Abstract
Entomopathogenic nematodes Steinernema carpocapsae were applied in a chitosan formulation (Biorend R®) to control the flat-headed rootborer Capnodis tenebrionis (Coleoptera: Buprestidae) in 5 field trials in the province of Valencia, Spain. Application was performed during spring and summer into the soil around apricot trees at densities of 1 and 1.5 million infective dauer juveniles per tree through drip irrigation, injection or by a drench. For evaluation of the control effect the roots of 106 trees were excavated, the cortex removed and living and dead insects were sampled. Dead larvae were dissected and checked for nematode infestation. Control of C. tenebrionis larvae was between 75% and 90%. No influence of the application method, nematode density or time of application on the control effect was recorded. Recovery of the infested trees was observed already in the season following nematode application. Due to the two year life cycle of the insect with egg laying from May to August, applications during spring and autumn are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capnodis tenebrionis (Linné) (Coleoptera: Buprestidae) is a common pest in stone fruit (apricot, plum, cherry, peach, nectarine and almonds trees) in Mediterranean countries. The major damage is caused by the larvae in the roots tunnelling between the wood and the bark. The pest is reported from Spain, Italy, Croatia, Turkey, Northern Africa, Israel, France and Palestine (Britvec, 2002; Garrido, 1984; Mahhou and Dennis, 1992; Mendel et al., 2003; Ulusoy et al., 1999; Viggiani, 1991; Chrestian, 1955; Hmimina et el., 1988; Martin, 1951; Rivnay, 1944; Ferrero, 1987; Colasurdo et al., 1997; Sanchez-Capuchino, 1987). The life cycle is biannual, the immature development lasting 13 months. In Spain, prepupa and pupa are found in the tree trunk from July to September (Garrido, 1984). Adults emerge in late August feeding on leaves and return to the soil for over wintering in September. They become mature after returning to the surface in April/May and feeding on spring shoots. Oviposition is from June until the end of August. One female can lay between 200 to 1,000 eggs in a distance of approximately 60 cm around the tree trunk usually in cracks of dry soil. Neonates hatch and immediately bore into the roots or tree trunk. Larvae are found throughout the year (Garcia et al., 1996; Hmimina et al., 1988). Damage becomes obvious when young shoots start to desiccate and resin occurs on the trunk and galleries or holes at the end of the trunk. As larvae can reach 10 cm of size they can cause major damage to the roots, why only few individuals are able to kill a tree within 2 years. C. tenebrionis is known as a pest for many years but it was only during the 80’s when some persistent soil insecticides were prohibit and this pest became a serious problem. Drought over several seasons and the increasing number of abandoned almond orchards have contributed to the problem in Spain. Chemical control is targeted to adult maturity feeding and oviposition with frequent insecticide applications on the foliage. (Garrido et al., 1990; Ben-Yehuda et al., 2000). Control of larvae with chemical insecticides is not effective as the compounds cannot reach the larvae inside the roots and is only successful against neonates when they hatch and move to the roots (Ben-Yehuda et al., 2000; Sanna-Passino and Delrio, 2001). Alternative control methods are therefore needed to limit the damage of this devastating pest.
Entomopathogenic nematodes (EPNs) within the families Steinernematidae and Heterorhabditidae are effective biological control agents of soil inhabiting insects (Grewal et al., 2005). They are safe for non-target vertebrates and to the environment (Ehlers, 2003). Since they are produced in large scale liquid culture, production costs have been significantly reduced and the application in horticulture, agriculture and forestry is increasing (Ehlers, 2001, Grewal et al., 2005). EPN have been successfully tested in laboratory assays against larvae of C. tenebrionis (García del Pino and Morton, 2005; Lobatón et al., 1998; Marannino et al., 2003). The objective of this study was to test S. carpocapsae in field trials and to define the best method, time for application, the application density and dose against populations of C. tenebrionis in apricot (Prunus armeniaca). Nematodes were applied together with the chitosan adjuvant Biorend R®. Chitosan is an organic biodegradable product with the active ingredient N-acetyl-glucosamine. It activates defence mechanisms in the plant (Hadwiger and Loschke, 1981), increases lignification and promotes the development of roots (Ait Barka et al., 2004). The use of nematodes with chitosan is patented (Martinez Peña, 2002).
Materials and methods
Field trials were done in five different apricot orchards in the province of Valencia, Spain. In all trials nematodes of the Spanish strain of S. carpocapsae (SSA3) were applied together with Biorend R® (1.25% chitosan diluted in acetic acid) according to Martinez Peña (2002). Nematodes had been produced in liquid culture according to Ehlers et al. (1998) in bubble columns, were harvested by centrifugation and formulated with clay soil for transport to the field.
