Abstract
The predatory mirid Dicyphus hesperus Knight (Hemiptera: Miridae) is native to North America. The species has been used for the control of glasshouse whitefly on aubergine in the Netherlands, and is currently being evaluated for continued and wider release in Europe. Field and laboratory studies were conducted on a population collected from southern California, USA, to assess the cold tolerance and potential for outdoor establishment under prevailing northern European climates. The supercooling points (whole animal freezing temperatures) of nymphal and adult insects were around −20°C. The lethal temperatures (LTemp50) of non-diapausing nymphs and adults and diapausing adults were close to their respective freezing temperatures at −17.6, −17.6 and −19.2°C. At 5°C, the LTime50 was 54, 101.7 and 117.5 days for fed nymphs, non-diapausing and diapausing adults respectively. When first instar nymphs were placed in the field in winter, starved samples died out after 70 days, but 5% of the fed nymphs survived until the end of winter (140 days) and developed to adult on return to the laboratory. After a similar 5-month field exposure, 50% of fed diapausing adults and 15% of fed non-diapausing adults were still alive at the end of winter, whereas starved diapausing adults died after 140 days. On return to the laboratory after 5 months in the field, both diapausing and non-diapausing adults mated and laid eggs, forming viable populations. Overall, the field and laboratory experiments indicate that this population of D. hesperus is able to enter diapause and that winter temperatures are not a barrier to establishment in northern Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Augmentative biological control is widely used as a method of pest management on many crops grown under protected cultivation in northern Europe. At least half of the predatory insects and mites and insect parasitoids used in glasshouse biocontrol in northern Europe are non-native species (De Clercq 2002). Therefore depending on the country of release, non-native biocontrol agents may be subject to some form of ‘licence’ or ‘permit’, often requiring an environmental risk assessment (ERA) as part of the licensing process (van Lenteren and Loomans 2006). Licences for the release of non-native biological control agents in Europe are regulated by national authorities. To date eight EU countries have implemented regulation (Austria, Czech Republic, Denmark, Hungary, Norway, Sweden, Switzerland, UK), with a further six preparing documents (Finland, Germany, Ireland, Netherlands, Slovenia, Spain). No regulation currently exists in six countries (Belgium, France, Greece, Italy, Poland, Portugal) (Loomans 2007).
Most of the glasshouse biocontrol agents used in northern Europe originate from tropical, semi-tropical or Mediterranean climates (van Lenteren 1997). For this reason, it has been widely assumed on the basis of ‘climate matching’, that imported species that escape from glasshouses would be unable to survive northern European winters. Based on introductions into the UK, this assumption is now known to be untrue. The predatory mite Neoseiulus californicus McGregor (Acari: Phytoseiidae) was first released in the UK in 1991, and was found to have established outdoors by 1999 (Jolly 2000). Similarly, the predatory mirid Macrolophus caliginosus Wagner (Hemiptera: Miridae), first released in the UK in 1995, has been found outside of glasshouses in winter, though full establishment has not yet been confirmed (Hart et al. 2002b). The impact of such exotic species on native ecosystems is unknown, but establishment of non-native species is considered to be undesirable.
A series of studies on non-native species released in the UK for the past 5–10 years (Hart et al. 2002a, b; Hatherly et al. 2004, 2005a; Tullett et al. 2004), revealed a strong correlation between indices of laboratory cold hardiness and duration of winter survival in the field. This provided a retrospective physiological explanation for the establishment of N. californicus, and the failure of other species to survive long-term outside of the glasshouse (Hatherly et al. 2005b). The development of such a predictive index and associated ERA methods is timely against the backdrop of EU policies to reduce pesticide use, alongside the view that whilst biocontrol may be preferable to chemicals, there is a need for ‘regulatory guidelines’ to prevent the release of ‘risky species’ (Babendreier et al. 2006; Bale 2005). There is an emerging consensus that regulation should be set at a minimum level that does not inhibit the discovery and development of new agents, without compromising environmental safety (Bale 2005).
