (Difco, Le Pont de Claix, France) at −20°C. A monoconidial culture was prepared from the isolate and stored on PDA at 4°C for the duration of this project.
Inoculation with mycelium blocks
B. cinerea colonies were grown on 1/5 strength PDA in 9 cm, unsealed Petri dishes at 20°C for 4 days in the dark. Inoculation was performed by placing 3 mm discs, excised from the growing edge of colonies, on the upper leaf surfaces, one plug per cauline leaf (leaves on the lower part of the inflorescence shoot). Leaves which formed later were not used. Following inoculation, the pots with the plants were placed in a plastic box with some water on the bottom, covered with transparent polypropylene to maintain close to 100% relative humidity and incubated at 20°C with a 12-h light/12-h dark cycle. Disease severity was scored 24 h after inoculation by measuring lesion diameters and the results were expressed as lesion size (mm) or as lesion size relative to that of the lesions forming on the corresponding background line (%).
Inoculation with conidia suspension
Conidia were washed with 1/2 strength potato dextrose broth (PDB) (Difco, Le Pont de Claix, France) from two-week-old colonies of B. cinerea grown on PDA. The conidia suspension was filtered through cotton wool and diluted with 1/2 strength PDB to 1 × 106 conidia/ml. Leaves were inoculated with droplets of 10 μl of this suspension, one droplet per leaf. Following inoculation, plants were incubated as described above. Disease severity was scored 7 days after inoculation by measuring lesion diameters and the results were expressed as described above.
Trichoderma treatments
A suspension of T. harzianum T39 (0.4% Trichodex in water) (Dik and Elad 1999; Elad, 2000) was applied to the plants three days before inoculation, as a soil drench (to saturation) or as a foliar spray. Plants were then inoculated with B. cinerea mycelia blocks or drops of conidia suspension, incubated and monitored for disease development as described above. To study the influence of T. harzianum on plant growth, diameters of plant rosettes were measured at 10 days after treatment.
Experimental design and data analysis
Each experiment included 5–10 different mutants and their background lines; 3–10 plants of each genotype were subjected to each treatment. All treatment plants were inoculated with B. cinerea. Before inoculation, plants were pretreated with soil applied T. harzianum or foliar applied T. harzianum. Control plants received no pretreatment. The soil applied pretreatment was used to evaluate whether T. harzianum could provide any systemic protective effect (ISR). The foliar pretreatment was used to evaluate whether T. harzianum could provide any localized protective effect against B. cinerea infection. Experiments were performed at least three times for every genotype. Data sets for experiments where disease development did not vary by background line were combined and data on lesion diameters and rosette diameters were compared using contrast t-tests, P ≤ 0.05. All tests were performed using the JMP 4.0.0 statistical software package (SAS Institute Inc., Cary, NC, USA).
Results
Susceptibility of background lines to B. cinerea and their responses to T. harzianum (inoculation with mycelium blocks)
Four background lines, Col-0, Ler-0, No-0 and Ws-4, were included in these experiments as non-manipulated hosts. Col-0 was rather resistant to B. cinerea; Ler-0, No-0 and especially Ws-4 were highly susceptible (Table 2). Trichoderma application at sites spatially separated (roots) from the B. cinerea inoculation (leaves) resulted in a 30% average reduction (up to 50% in some experiments) of grey mold symptoms on ecotype Col-0. Ecotypes Ws-4, No-0 and Ler-0 did not express ISR; disease on these lines was often strengthened after pretreatment of roots with T. harzianum. Foliar applications of T. harzianum (preceding the application of B. cinerea to the leaves) resulted in a reduction of disease on the ISR-inducible ecotype Col-0, whereas there was little to no reduction of disease on the ISR-non-inducible ecotypes Ler-0, No-0 and Ws-4. In Col-0, the mean disease reduction following root or leaf application of T. harzianum was about 30% (up to 50% or even more in some experiments) (Table 2). Significant variability in the extent of plant protection following soil or leaf application of T. harzianum, as well as some variation in basal susceptibility, were recorded across the experiments. However, the relative susceptibilities of the different ecotypes were stable: within each experiment, Col-0 was more resistant than the three other ecotypes.