In the first trial in Cocentaina (Alcoy) 3 different application methods were compared. Three nematode doses and Biorend R® concentrations were tested at different times during the season: 0.5 × 106 dauer juveniles (DJs) + 0.3 g chitosan, 1 × 106 DJs + 0.6 g chitosan and 1.5 × 106 DJs + 0.9 g chitosan per tree (Table 1). One treatment was done through a drip irrigation system with four holes around each tree. Nematodes and chitosan were applied in 36 l of water per tree within 2 h. Prior to drip application the homogenous distribution of the nematodes was confirmed by taking samples at each drip hole. Another treatment was done by applying nematodes into a 25 cm deep ditch excavated around the trunk of the trees. Prior to application the soil was moistened with 34 l of water and then nematodes and chitosan were applied in 2 l of water. When the water had drained into the soil the ditch was closed again. The third method used an injection system for subterranean application consisting of a 2 cm Ø injector with four holes of 3 mm Ø at the end. Before treatment, the sand was moistened with 34 l of water per tree. Nematodes and chitosan were mixed in 2 ls of water and applied through the injector.
In the second trial in Belgida (Valencia) injection and application into the ditch was compared in two different fields (Table 2) and in the third trail also in Belgida but on a different farm different application times by injection were compared in two fields (Table 3). In all controls only 36 l of water per tree were applied.
To evaluate the C. tenebrionis population the cortex of the roots of all trees in controls and treatments was removed and living and dead larvae were sampled from the complete root system. All trees in one trial were excavated at the same day. Dead larvae were dissected and checked for nematode infestation.
To evaluate the efficacy of treatments Abbot corrected mortality was calculated. The square root transformed number of living larvae in control and treatments was analysed by ANOVA and post-hoc Tukey test for unequal numbers of replicates.
To detect the presence of applied nematodes, at least 5 soil samples were taken from 0 to 25 cm depth with soil borers and the samples were mixed. 100 g soil was taken from each of the treated and untreated control trees. Nematodes were first extracted by the sieving techniques and then concentrated by the centrifugal flotation techniques using sucrose (Kaya and Stock, 1997).
Results
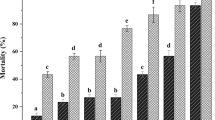
In the first trial all treatments significantly reduced the population of C. tenebrionis in apricot trees (F = 31.2, df = 4, 17, P < 0.0001). As the mean number of living insects recorded from the treated trees was similar in all treatments (Fig. 1) there was no influence of the application date, dose or number of injections on the success of the treatment (P ≥ 0.95). No dead individuals were found in the control. For the analysis of the results plots with one injection and plots with two injections were analysed together. The control ranged from 95% recorded for drip irrigation, 92% for drench and one injection, 90% for one injection and 89% for two injections (Fig. 1). A considerable number of dead, nematode-infected larvae were found in all treatments but none in the control trees (3.3 in drip irrigation, 12 in drench, 12 in one injection and 10 in two injections). S. carpocapsae were recovered from the soil around the trees until 169 days after the last treatment (26 – 282/100 g of soil) in decreasing numbers over time.
Mean number (± SD) of living and dead larvae per apricot tree of C. tenebrionis and efficacy (Abbott corrected mortality in %) after application of Steinernema carpocapsae by drench of 1 × 106 nematodes/tree followed by an injection of 0.5 × 106/tree (D + INJ), application of 1 × 106 /tree through drip irrigation (DRIP), injection of 1 × or 1.5 × 106 million/tree (INJ1) or split injection of 1 × and 0.5 × 106 /tree (INJ2)
In the second trail a split drench application of a total of 1.5 million DJ per tree was compared with an injection of 1 million or a split irrigation of 1 and 0.5 million per tree in two fields. In one field (Fig. 2A) the mean number of individuals in the control was low (7.8). Nevertheless, a significant effect (F = 8.7, df = 3, 20, P = 0.001) between treatments and control was recorded (1.3 in drench, 1.0 in one injection and 1.2 in two injections), but not between the treatments. In the second field (Fig. 2B) the mean number of individuals in the control (25.7) was significantly different (F = 8.73, df = 3, 8, P = 0.007) from the treatments (5.0 in drench, 5.7 in one and 4.0 in two injections). Nematode infected insects were recorded in all treatments but not in the control. Only in the control of field A a mean of 0.16 insect per tree was recorded dead but without nematodes. The efficacy was 83% in drench application, 87% in one and 85% in two injections (Fig. 2A) and 80% in drench application, 78% in one and 84% in two injections (Fig 2B). Nematodes were recovered from the soil around the trees until 115 days after the last treatment with 20 – 220/100 g of soil in field A and 19–73 in field B.