Dicyphus hesperus Knight (Hemiptera: Miridae) is native to North America and is an omnivorous predator that has shown potential for glasshouse biocontrol on crops such as tomato, controlling glasshouse whitefly Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) and glasshouse red spider mite Tetranychus urticae Koch (Acari: Tetranychidae) (McGregor et al. 1999). It is currently used on glasshouse tomato crops in British Columbia, Ontario and Quebec (all Canada). Its development as a biocontrol agent was driven by the success of M. caliginosus on tomato crops in Europe, and the reluctance of the North American regulatory authorities to licence M. caliginosus for release in their territories, despite strong lobbying from Canadian tomato growers (Gillespie, D., Agriculture and Agri-Food Canada, personal communication). Additionally, naturally occurring populations of the native European mirid Dicyphus tamaninii Wagner (Hemiptera: Miridae) successfully controlled T. vaporariorum on outdoor tomatoes in Spain (Gabarra et al. 1988). This further strengthened the view that a mirid that was native to North America may be able to control tomato pests in glasshouses.
There has been recent interest in introducing D. hesperus for glasshouse biocontrol in northern Europe and in its wider potential as a biocontrol agent. It has been released in The Netherlands for the past 4 years for the control of whitefly on aubergine, but is now being used only by assigned growers and under certain conditions; for example, with a requirement to conduct post-release monitoring outside of glasshouses. There is no evidence that D. hesperus had established in The Netherlands. A small number of individuals were found outside of glasshouses in 2006, but it is unclear whether this species can overwinter outdoors (Loomans, A. J. M., Plant Protection Service, Wageningen, The Netherlands, personal communication). In the light of current interest in this species and its local release in one northern European country, studies were conducted on D. hesperus, using the protocols established for other non-native agents (Hatherly et al. 2005b).
Previous studies on North American populations of D. hesperus reported a developmental threshold of around 8°C (Gillespie and Sanchez 2004), with adults overwintering in reproductive diapause (Gillespie, D., Agriculture and Agri-Food Canada, personal communication). Dicyphus hesperus ranges from northern British Columbia to Mexico and differences in the critical daylength for diapause induction have been found between northern (British Columbia, Canada) and southern (California, USA) populations (Gillespie and Quiring 2005). The experiments in this study were conducted on the most southerly population of D. hesperus yet collected for experimentation, in which the adult diapausing ability was unknown. Various studies have shown that populations overwintering in diapause may be more cold tolerant than their non-diapausing counterparts (Denlinger 1991; Slachta et al. 2002). Thus, if the southern Californian population was found to be lacking in diapause ability, it might also be less likely to survive through cold winters in northern Europe. With this in mind, the aim of this study was to determine the establishment potential of the sampled population of D. hesperus in northern Europe, using a risk assessment protocol that had been applied successfully to other non-native species.
Materials and methods
Rearing of Dicyphus hesperus
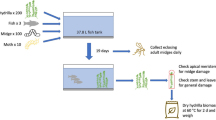
Dicyphus hesperus were obtained from Koppert, The Netherlands from a source population that had originally been collected from the San Bernardino Mountains, in southern California (USA) in 1999. They were reared under quarantine at 23°C, LD 18:6 h on Nicotiana tabacum L. plants and supplemented with eggs of Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae). Field trials were conducted with first instar nymphs and adults directly upon receipt from Koppert, in the same physiological state as would be supplied to commercial growers, and randomly assigned to the various treatments. For laboratory trials, in excess of 100 D. hesperus mated adults were placed on large N. tabacum plants sprinkled with E. kuehniella eggs. After 24 h, all adults were removed. Of the emerging first instar nymphs, some were used in laboratory experiments and some were kept at 23°C, LD 18:6 h and reared to adult. Some of the plants that had been used for oviposition on by D. hesperus were placed at 18°C, LD 12:12 h and the emerging nymphs were reared to adult as a ‘diapausing population’. The temperature and lighting regime were based on a previous study on diapause of D. hesperus (Gillespie and Quiring 2005). The diapausing and non-diapausing adults were then used in the field and laboratory experiments as required. To determine if adults were in diapause, insects were allowed to mate and left at 18°C, LD 12:12 h for 20 days. Females that did not lay eggs over this time period were considered to be in diapause, although they were not dissected to check for egg development within the ovaries (Gillespie and Quiring 2005). Temperatures were recorded throughout all experiments using Tinytalk® dataloggers (Gemini, UK).