Susceptibilities of mutants to B. cinerea and their responses to T. harzianum (inoculation with mycelium blocks)
ABA-impaired mutants
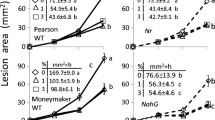
Three ABA-deficient and three ABA-insensitive mutants were tested. ABA-insensitive mutants were derived from Ler-0 (abi1-1, abi2-1 and abi3-1); ABA-deficient mutants were derived from Ler-0 (aba1-3), or from Col-0 (aba2-1 and aba3-1). All mutants were more susceptible to B. cinerea than their corresponding background lines. Ler-0-derived mutants were more susceptible than Col-0-derived ones. Root application of T. harzianum resulted in a significant reduction in the amount of disease on the background line Col-0, but not on Ler-0 and not on ABA-impaired mutants, irrespective of their type or origin. Pretreating the roots of Ler-0-derived mutants with T. harzianum often resulted in disease levels which were higher than those of the control. Foliar applications of T. harzianum protected Col-0 and Col-0-derived mutants, whereas any reduction in the amount of disease on Ler-0 or Ler-0-derived mutants was mostly insignificant (Fig. 1 ABA).
Susceptibilities of phytohormone-impaired mutants of Arabidopsis thaliana and their background lines to Botrytis cinerea and their responses to treatments with Trichoderma harzianum. B4 = inoculation with B. cinerea; B4Tsoil = pretreatment of roots by T. harzianum with subsequent inoculation with B. cinerea; B4Tspray = pretreatment of leaves by T. harzianum with subsequent inoculation with B. cinerea. Y-axis: Lesion size (mm2); X-axis: Lines and mutants tested, mutants follow their background. Asterisks indicate statistically significant reductions in disease after T. harzianum treatment relative to non-treated control plants; plus signs indicate mutant susceptibilities to B. cinerea significantly higher than those of their respective background lines (t tests at P ≤ 0.05). B. cinerea was applied as mycelium plugs to cauline leaves; T. harzianum was applied as Trichodex soil drench or foliar spray. Abbreviations: ABA = abscisic acid; GA = gibberellic acid; JA = jasmonic acid; SA = salicylic acid
Auxin-impaired mutants
Seven of the eight auxin-impaired mutants were derived from ecotype Col-0; they included auxin-resistant mutants (axr1-3, axr4-1 and axr4-2), mutants resistant to auxin and ethylene (aux1-7, aux1-7 axr4-2 and aux1-7 ein2), and a mutant resistant to auxin transport inhibitor (tir1-1). The mutants axr1-3 and aux1-7 ein2 were significantly more susceptible to B. cinerea than the background line Col-0, whereas the susceptibilities of other mutants did not differ significantly from their respective parental lines. Mutants resistant to both auxin and ethylene exhibited ISR-non-inducible phenotypes. Auxin-resistant mutants and tir1-1 were ISR- inducible, and root and often leaf applications of T. harzianum protected these mutants. The background line Ws-4 and its mutant ilr1-1 (resistant to indole-3-acetic acid (IAA)-leucine conjugate) were both highly susceptible to B. cinerea. Neither soil nor foliar applications of T. harzianum succeeded in protecting Ws-4 or its mutant (Fig. 1 Auxin).
Ethylene- or JA-impaired mutants
This group included 14 mutants: one JA-resistant (jar1-1) and 13 impaired in ethylene response or production. Ethylene-impaired mutants included plants which were ethylene-insensitive (ein2, ein2-1, ein3-1, ein4, ein5-1, ein6, etr1-1, etr1-3), had ethylene-insensitive roots (eir1-1), overproduced ethylene (eto1-1, eto2, eto3) and under produced ethylene (hls1-1). All of these mutants were derived from Col-0, except ein6 which was derived from Ler-0. All mutants were affected more severely than the background lines and all were unable to develop T. harzianum-induced ISR. Mutant eir1-1, with ethylene-insensitive roots, did not express ISR following pretreatment of its roots with T. harzianum, whereas pretreatment of the leaves significantly reduced disease. Reductions in disease after foliar applications of T. harzianum were also recorded for eto3, etr1-3, hls1-1, jar1-1 and their background Col-0 (Fig. 1 Ethylene/JA).
