Abstract
Previous research suggests that both genetic and environmental influences are important for antisocial behavior across the life span, even though the prevalence and incidence of antisocial behavior varies considerably across ages. However, little is known of how genetic and environmental effects influence the development of antisocial behavior. A total of 2,600 male and female twins from the population-based Swedish Twin Registry were included in the present study. Antisocial behavior was measured on four occasions, when twins were 8–9, 13–14, 16–17, and 19–20 years old. Longitudinal analyses of the data were conducted using structural equation modeling. The stability of antisocial behavior over time was explained by a common latent persistent antisocial behavior factor. A common genetic influence accounted for 67% of the total variance in this latent factor, the shared environment explained 26%, and the remaining 7% was due to the non-shared environment. Significant age-specific shared environmental factors were found at ages 13–14 years, suggesting that common experiences (e.g., peers) are important for antisocial behavior at this age. Results from this study show that genetic as well as shared environmental influences are important in antisocial behavior that persists from childhood to emerging adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
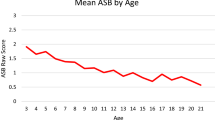
The prevalence of antisocial behavior varies considerably across the life span, typically, it increases in early adolescence, peaks in mid-adolescence, and then drops sharply by young adulthood (Moffitt 1993). Cross-sectional studies have shown that genetic and environmental effects are important for antisocial behavior during childhood (Arseneault et al. 2003), adolescence (Slutske et al. 1997; Taylor et al. 2000), and in adulthood (Lyons et al. 1995). Rhee and Waldman (2002) conducted a comprehensive meta-analysis by summarizing 51 twin and adoption studies that focused on different dimensions of antisocial behavior in children, adolescents, or adults. These included studies of trait aggression, criminal offending, and symptoms for the major psychiatric disorders (i.e., conduct disorder, oppositional defiant disorder, antisocial personality disorder), and involved a variety of methods of assessment (e.g., self-report, parent ratings, and official records). In their meta-analysis, heritable effects explained 41% of the total variance in antisocial behavior. Environmental effects shared by relatives accounted for 16%, with the remaining variance (43%) being explained by the non-shared environment (i.e., non-genetic influences that contribute to within-pair dissimilarity of twins). These effects were comparable for males and females, although they differed significantly according to definition, method of assessment of antisocial behavior, and age of the subjects (Rhee and Waldman 2002).
Evidence from studies using a longitudinal design suggests that the effect of genes and environments may be different when studied in a developmental perspective. Studies examining the genetic and environmental stability of antisocial and externalizing behavior in smaller samples (i.e., samples including less than 1,500 twins) generally report that the stability is largely due to genetic influences (Burt et al. 2007).
In contrast, larger studies (i.e., studies of more than 1,500 twins) report that the stability of antisocial behavior is due to genetic as well as shared environmental influences (Bartels et al. 2004; Jacobson et al. 2002; van Beijsterveldt et al. 2003; van der Valk et al. 2003). For example, a retrospective study found that common genetic influences were important for adolescent (age 15–17) and adult (>age 18 years) antisocial behavior, but did not overlap with antisocial behavior occurring prior to age 15. In addition, significant shared environmental influences were found for childhood, adolescent and adult antisocial behavior (Jacobson et al. 2002). Another study examining the development of externalizing behavior from childhood to adolescence, reported that genetic factors explained on average 67 and 53% of the stability in boys and girls, respectively. Shared environmental influences accounted for, on average, 27 and 40% of the stability (Bartels et al. 2004). Despite the large number of participants in these longitudinal studies, the influence of genetic and environmental effects on the development of antisocial behavior has mainly been investigated at separate time periods across development, either from childhood to adolescence or from adolescence to adulthood. Thus, there is a need for prospective longitudinal studies investigating the genetic and environmental etiology of antisocial behavior throughout the entire developmental period.