Treatments
For both laboratory and field experiments there were 6 treatments. Treatments 1 and 2 were fed and unfed first instar nymphs, 3 and 4 were fed and unfed adults, and 5 and 6 were fed and unfed adults that had been reared under a diapause inducing regime.
Supercooling points
Supercooling points (SCPs) were measured for treatments 2, 4 and 6 to provide an initial indication of the cold tolerance of D. hesperus and also to determine whether it was a freeze tolerant or intolerant species. For treatments 4 and 6 (adults), each individual was placed into a size 3 Beem capsule (n = 23) (Agar Scientific Ltd, UK) with a temperature probe inserted in the tube and the temperature lowered at 0.5°C min−1 to the SCP (onset of the freezing exotherm). SCPs were recorded directly to a computer to an accuracy of 0.1°C. The capsules were then opened and individual D. hesperus placed into glass vials, with eggs of E. kuehniella at 20°C, LD 18:6 h and mortality recorded after 24 and 48 h. The same procedure was repeated for treatment 2 (n = 26), with nymphs attached to the temperature recording probe with Vaseline grease. The SCPs of each treatment were tested for a normal distribution, analysed using a one-way ANOVA, and differences between treatments compared using Tukey’s HSD test.
Lower lethal temperatures
Assessment of the lower lethal temperature of D. hesperus enabled a comparison with the SCPs to identify the occurrence of any pre-freeze mortality. Fifty D. hesperus of each unfed treatment group were placed individually into Beem capsules. The mirids were exposed singly within each replicate as preliminary control experiments had shown that D. hesperus adults became entangled with other individuals at higher exposure temperatures when movement was still possible, leading to higher mortality than expected. Ten capsules were placed in each of five boiling tubes (constituting five replicates of ten insects each) and suspended in a low temperature programmable alcohol bath (Haake C50P), (Haake, Germany). Each treatment was cooled from 15°C at 0.5°C min−1 to a range of temperatures between 0 and −25°C. After being held at the required minimum exposure temperature for 1 min, the tubes were re-warmed to 15°C at 1°C min−1. Individual D. hesperus from each treatment were then placed in glass vials (3.5 × 2.5 cm2) with E. kuehniella eggs at 20°C, LD 18:6 h and mortality recorded after 24 and 48 h. A control treatment of 50 insects was held in the alcohol bath at 15°C for 2 h 10 min, the maximum time any insects would have been in the bath during experimental exposures. The results were assessed using Probit analysis (Finney 1971) to estimate the temperatures required to kill 10, 50 and 90% of the population (LTemp10,50,90) and significant differences identified by non-overlapping fiducial limits (Hart et al. 2002a).
Lower lethal time
Lower lethal time experiments complement the lethal temperature work by investigating the response of D. hesperus to temperatures likely to be experienced in the field over longer periods of time. Replicate samples for treatments 1–6 were taken from the culture regime, held for 1 h at 10°C to overcome possible initial mortality due to cold shock, and were then transferred to low temperature incubators (INF-750, Sanyo Gallenkamp) at −5, 0 and 5°C at LD 18:6 h. Four replicates of ten insects each were removed from each exposure temperature, held for 1 h at 10°C to overcome possible heat shock mortality, and mortality was then recorded after 24 and 48 h at 20°C, LD 18:6 h. Sampling was after 2, 4 and 6 days and then weekly until 100% mortality was observed or the experiment was terminated (after 146 days). The results were analysed using Probit analysis to estimate the time required to kill 10, 50 and 90% of the individuals at each temperature (LTime10,50,90) and significant differences identified as described above.