GA-impaired mutants
The GA-deficient mutants ga1-4 and ga2-1 were derived from ecotype Ler-0; the GA-resistant mutant spy3 was derived from Col-0. All mutants were very susceptible to infection by B. cinerea and exhibited ISR-non-inducible phenotypes. The Ler-0-derived mutants were more severely infected than the Col-0-derived one. Foliar applications of T. harzianum did not provide any protection for Ler-0 derived mutants, whereas they did have a protective effect on spy3 (Fig. 1 GA).
SA-impaired mutants
SA-impaired mutants of arabidopsis included mutants which did not express PR-genes (npr1, npr1-2, and npr1-5), a mutant with constitutive expression of PR genes (cpr5-2) and a mutant with enhanced disease susceptibility (eds5-1). The mutant npr1-5 was derived from No-0; other mutants were derived from Col-0. The mutant npr1 was slightly more susceptible to B. cinerea than its background line; other mutants retained the basal level resistance of their parent lines. Root applications of Trichodex provided protection for Col-0-derived mutants, but No-0-derived npr1-5 was ISR-non-inducible. Foliar applications of T. harzianum provided protection for all lines, except No-0 (Fig. 1 SA).
ISR in response to challenge inoculation with a conidia suspension
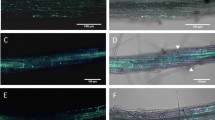
The results of challenge inoculations using conidia suspensions of B. cinerea were similar to the results of inoculations using mycelia plugs (Table 3). Compared to inoculation with mycelia blocks, inoculations with conidia suspensions caused slower disease development. Following inoculations with conidia suspensions, lesion spots covered the whole leaf square after 7–10 days of incubation, as compared to 24–48 h after inoculation with mycelia blocks. The difference in disease between treated and control plants of ISR-inducible ecotype Col-0 was remarkable (Fig. 2).
Responses of the ISR-inducible ecotype Col-0 and the ISR-non-inducible ecotype Ler-0 to induction of Trichoderma harzianum -mediated ISR. In each picture, top: disease in control; bottom: disease on plants pretreated with T. harzianum. Two weeks after inoculation with B. cinerea conidial suspension. T. harzianum was applied as Trichodex soil drench three days before the plants were inoculated with the pathogen
Effect of T. harzianum on plant growth
All arabidopsis genotypes treated with T. harzianum, including the ISR-inducible and ISR-non-inducible ecotypes and their mutants, grew more quickly following the treatment of roots with T. harzianum. At 10 days after treatment, treated plants had formed rosettes 11–33% larger in size than those of untreated plants. The mean growth enhancement across the tested lines was about 22% (Figs. 3, 4).
Response of arabidopsis rosette growth (mm diameter) to treatment with T. harzianum. Control: non-treated plants; T. harzianum: plants treated with Trichodex (0.4% in water) as soil drench until saturation. Mutants follow their respective background lines. Asterisks indicate statistically significant enhancement of rosette diameter after T. harzianum treatment relative to non-treated plants (t test at P ≤ 0.05)
Discussion
Ecotypes of arabidopsis differ in their susceptibilities to B. cinerea. Within this project, Col-0 was rather resistant, while Ler-0, No-0 and especially Ws-4 were susceptible to B. cinerea strain B4. In previously published work, Col and Ws did not differ significantly in terms of number of dead plants after inoculation with B. cinerea, but Ws was considerably more susceptible to ozone exposure; similar mechanisms were proposed for sensitivity to ozone and susceptibility to necrotrophic pathogens (Nickstadt et al. 2004). Earlier, arabidopsis was shown to exhibit a degree of isolate specificity. When 16 different ecotypes were screened for basal susceptibility to two B. cinerea isolates, most ecotypes showed a similar response to both isolates, whereas a few ecotypes showed differential susceptibility. Susceptibility was associated with lower camalexin accumulation, and, in contrast to our data, ecotype Ler-0 was less susceptible than Col-0 (Denby et al. 2004). Isolate specificity, as described in the cited work, could explain how a given arabidopsis ecotype could react differently with different pathogen isolates. Ler ecotype is reportedly much more susceptible than Col-0 to Fusarium oxysporum Schlect., with plants dying more rapidly and at lower spore concentrations (Anderson et al. 2004). Ten different ecotypes of arabidopsis were screened for their potential to express