Mean number (±SD) of living and dead larvae per apricot tree of C. tenebrionis and efficacy (Abbott corrected mortality in %) after application of Steinernema carpocapsae by drench of 1 × 106 nematodes/tree followed by a drench of 0.5 × 106/tree (DRENCH) or injection of 1 × 106 million/tree (INJ1) or split injection of 1 × and 0.5 × 106 /tree (INJ2) in field 1 (A) and field 2 (B)
In the third trial an injection of 1.0 million DJ per tree was compared with a split injection of 1 million followed by 0.5 million DJ. Two fields were treated. In one field (Fig. 3A) the mean number of individuals in the control was 29.6. A significant effect (F = 46.4, df = 2, 6, P = 0.0002) between treatments and control was recorded (only 3.0 individuals in one injection and 4.0 in two injections), but not between the treatments. In the second field (Fig. 3B) the mean number of individuals in the control (15.2) was significantly different (F = 8.8, df = 2, 15, P = 0.003) from the treatments (3.8 in one and 3.0 in two injections). Again, nematode infected insects were recorded in all treatments but not in the control. The efficacy was 90% in one and 86.5% in two injections (Fig. 3A) and 75% in one and 80% in two injections (Fig. 3B). Nematodes were recovered from the soil around the trees until 116 days after the last treatment with 17–82/100 g of soil in field A and 22–82 in field B.
Discussion
These results demonstrate the potential of the entomopathogenic nematode S. carpocapsae to control C. tenebrionis larvae under field conditions. All trials resulted in control results of at least 80%. The different application dates in spring or summer seem to have no influence on the success. As the insect C. tenebrionis has a two year life cycle, susceptible larvae are found throughout the year. García del Pino and Morton (2005) reported that neonate larval stages are susceptible to EPNs. During all application dates from end of February to the end of August the nematodes encountered susceptible larval stages. However, it is recommended to apply during spring and autumn when soil moisture is usually higher that in the summer. Decreasing soil moisture can significantly reduce nematode activity (Alekseev et al., 2006). During these trials soils were irrigated before and during nematode application and nematodes were applied with much water, hence optimal conditions for nematode movement were produced. Whether EPNs were applied by drench, injection or drip irrigation had no significant influence on the results. As almost all orchards in commercial production have drip irrigation systems installed, this method seems to be most practicable for the users. No increase of insect control was recorded when nematodes were applied at higher concentration surpassing 1 million per tree or by an addition application. However, infested orchards should be treated twice a year, in spring and autumn, in order to control over-wintering larvae and also the new generation resulting from egg laying of adults from May to August.
Dead and nematode infested larvae were recorded even 170 days after nematode application. Two reasons might be responsible for the long-lasting effect. Nematodes either propagate in cadavers of C. tenebrionis, then emigrated, moved between the galleries and infested surviving larvae or the conditions inside the root preserved cadavers, which did not decay due to limited moisture (Koppenhofer et al., 1997).
In the year following the application, nematode treated trees recovered from the infestations with C. tenebrionis. The damaged roots were healing and the trees produced new branches and dense foliage. This effect is due to the growth promoting effect of the adjuvant Biorend R®. The same control effect can be reached applying nematodes without Biorend R®; but the recovery of the plant is occurring a year later and is less expressed.
The use of nematodes against larvae of the flat-headed root borer provides effective control against this deadly stone fruit pest in Mediterranean countries and can also substitute for ineffective chemical control measures.