Field experiments
The field experiments were conducted to measure the effect of fluctuating temperatures on outdoor populations. Five D. hesperus individuals of each treatment were placed in glass vials (7 × 2.5 cm2) on a 0.5-cm deep layer of agar (2%) (Oxoid Ltd technical agar, UK), with a circular piece of filter paper (2 cm in diameter) resting on the agar which provided a source of moisture. Each vial was sealed with a ventilated plastic lid covered in 75-μm muslin, (Lockertex, UK). Unfed treatments had no food placed in the vials, whereas fed treatments had approximately 100 E. kuehniella eggs added to each vial. Two hundred vials of each treatment sufficient for a 5-month field trial were placed in plastic boxes and sealed, except for four ventilation holes covered in muslin in the side of the box (3 cm in diameter), and placed in a sheltered field location (away from direct winds and sunlight) at the University of Birmingham. Air temperature was recorded within the boxes amongst the vials with Tinytalk® dataloggers (Gemini, UK). For each treatment, four replicates of two vials each (ten insects per replicate) were collected at random from the field and mortality (%) recorded after return to the laboratory. Sampling was carried out on three occasions in the first week, weekly for the next month, and finally every 2 weeks until the end of the experiment. Treatments 1–4 were placed in the field on 16 November 2005 and treatments 5 and 6 on 7 December 2005. Live adults from treatments 3 to 6 collected from the field on and after 13 April 2006 (when the experiment was terminated) were placed on N. tabacum plants sprinkled with E. kuehniella eggs and observed for oviposition. Any live nymphs recovered from the field were kept in the laboratory with food and development followed through until adulthood. Throughout the field experiments, food supply was changed at approximately 3 week intervals (depending on air temperature) to stop insects becoming entangled in fungal infections and to ensure that excess food was always available. To do this, 50 vials of each treatment were moved into a quarantine area at 8°C, the insects transferred to fresh vials and then returned to the field within 20 min. Cold tolerance data obtained for D. hesperus were also compared with those of other biocontrol agents to identify relative differences in cold tolerance indicative of establishment potential.
A control sample of 30 first instar D. hesperus in individual vials was maintained at 23°C, LD 18:6 h and development to adult observed to ensure that the experimental set up was not deleterious to the insects.
Data comparison of D. hesperus and M. caliginosus
Differences in cold tolerance and winter survival of D. hesperus and M. caliginosus were compared (Gillespie and Sanchez 2004; Hart et al. 2002b), in the knowledge that M. caliginosus is found outside of UK glasshouses in winter, although year round establishment is yet to be confirmed (Hart et al. 2002b).
Results
Diapause induction regime
A total of 73.3% of the D. hesperus reared from egg to adult at 18°C, LD 12:12 h (n = 30) did not lay any eggs over a 20-day period after becoming adult and were considered to be in diapause. In a comparative population reared at 23°C, LD 18:6 h, 96.6% of the females laid eggs. The diapause induction regime therefore induced diapause in a high proportion of the sample population.
Supercooling points
No significant differences were detected in the SCPs of nymphal, non-diapausing and diapausing adult D. hesperus (F 2,70 = 2.4, P > 0.05), with all life stages freezing at around −20°C. The mean and range of SCPs for each treatment are shown in Table 1. All individuals were dead after freezing.
Lower lethal temperature
Survival of D. hesperus adults and nymphs after the control exposure at 15°C for 2 h 10 min was 100%. The lethal temperatures for 10, 50 and 90% mortality (LTemp10,50,90) were −15, −17.6, −21°C for nymphal D. hesperus, −15, −17.6, −20.7°C for non-diapausing adults and −17.9, −19.2 and −20.4°C for diapausing D. hesperus.