Several theories have been proposed to understand the development of antisocial behavior. The fact that antisocial behavior peaks in adolescence, and that age of onset is related to persistence, provides the basis for Moffitt’s developmental taxonomy of antisocial behavior. The theory differentiates the most deviant offenders over the life course from those likely to show temporary difficulties during adolescence. ‘Life-course persistent’ and ‘adolescent-limited’ antisocial behavior differs in terms of etiology, developmental course, prognosis, and classification of behavior as pathological versus normative (Moffitt 1993). Life-course persistent antisocial behavior starts at a young age and continues through adolescence into adulthood. This form of antisocial behavior is thought to be associated with early neuropsychological problems that result in cognitive impairment and impulsivity/hyperactivity as well as family related risk factors. Adolescence-limited antisocial behavior on the other hand, is less likely to be associated with neuropsychological impairments and family adversity, and more strongly influenced by social peer pressure (Moffitt 1993). That is, the same risk factors are important for both types of antisocial behavior, but neuropsychological impairments and family adversity are much more weakly associated with adolescent-limited antisocial behavior. Further, DiLalla and Gottesman (1989) have suggested, in addition to early-onset or continuous antisocials and late-onset or transitory delinquents, a third type of offenders called ‘late bloomers’. Late bloomers are thought to begin their offending in adulthood (DiLalla and Gottesman 1989).
Only a few studies using a genetically informative design have reported findings that can be interpreted in support of these developmental theories, or at least in support of different aspects of these theories. An early study found that shared environmental influences were more important for adolescent antisocial behavior, whereas genetic influences were stronger for antisocial behavior occurring in adulthood (Lyons et al. 1995). Further, early-onset delinquent behavior in 11 year old boys was more strongly influenced by genetic effects than late-onset delinquent behavior (Taylor et al. 2000). These findings suggest that genetic etiological processes may contribute more to early-onset or persistent antisocial behavior, than to transitory antisocial behavior. In line with this, a recent study found that a common genetic factor influenced antisocial behavior lasting from age ten through young adulthood, thus reflecting persistent antisocial behavior. Shared environmental influences only became important when the youth reached their adolescent years, which would reflect adolescence-limited antisocial behavior. These shared environmental influences are likely to reflect a combination of peers’ effects and family environment (e.g., poor child rearing practices) (Silberg et al. 2007). However, one shortcoming common to these studies is the exclusion of females. Hence, it is not clear how genetic and environmental effects influence antisocial behavior in females across the development.
Clearly, there is a lack of prospective studies investigating the genetic and environmental etiology of antisocial behavior in both males and females. We therefore attempted to fill this research gap by examining how genetic and environmental effects influence antisocial behavior using data from a large longitudinal sample of both male and female twins. Antisocial behavior was measured on four occasions, when twins were 8–9, 13–14, 16–17, and 19–20 years old. In agreement with the developmental theories of antisocial behavior (Moffitt 1993; DiLalla and Gottesman 1989) and findings from previous longitudinal studies (Silberg et al. 2007), we expected that a common latent factor would explain the covariance in antisocial behavior across development, reflecting more persistent forms of antisocial behavior. The variance in this latent factor would primarily be explained by genetic influences and to a lesser extent by shared environmental factors. We further hypothesized that any age-specific effects would be related to adolescence-limited antisocial behavior. Specifically, we expected that age-specific shared environmental effects would be more influential for antisocial behavior in adolescence and fade out toward young adulthood. Thus, reflecting the influence that peers and other shared environmental experiences may have for development of adolescence-limited antisocial behavior.