rhizobacteria (Pseudomonas fluorescens Trev. WCS417r)-mediated ISR. Ecotype Ws was unable to express ISR and had a low level of basal resistance to P. syringae pv. tomato (Pst), whereas Col and Ler were found to be ISR-inducible and resistant to Pst. Genetic crosses between ISR-inducible and ISR-non-inducible arabidopsis genotypes revealed that the traits of ISR-inducibility and basal resistance against Pst correlated and were inherited as monogenic, dominant traits genetically linked at the locus ISR. ISR-non-inducible ecotypes Ws and RLD exhibited reduced sensitivity to ethylene, suggesting that the ISR1 locus encodes a component of the ethylene-response pathway that plays an important role in ethylene-dependent resistance mechanisms (Ton et al. 2001). In the present work, we tested the abilities of four arabidopsis ecotypes to develop Trichoderma-mediated ISR against B. cinerea. In ecotype Col-0, which is relatively resistant to the challenging pathogen, T. harzianum T39 application at sites spatially separated (roots) from the B. cinerea inoculation site (leaves), reduced the severity of grey mold infection. Ecotypes Ler-0, No-0 and especially Ws-4 had low levels of basal resistance to B. cinerea and were unable to express Trichoderma-mediated ISR. In arabidopsis, some common features were evident in ISR induction by P. fluorescens spp. (Ton et al. 2001) or by T. harzianum. Ecotypes which were relatively resistant to the challenge pathogen (Pst or B. cinerea) were able to develop ISR, whereas susceptible lines were not. Ecotype Ws was highly susceptible to both B. cinerea (present work) and Pst, whereas Ler-0 was relatively resistant to Pst but rather susceptible to B. cinerea. Col-0 was relatively resistant to both pathogens.
The high susceptibility of ethylene/JA-impaired arabidopsis mutants to B. cinerea was shown earlier. The mutants coi1, jar-1 and etr1 showed enhanced susceptibility to infection by the necrotrophic pathogens A. brassicicola, B. cinerea and Pythium sp., but not to the obligatory parasite P. parasitica (Staswick et al. 1998; Thomma et al. 1998). The mutant ein2-1 was much more susceptible than the wild-type to infection by two different strains of B. cinerea (Thomma et al. 1998). Infections caused by B. cinerea in rosette leaves of ein2-1, etr1-1, jar1-1 and coi1-1 resulted in lesions about twice as large as those formed on wild-type leaves, and about three-fold larger in the double mutant ein2-1 jar1-1 (Ferrari et al. 2003). ISR, induced by P. fluorescens WCS417r towards infection of arabidopsis with Pst, required responsiveness to JA and ethylene, and was shown to be blocked in those arabidopsis mutants whose JA or ethylene pathways had been altered (Pieterse et al. 2001). To study the roles of ethylene/JA-signaling in basal resistance of arabidopsis to B. cinerea and in Trichoderma-mediated ISR, we tested 14 mutants with impaired ethylene/JA responses or production. All mutants were affected more severely than their background lines and none of these mutants developed Trichoderma-induced ISR in response to pretreatment of roots with T. harzianum. It can be concluded that Trichoderma-induced ISR is ethylene/JA mediated, like P. fluorescens WCS417r induced ISR. In other systems, ISR is likely to involve a variety of signaling routes, depending on the biocontrol agent and the target pathogens. For example, the ISRs elicited by several strains of Bacillus spp. are independent of SA but dependent on JA, ethylene and the regulatory gene NPR1. These results are in agreement with the model for ISR elicited by Pseudomonas spp. In other cases, ISR elicited by Bacillus spp. is dependent on SA and independent of JA and NPR1. In addition, while ISR by Pseudomonas spp. does not lead to the activation of the defense gene PR1 in plants, in some cases, ISR by Bacillus spp. does (reviewed by Kloepper et al. 2004). The signaling pathway activated in arabidopsis by volatiles from B. subtilis Cohn GBO3 against E. carotovora is dependent on ethylene, albeit independent of the SA or JA signaling pathways (Ryu et al. 2004). ISR induced in arabidopsis by P. fluorescens CHAOr in response to the pathogen P. parasitica was dependent on NPR1, JAR1 and EIR1 proteins, but independent of EIN2 (Iavicoli et al. 2003). Paenibacillus alvei K-165 protected arabidopsis against Verticillium dahliae Kleb. by means of an ISR response which was blocked in the SA-affected mutants NahG, npr1, sid2 and eds5/sid1, whereas significant protection was induced in etr1-1 or jar-1 (Tjamos et al. 2005).