Lower lethal time
The lethal times for 10, 50 and 90% mortality (LTime10,50,90) at −5, 0 and 5°C of fed and unfed nymphal, non-diapausing and diapausing adult D. hesperus are shown in Fig. 1a–c. Fed and unfed nymphs survived significantly less time than both non-diapausing and diapausing adults at −5 and 0°C (as indicated by non-overlapping fiducial limits). At 5°C (LTime50), fed individuals of all treatments survived longer than the equivalent unfed samples. Fed non-diapausing and diapausing adults survived significantly longer than fed nymphs. At 5°C the LTime50 was 54, 101.7 and 117.5 days for fed nymphs, non-diapausing and diapausing adults respectively.
Field experiments
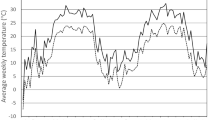
Mortality increased in all treatments during the field exposures, but at different rates and with different final levels of survival (Fig. 2). The mean, maximum and minimum temperatures from 16 November 2005 until 13 April 2006 were 4.3, 17 and −3.1°C respectively; mean, maximum and minimum temperatures for each month are shown in Table 2. On 29 December 2005 the temperature was never above −1°C. Between 18 November 2005 and 23 March 2006 (first and last days with temperatures below 0°C), temperatures fell below 0°C on 34 of a possible 125 days.
After 140 days in the field, 5% of the fed nymphs were still alive (unfed nymphs all died after 70 days), and when returned to the laboratory developed to adulthood. After 148 days (5 months), 15% of fed non-diapausing adults were still alive (unfed non-diapausing adults were all dead after 110 days). Fed non-diapausing adults that were returned to the laboratory at 23°C after this period of time in the field laid eggs which hatched and developed to adults. They continued to lay viable eggs for 32 days. Dicyphus hesperus reared under the diapause inducing regime all died after 140 days when maintained without food, but 50% of the fed individuals were still alive after this time. When returned to the laboratory, these individuals mated and laid eggs which hatched and developed to adulthood. They survived and laid viable eggs for 23 days. All nymphs in the control experiments moulted to adult.
Survival of D. hesperus was higher at the end of the winter experiment than in any other species tested so far (Table 3) using directly comparable methodology. Most notably, 50% of diapausing D. hesperus were still alive after 140 days in the field.
Data comparison of D. hesperus and M. caliginosus
A comparison of laboratory and field data obtained for adult non-diapausing D. hesperus and M. caliginosus is shown in Table 4.
Discussion
Establishment of a non-native biocontrol agent in northern Europe is dependent on a number of factors, the most important of which are an adequate thermal budget to support development and reproduction, cold tolerance for winter survival, and access to sources of food (Hatherly et al. 2005b). The experiments conducted in this study assessed the cold tolerance of different life cycle stages of D. hesperus in the laboratory and field, and together with previously published information on its developmental threshold and rate of development, uses this combined data to evaluate its establishment potential outside of the glasshouse environment in northern Europe. To put the results on D. hesperus into context, comparisons are made with another previously released non-native (to the UK) mirid (M. caliginosus), and with other species that have been evaluated using the same experimental protocols.
The developmental threshold of D. hesperus (8°C) and development time from egg to adult (about 35 days at 22°C) are both very similar to the values reported for M. caliginosus (Gillespie and Sanchez 2004; Hart et al. 2002b). Given that M. caliginosus was estimated to be able to complete two generations outdoors under UK conditions (Hart et al. 2002b), it seems likely that D. hesperus would be able to develop and reproduce outdoors in northern Europe, at least during the summer.