References
Ait Barka E, Eullaffroy P, Clement C, Vernet G (2004) Chitosan improves development and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep 22:608–614
Alekseev E, Glazer I, Samish M (2006) Effect of soil texture and moisture on the activity of entomopathogenic nematodes against female Boophilus annalatus ticks. BioControl 51:419–438
Ben-Yehuda S, Assael F, Mendel Z (2000) Improved chemical control of Capnodis tenebrionis and C. carbonaria in stone fruit plantations in Israel. Phytoparasitica 28:27–41
Britvec B (2002) The biome and scientific investigations in Croatia. Entomologia Croatica 6:75–96
Chrestian P (1955) Le Capnode noir des Rossacées. Protectorat de la République Française au Maroc, Service de la Défence des Végétaux Rabat. Travaux originaux 6 141 pp
Colasurdo G, Vallillo E, Berchicci D, Romualdi G, de Lillo E (1997) Prime esperienze di controllo degli adulti di Capnodis tenebrionis in Molise. Inf Fitopatol 10:53–57
Ehlers RU, Lunau S, Krasomil-Osterfeld K, Osterfeld K-H (1998) Liquid culture of the entomopathogenic nematode-bacterium-complex Heterorhabditis megidis/Photorhabdus luminescens. BioControl 43:77–86
Ehlers RU (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Ehlers RU (2003) Biocontrol nematodes. In: Hokkanen HMT, Hajek AE (eds) Environmental impacts of microbial insecticides. Kluwer Acedemic Publisher, Dordrecht, NL, (pp 177–220)
Ferrero F (1987) Trois bupresten ennemies de l’abricotier en Roussillon. Phytoma 384:43
García MT, Perez JA, Arias A, Martinez de Velasco D (1996) Adult population and period of oviposition of Capnodis tenebrionis (L.) (Col.: Buprestidae) in cherry orchards in the Jerte valley. Bol Sanid Veg, Plagas 22:451–463
García del Pino F, Morton A (2005) Efficacy of entomopathogenic nematodes against neonate larvae of Capnodis tenebrionis (L.) (Coleoptera: Buprestidae) in laboratory trials. BioControl 50:307–316
Garrido A (1984) Bioecology of Capnodis tenebrionis L. (Coleop.: Buprestidae) and approaches to its control. Boletin del Servicio de Defensa contra Plagas e Inspeccion Fitopatologica 10:205–221
Garrido A, Malagon J, Busto T del (1990) Toxicity of pesticides by contact and ingestion against adults of Capnodis tenebrionis (L.), (Coleoptera: Buprestidae). Bol Sanid Veg, Plagas 16:165–172
Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) (2005) Nematodes as biocontrol agents. CABI Publisher, Wallingford, UK
Hadwiger LA, Loschke DC (1981) Molecular communication in host-parasite interactions: Hexosamine polymers chitosan as regulator compounds in race specific and other interactions. Phytopathology 71:756–762
Hmimina M, Sekkat A, Lahfa L, Histane M (1988) Biological cycle of Capnodis tenebrionis L. (Coleoptera, Buprestidae) in the region of Meknes. Actes de l’Institut Agronomique et Vétérinaire Hassan II 8:41–49
Koppenhofer AM, Baur ME, Stock SP, Ho YC, Chinnasri B, Kaya HK (1997) Survival of entomopathogenic nematodes within host cadavers in dry soil. Appl Soil Ecol 6:231–240
Lobatón CS, Vela JRG, Lopez MPL, Roca AC (1998) Bioassays with a strain of Steinernema carpocapsae (Filipjev) detected on Capnodis tenebrionis L. larvae. Bol Sanid Veg, Plagas 24:679–686
Marannino P, Tarasco E, De Lillo E (2003) Biological notes on larval hatching in Capnodis tenebrionis (L.) (Coleoptera Buprestidae) and evaluation of entomopathogenic nematodes in controlling neonate larvae. Redia 86:101–105
Mahhou A, Dennis FG Jr (1992) The almond in Morocco. Horticulture Technology 2:488–492
Martin H (1951) Contribution à l’étude du Capnode noir des arbres fruitiers (Capnodis tenebrionis L.) dans la région d’Alger. Revue de Pathologie Végétale et d’Entomologie Agricole 30:97–113
Martinez Peña A (2002) Biological pesticide based on chitosan and entomopathogenic nematodes. WO Patent 037966
Mendel Z, Assael F, Ben Yehuda S (2003) Host selection and root colonization of cyanogenic stonefruit species by Capnodis spp. (Coleoptera: Buprestidae). Ann Entomol Soc Am 96:127–134
Rivnay E (1944) Physiological and ecological studies on the species of Capnodis in Palestine (Col., Buprestidae). I. Studies on the eggs. Bull Entomol Res 33:235–242
Sanchez-Capuchino JA, Garcia S, Salazar DM, Miro M, Martinez R, Melgarejo P (1987) El almedro como patròn en secano del albaricoquero frente al ataque dal gusano cabezudo. Agricola Vergel 62:80–84
Sanna Passino G, Delrio G (2001) Efficacy of insecticides on larvae of Capnodis tenebrionis (L.). Bol Sanid Veg, Plagas 27:59–64
Ulusoy MR, Vatansever G, Uygun N (1999) The cherry pests, their natural enemies and observations on some important species in Uluksla (Nigde) and Pozant (Adana) province of Turkey. Turkiye Entomoloji Dergisi 23:111–120
Viggiani G (1991) Pests of apricots. Acta Horticulturae 2:481–486
Acknowledgement
Thanks are due to the EU COST Action 850 “Biocontrol Symbiosis” for the support of a travel grant of the first author to the University Kiel, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez de Altube, M.M., Strauch, O., Fernandez De Castro, G. et al. Control of the flat-headed root borer Capnodis tenebrionis (Linné) (Coleoptera: Buprestidae) with the entomopathogenic nematode Steinernema carpocapsae (Weiser) (Nematoda: Steinernematidae) in a chitosan formulation in apricot orchards. BioControl 53, 531–539 (2008). https://doi.org/10.1007/s10526-007-9094-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9094-0