Salicylic acid is an important regulator of plant defense responses, and a variety of arabidopsis mutants with impaired resistance to bacterial and fungal pathogens show defects in SA accumulation, perception, or signal transduction. The SA-impaired mutants tested in this work (npr1, npr1-2, npr1-5, cpr5-2, and eds5-1) retained basal level resistance to B. cinerea, confirming the minor role of an SA-mediated pathway in resistance to B. cinerea (Govrin and Levin 2002). The mutant npr1 has been reported to be highly susceptible to infection by the obligatory pathogen Pst (Pieterse et al. 2001) and the systemic pathogen V. dahliae (Tjamos et al. 2005), whereas it does not differ from its wild-type progenitor in its susceptibility to the necrotrophs A. brassicicola and B. cinerea (Thomma et al. 1998; Ferrari et al. 2003). Mutant eds5-1, with blocked SA biosynthesis, showed wild-type level susceptibility to B. cinerea, whereas cpr1-1 which constitutively accumulates high levels of SA, was even more resistant than the wild-type (Ferrari et al. 2003). In the experiments with rhizobacteria-mediated ISR, the SA-impaired mutants derived from Col-0 had an ISR-inducible phenotype, except for npr1-1 which was blocked in both SAR and P. fluorescens WCS417r-mediated ISR (Pieterse et al. 2001; Ton et al. 2002; Iavacoli et al. 2003). The induction of ISR by the biocontrol bacterium P. alvei K165 against V. dahliae was also blocked in npr1-1 (Tjamos et al. 2005). In this work, SA-impaired mutants derived from the ISR-inducible ecotype Col-0 developed Trichoderma-induced ISR following the pretreatment of roots with T. harzianum, whereas mutant npr1-5, derived from the ISR-non-inducible ecotype No-0, was ISR-non-inducible.
SA, JA and ethylene are the major signal molecules involved in regulating defense responses in plants. In arabidopsis, an intact ethylene/JA signaling pathway is thought to be necessary for resistance to necrotrophic pathogens, such as B. cinerea and E. carotovora, whereas the SA signaling pathway is believed to mediate resistance to biotrophic pathogens, such as P. parasitica and Pst. Genetic analyses of arabidopsis mutants with altered ABA biosynthesis or signaling have identified a complex interplay between ABA and other phytohormone signaling pathways. This analysis has also demonstrated that ABA signaling antagonizes both the ethylene/JA responsive defense gene expression and resistance to fungal disease (Anderson et al. 2004). ABA involvement in plant disease resistance is complex and dependent on the type of pathogen. Treatment of wild-type arabidopsis with ABA has been reported to increase its susceptibility to the biotrophic pathogens Pst and P. parasitica (Mohr and Cahill 2003) and induce callose accumulation and resistance to the necrotrophic pathogens A. brassicicola and Plectosphaerella cucumerina (Ton and Mauch-Mani 2004). ABA-deficient mutants of arabidopsis (aba2-1, aba1-1) were more resistant than the wild-type to Pst, P. parasitica and Fusarium oxysporum, while ABA-insensitive mutants (abi1-1, abi1-2) did not differ from the wild-type (Mohr and Cahill 2003; Anderson et al. 2004). Disease caused by A. brassicicola or P. cucumerina in both ABA-insensitive and ABA-deficient mutants of arabidopsis did not differ from the disease in the wild-type (Ton and Mauch-Mani 2004). Mutants abi1, abi3, abi4, and aba2 were more susceptible than the wild-type plants to infection by the oxalate-deficient mutant of Sclerotinia sclerotiorum (Lib.) DBy. (Guimaraes and Stotz 2004). Similarly, the ABA-insensitive and ABA-deficient arabidopsis mutants examined in this work were more susceptible to B. cinerea than their corresponding background lines. One possible explanation of these results is that the ethylene/JA pathway necessary for the resistance of arabidopsis to B. cinerea may be affected in ABA-related mutants. Root applications of Trichodex caused significant reductions in disease in the background line Col-0, but not in Ler-0 or ABA-impaired mutants irrespective of their type or origin. Similarly, ABA-induced resistance to the necrotrophic pathogens A. brassicola and P. cucumerina was blocked in both ABA-deficient (aba1-5) and ABA-insensitive (abi1-4) mutants. Previous reports have shown ABA-induced resistance to be based on enhanced callose accumulation, which is controlled by an ABA-dependent defense pathway (Ton and Mauch-Mani, 2004).