The SCPs of D. hesperus were similar to those previously reported for M. caliginosus. There were no differences in lethal temperatures between D. hesperus nymphs, and non-diapausing and diapausing adults. Comparison of the lethal temperature data with the respective SCPs suggests that any pre-freeze mortality is minimal, with a mean SCP for diapausing adults of −21°C and an LTemp50 of −19.2°C. The level of pre-freeze mortality was slightly lower than for M. caliginosus (Hart et al. 2002b). Whilst the observed SCP and LTemp50 values are lower than temperatures that would be experienced in The Netherlands or the UK, they indicate that D. hesperus can survive acute exposures at lower temperatures that occur routinely in colder climates.
The LTime50 at 5°C for unfed D. hesperus adults was 60 days. In a similar experiment, the equivalent value for unfed M. caliginosus adults was 32.4 days (Hart et al. 2002b). At all three exposure temperatures (−5, 0 and 5°C), nymphs survived for less time than non-diapausing and diapausing adults. This is partly due to the lower cold tolerance of nymphs, but may also be attributable to different levels of energy reserves at the start of the experiments. Nymphs were used directly after emergence from the egg and therefore, may have not accumulated any fat reserves. At 5 °C, the LTime50 was 54, 101.7 and 117.5 days for fed nymphs, non-diapausing and diapausing adults respectively. Although there was no significant difference in survival between non-diapausing and diapausing adults, the latter still survived longer and this may be due to increased cold tolerance in diapausing populations. At −5 and 0°C, there were no significant differences between fed and unfed individuals; however at 5°C (LTime50), survival of fed individuals was longer in all treatments when compared to the equivalent unfed treatment. This suggests that feeding was not possible at the two lower temperatures, but at 5°C some feeding occurred, and increased survival time, which was also apparent in all field exposures.
All life cycle stages of D. hesperus were able to survive throughout a colder than average UK winter, with 50% survival in fed diapausing adults after 148 days in the field (end point of experiment). Both the British Columbian and the Californian strain of D. hesperus can enter diapause (Gillespie and Quiring 2005). The non-diapausing and diapausing adults maintained without food survived for 110 and 140 days respectively, which are relatively long periods of time given their starved condition. The addition of a food resource increased field survival; the polyphagous nature of D. hesperus suggests that it is likely to be able to find food outdoors in winter should it be required. By comparison, unfed adult M. caliginosus survived for a maximum of 75 days in the field (Hart et al. 2002b).
Although the diapause trial was started 3 weeks later than the non-diapause field exposures, sufficiently low temperatures were experienced during December, January and February 2005–2006 to suggest that the higher survival of diapausing adults was related to the diapause state rather than differences in exposure temperature. Nevertheless, the current study demonstrates that D. hesperus can survive an entire winter in a non-diapause state. Other mirids such as M. caliginosus actively seek shelter during winter months (Hart et al. 2002b) and as this is likely to be the case with D. hesperus, diapause may not be an essential requirement for winter survival in northern Europe. Field data from previous studies shows that survival of D. hesperus was much higher than for other species at the end points of winter experiments, including N. californicus, which is now established in the UK (Hatherly et al. 2005b).
Whilst this study has shown that D. hesperus is sufficiently cold hardy to survive outdoors through northern European winters, for long term establishment to occur there is another essential requirement: food. On return to the laboratory after nearly 5 months in the field and provided with food, non-diapausing and diapausing fed D. hesperus adults mated and laid eggs that formed viable populations. Similarly, fed nymphs returned to the laboratory at the end of winter and given food also developed to adulthood. It is likely that post-winter survivors of D. hesperus would lay eggs in the field as long as a suitable prey and host plant combination was available. Even unfed adults that survived for 110 days in the field are likely to be able to obtain food by re-entering a glasshouse or consuming prey such as field populations of aphids that will begin to build up in early spring. Also, some individuals may resort to cannibalism in times of prey shortage (Laycock et al. 2006). Subject to sufficiently high temperatures to enable mobility, all life cycle stages, whether fed or non-fed, diapausing or non-diapausing, survived for periods of time that would allow them to seek periodic refuge and food in glasshouses that continue production through winter. In this respect, recent studies have shown D. hesperus females feed on Encarsia formosa (Hymenoptera: Aphelinidae) larvae and pupae (parasitoid used as a control agent against T. vaporariorum) in laboratory and glasshouse trials (Labbe et al. 2006; McGregor and Gillespie 2005).
Whilst this study did not include any host range (prey) experiments, D. hesperus is known to be polyphagous and feeds on T. vaporariorum, T. urticae (McGregor et al. 1999) and Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) (Shipp and Wang 2006) in glasshouses. Outdoors, it is likely that D. hesperus will be able to locate and feed on related prey such as the cabbage whitefly, Aleyrodes proletella (Linnaeus) (Hemiptera: Aleyrodidae), and on common aphids such as Myzus persicae (Sulzer) (Hemiptera: Aphididae), as has recently been shown for M. caliginosus (Hatherly unpublished data). The ability of mirid predators to successfully feed on plant sap (Sampson and Jacobson 1999) may also further enhance the chances of post-winter survival.
In conclusion, this study has shown that a population of D. hesperus originating from southern California, USA can enter diapause. Nymphs and non-diapausing and diapausing adults are able to survive through an entire UK winter outdoors, and survival is enhanced with access to food. After nearly 5 months in the field, surviving nymphs developed to adult and non-diapausing and diapausing adults were able to mate and form viable populations. Data are now available on five other non-native biocontrol agents that have been screened by the same protocol and in the same laboratory. Analysis of these combined datasets indicates that there is a strong correlation (R 2 = 0.916) between LTime50 at 5°C and maximum field survival (Fig. 3), which further strengthens the relationship reported in Hatherly et al. (2005b). In the context of this study it demonstrates that D. hesperus is the most cold tolerant of the six species tested and that with continued releases into glasshouses over longer periods of time, establishment of this species outdoors is likely in northern Europe.
Relationship between maximum field survival (days) and LTime50 at 5°C (days) for six non-native biocontrol agents. Data refer to unfed adults of all species except E. eremicus that were exposed as unfed larvae (adapted from Hatherly et al. 2005b)
References
Babendreier D, Bigler F, Kuhlmann U (2006) Current status and constraints in the assessment of non-target effects. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods. Methods and risk assessment. CABI publishing, Wallingford, UK
Bale JS (2005) Effects of temperature on the establishment of non-native biocontrol agents: the predictive power of laboratory data. In Hoddle MS (Compiler) Proceedings of the second international symposium on biological control of arthropods, vol. 2, USDA Forest Service, pp 593–602
De Clercq P (2002) Dark clouds and their silver linings: exotic generalist predators in augmentative biological control. Neotrop Entomol 31:169–176
Denlinger DL (1991) Relationships between cold hardiness and diapause. In Lee RE, Denlinger DL (eds) Insects at low temperature. Chapman and Hall
Finney DJ (1971) Probit analysis. University Press, Cambridge
Gabarra R, Castane C, Bordas E, Albajes R (1988) Dicyphus tamaninii as a beneficial insect and pest in tomato crops in Catalonia, Spain. Entomophaga 33:219–228
Gillespie DR, Sanchez JAS (2004) Cumulative temperature requirements and development thresholds in two populations of Dicyphus hesperus. (Hemiptera: Miridae). Can Entomol 136:675–683
Gillespie DR, Quiring DMJ (2005) Diapause induction under greenhouse conditions in two populations of Dicyphus hesperus (Hemiptera: Miridae). Biocontrol Sci Technol 15:571–583
Hart AJ, Bale JS, Tullett AG, Worland MR, Walters KFA (2002a) Effects of temperature on the establishment potential of the predatory mite Amblyseius californicus McGregor (Acari: Phytoseiidae) in the UK. J Insect Physiol 48:593–599