Three GA-impaired mutants tested in this work were highly susceptible to B. cinerea and exhibited ISR-non-inducible phenotypes. Although two of these mutants were derived from the ISR-non-inducible ecotype Ler-0 and could be ISR-non-inducible for this reason, mutant spy3 was derived from the ISR-inducible ecotype Col-0 but was still ISR non-inducible. This suggests that components of the GA signaling pathways play a role in resistance to B. cinerea and ISR potential. Recently, it was found that the expression of gene tgas118, which encodes the defense protein defensin, is regulated by gibberellin (Van Heuvel et al. 2001). The plant defensin gene is induced by pathogens via an ethylene/JA-dependent pathway (Thomma et al. 1998). The high susceptibility of GA-related mutants to B. cinerea suggests a link between gibberellin- and ethylene/JA-signaling pathways.
Most of the auxin-affected arabidopsis mutants were derived from Col-0; they retained basal level resistance to B. cinerea and were ISR-inducible. The auxin-resistant mutant axr1-7 was highly susceptible and ISR-non-inducible. The AXR1 locus has been shown to be involved in methyl jasmonate response, providing a link between ethylene/JA- and auxin-signaling pathways (Tiryaki and Staswick 2002). The mutant axr1-3 was the only one of this set of mutants to be both susceptible to B. cinerea and ISR-inducible. The IAA-leucine-resistant mutant ilr1-1 was ISR-non-inducible, just like the parent ecotype Ws-4.
In this work, Trichoderma enhanced arabidopsis growth regardless of plant mutation or ISR inducibility. Earlier, plant growth promotion by Trichoderma spp. was reported in different plant species, although the effect was not always consistent (Ousley et al. 1994; Dissevelt and Ravensberg 2002). Enhanced plant growth and productivity were also observed for Rhizobacteria- and Bacillus spp.-treated hosts (Van Loon et al. 1998; Kloepper et al. 2004). In the case of Bacillus spp., it was found that the same strains that elicit ISR typically promote plant growth (Kloepper et al. 2004). In Pseudomonas fluorescens, ISR is not as closely associated with enhanced growth. PGPRs have been divided into two large classes, the biocontrol PGPRs that suppress plant pathogens and thereby indirectly benefit the plant, and the PGPRs that directly affect plant metabolism to increase plant growth, seed emergence or crop yield (Ping and Boland 2004). To date, no specific studies of the effects of different strains of Trichoderma on ISR and plant growth have been published.
Abbreviations
- ABA:
-
Abscisic acid
- GA:
-
Gibberellic acid
- HR:
-
Hypersensitive response
- IAA:
-
Indole-3-acetic acid
- ISR:
-
Induced systemic resistance
- JA:
-
Jasmonic acid
- SA:
-
Salicylic acid
- SAR:
-
Systemic acquired resistance
- PR:
-
Pathogenesis-related
- PGPR:
-
Plant growth promoting rhizobacteria
References
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Macleon DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16:3460–3479
Denby KJ, Kumar P, Kliebenstein DJ (2004) Identification of Botrytis cinerea susceptibility loci in Arabidopsis thaliana. Plant J 38:473–486
Dik JA, Elad Y (1999) Comparison of antagonists of Botrytis cinerea in greenhouse-grown cucumber and tomato under different climatic conditions. Eur J Plant Pathol 105:123–137
Dissevelt M, Ravensberg WJ (2002) The effect of cultural and environmental conditions on the performance of Trichoderma harzianum strain T-22. IOBC WPRS Bull 25(10):49–52
Elad Y (2000) Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot 19:709–714
Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM (2003) Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J 35:193–205
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10:751–757
Govrin EM, Levine A (2002) Infection of Arabidopsis with a necrotrophic pathogen, Botrytis cinerea, elicits various responses but does not induce systemic acquired resistance (SAR). Plant Mol Biol 48:267–276
Guimaraes RL, Stotz HU (2004) Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol 136:3703–3711
Hammerschmidt R (1999) Induced disease resistance: how do induced plants stop pathogens? Physiol Mol Plant Pathol 55:77–84
Harman GE, Howell CR, Vitebro A, Chet I, Lorito M (2004) Trichoderma species––opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Iavicoli A, Boutet E, Buchalla A, Metraux JP (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHAO. MPMI 16:851–858