Hart AJ, Tullett AG, Bale JS, Walters KFA (2002b) Effects of temperature on the establishment potential in the UK of the non-native glasshouse biocontrol agent Macrolophus caliginosus. Physiol Entomol 27:112–123
Hatherly IS, Bale JS, Walters KFA, Worland MR (2004) Thermal biology of Typhlodromips montdorensis: implications for its introduction as a glasshouse biological control agent in the UK. Entomol Exp Appl 111:97–109
Hatherly IS, Bale JS, Walters KFA (2005a) UK winter egg survival in the field and laboratory diapause of Typhlodromips montdorensis. Physiol Entomol 30:87–91
Hatherly IS, Hart AJ, Tullett AG, Bale JS (2005b) Use of thermal data as a screen for the establishment potential of non-native biological control agents in the UK. BioControl 50:687–698
Jolly RJ (2000) The predatory mite Neoseiulus californicus: its potential as a biocontrol agent for the fruit tree red spider mite Panonychus ulmi in the UK. Proceedings of the 2000 Brighton Conference – Pests & Diseases 2000 1:487–490
Labbe RM, Cloutier C, Brodeur J (2006) Prey selection by Dicyphus hesperus of infected or parasitized greenhouse whitefly. Biocontrol Sci Technol 16:485–494
Laycock A, Camm E, van Laerhoven S, Gillespie D. (2006) Cannibalism in a zoophytophagous omnivore is mediated by prey availability and plant substrate. J Insect Behav 19:219–229
Loomans AJM (2007) Regulation of invertebrate biological control agents in Europe: review and recommendations in its pursuit of a harmonised regulatory system. Report EU project REBECA [Regulation of Biological Control Agents]
McGregor RR, Gillespie DR (2005) Intraguild predation by the generalist predator Dicyphus hesperus on the parasitoid Encarsia formosa. Biocontrol Sci Technol 15:219–227
McGregor RR, Gillespie DR, Quiring DMJ, Foisy MRJ (1999) Potential use of Dicyphus hesperus Knight (Heteroptera: Miridae) for biological control of pests of greenhouse tomatoes. Biol Control 16:104–110
Sampson AC, Jacobson RJ (1999) Macrolophus caliginosus Wagner (Heteroptera: Miridae): a predator causing damage to UK tomatoes. IOBC Bull 22:213–216
Shipp JL, Wang K (2006) Evaluation of Dicyphus hersperus (Heteroptera: Miridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomato. J Econ Entomol 99:414–420
Slachta M, Vambera J, Zahradnickova H Kostal V (2002) Entering diapause is a prerequisite for successful cold-acclimation in adult Graphosoma lineatum (Heteroptera: Pentatomidae). J Insect Physiol 48:1031–1039
Tullett AG, Hart AJ, Worland MR, Bale JS (2004) Assessing the effects of low temperature on the establishment potential in Britain of the non-native biological control agent Eretmocerus eremicus. Physiol Entomol 29:363–371
van Lenteren JC (1997) Benefits and risks of introducing exotic macro-biological control agents into Europe. Bull OEPP/EPPO 27:15–27
van Lenteren JC, Loomans AJM (2006) Environmental risk assessment: methods for comprehensive evaluation and quick scan. In: Bigler F, Babendreier D, Kuhlmann U (eds) Environmental impact of invertebrates for biological control of arthropods. Methods and risk assessment. CABI Publishing, Wallingford, UK
Acknowledgements
We are grateful to Jenny Dryden for technical support. The study was funded by the Dutch Horticultural Product Board and Koppert, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hatherly, I.S., Pedersen, B.P. & Bale, J.S. Establishment potential of the predatory mirid Dicyphus hesperus in northern Europe. BioControl 53, 589–601 (2008). https://doi.org/10.1007/s10526-007-9099-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9099-8