Kloepper JW, Ruy CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Korolev N, Rav David D, Elad Y (2004) Involvement of plant hormones in the biocontrol achieved by Trichoderma harzianum. IOBC WPRS Bull 27(8):363–366
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
De Meyer G, Bigirimana J, Elad Y, Höfte M (1998) Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur J Plant Pathol 104:279–286
Mohr PG, Gahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30:461–469
Nickstadt A, Thomma BPHJ, Feussner I, Kangasjarvi J, Zeier J, Loeffler C, Scheel D, Berger A (2004) The jasmonate-insensitive mutant jin1 shows increased resistance to biotrophic as well as necrotrophic pathogens. Mol Plant Pathol 5:425–434
Okon Levy N, Elad Y, Korolev N, Katan J (2003) Resistance induced by soil biocontrol application and soil solarization for the control of foliar pathogens. IOBC WPRS Bulletin 27:171–176
Ousley MA, Lynch JM, Whipps J (1994) Potential of Trichoderma spp. as consistent plant growth stimulators. Microbiol Fertil Soils 17:85–90
Pieters CMJ, Van Wees SCM, Van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, Van Loon LC (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10:1571–1580
Pieterse CMJ, Van Pelt JA, Van Wees SCM, Ton J, Leon-Kloosterziel KM, Keurentjes JJB, Verhagen BWM, Knoester M, Van der Slius I, Bakker PAHM, Van Loon LC (2001) Rhizobacteria-mediated induced systemic resistance: triggering, signaling and expression. Eur J Plant Pathol 107:51–61
Ping L, Boland W (2004) Signals from the underground: bacterial volatiles promote growth in Arabidopsis. Trends in Plant Sci 9:263–266
Rusterucci C, Zhao Z, Haines K, Mellersh D, Neuman M, Cameron RK (2005) Age-related resistance to Pseudomonas syringae pv. tomato is associated with the transition to flowering in Arabidopsis and is effective against Peronospora parasitica. Physiol Mol Plant Pathol 66:222–231
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induced systemic resistance in Arabidopsis. Plant Physiol 234:1017–1026
Staswick PJ, Yuen GY, Lehman CC (1998) Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J 15:747–754
Thaler JS, Owen B, Higgins VJ (2004) The role of the jasmonate response in plant susceptibility to diverse pathogens with a range of lifestyles. Plant Physiol 135:530–538
Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue PA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Nat Acad Sci USA 95:15107–15111
Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130:887–894
Tjamos SE, Flemetakis E, Paplomatas EJ, Katinakis P (2005) Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. MPMI 18:555–561
Ton J, De Vos M, Robben C, Buchala A, Mètraux J-P, Van Lonn LC, Pieterse CMJ (2002) Characterization of Arabidopsis enchanced disease susceptibility mutants that are affected in systemically induced resistance. Plant J 29:11–21
Ton J, Mauch-Mani B (2004) β-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38:119–130
Ton J, Davidson S, van Loon LS, Pieterse CMJ (2001) Heritability of rhizobacteria-mediated induced systemic resistance and basal resistance in Arabidopsis. Eur J Plant Pathol 107:63–68
Van Heuvel KHPT, Hulzink JNR, Barense GWM, Wullems GJ (2001) The expression of tgas118, encoding a defensin in Lycopersicon esculentum, is regulated by gibberellin. J Exp Bot 52:1427–1436
Van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by Rhizosphere bacteria. Ann Rev Phytopathol 36:453–83
Weigel D, Glazenbook J (2002) Arabidopsis. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Acknowledgements
The study of the susceptibilities of arabidopsis mutants to B. cinerea was supported by grant No. TU 50/9-4 from the Deutsche Forschungsgemeinschaft (DFG). N. Korolev’s work was supported by a fellowship from the Israeli Ministry of Immigration. We thank the Nottingham Arabidopsis Stock Center and the Arabidopsis Biological Resource Center (Columbus, OH) for the arabidopsis seeds and the P. Tudzynski from the Institute Fuer Botanik, Muenster, Germany for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korolev, N., Rav David, D. & Elad, Y. The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana . BioControl 53, 667–683 (2008). https://doi.org/10.1007/s10526-007-9103-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9103-3