Method
Participants
Ascertainment procedures for the Twin study of Child and Adolescent Development (TCHAD) have recently been described (Lichtenstein et al. 2007). In brief, the TCHAD study is an ongoing prospective longitudinal twin study concerning health and behavior in twins from childhood to early adulthood. The study is a sub-sample of the Swedish Twin Registry (Lichtenstein et al. 2002), and contains all 1,480 twin pairs born in Sweden between May 1985 and December 1986. The twins and/or their parents have been contacted on four different occasions via mailed questionnaires. During the first wave of assessment in 1994, only parents were used as informants [response rate = 75% (n = 1,103)]. In Wave 2 in 1999, when the twins were 13–14 years old, data was collected from both parents and the twins [parent’s response rate = 73% (n = 1,063); child’s response rate = 78%, (n = 2,261)]. The same procedure was reiterated at Wave 3 in 2002 [twins were 16–17 years old; parent’s response rate = 74%, (n = 1,067); child’s response rate = 82%, (n = 2,368)], and during Wave 4 in 2005 [twins were 19–20 years old; parents = 51% (n = 1,197); twin’s response rate = 59% (n = 1,698)]. During Wave 4, consent to contact the parents was obtained from the twins. The present study utilized data from Waves 1–4: n = 2,600 twins (506 MZ male twins, 363 DZ male, 431 DZ male opposite-sex twins, 534 MZ female, and 397 DZ female, and 369 DZ female opposite-sex twins).
Fifteen percent of the families in our sample were unskilled workers, 29% were skilled workers, 28% were intermediate non-manual employees, and 28% were employed and self-employed professionals, higher civil servants or executives, which is consistent with the population of Sweden. Middle school was completed by 7% of the parents, high school by 29%, junior college by 18%, and university/college by 46%. There were slightly more parents with a college degree compared with average Swedish population. The majority of the parents were born in Sweden (86%), 12% were born in Europe, and 2% were born outside Europe, North America or Australia/New Zealand (Tuvblad et al. 2006).
The zygosity of twins was assessed by DNA testing. The DNA was extracted from saliva samples that were collected using OraGene® DNA (DNA Genotek Inc., Ontario, Canada) self-collection kit. For twins who did not provide DNA, zygosity was determined based on an algorithm derived from discriminant analyses of twin’s and parents’ responses to questionnaire items. The validated questionnaire items addressed the twins’ physical similarity and the frequency with which people confuse them. The algorithm only classified cases that had a 95% probability of being correctly classified as monozygotic (MZ) and dizygotic (DZ) (Lichtenstein et al. 2002).
Measures
Antisocial behavior measured at ages 8–9 years
During the first wave of assessment, antisocial behavior was measured using parent reports on the externalizing scale from the Child Behavior Checklist (CBCL) (Achenbach 1991). The CBCL has been shown in several studies to be a reliable and valid instrument for assessment of behavioral and emotional problems in children and adolescents (Achenbach and Rescorla 2000). The Externalizing Behavior scale is comprised of both aggressive and delinquent items. The aggressive items include such behaviors as destroying one’s own and other’s belongings, fighting with other children, attacking others, argues a lot, and brags and boasts. The delinquent items include such behaviors as lying, and stealing at home or elsewhere. The items had a three-point response format: 0 if the item is not true, 1 if it is sometimes or somewhat true and 2 if is very true or often true. The externalizing scale was created by summing the items (Cronbach’s alpha (α) = 0.89). The scale was skewed (skewness: 2.04, kurtosis: 6.44), and was transformed (log10(x + 1)) to approximate normal distribution (skewness: −0.18, kurtosis: −0.69).
Antisocial behavior measured at ages 13–14, 16–17, and 19–20 years
Antisocial behavior during Waves 2 through 4 was measured using a self-report delinquency questionnaire (Ring 1999). The questionnaire roughly covers three different types of law-breaking behavior: (i) Property offenses and truancy including shoplifting, breaking and entering, vandalism, motor vehicle theft; (ii) Violent offenses, including simple assault, fighting, and robbery; (iii) Drug-related offenses including the use and selling of various types of illicit drugs. During the second and third wave of assessment, the questionnaire was slightly revised by collapsing items tapping into similar areas: Wave 2 (34 items); Wave 3 (32 items), and Wave 4 (31 items). Factor analyses of antisocial behavior items resulted in a single factor solution with a high internal consistency: Wave 2: α = 0.87; Wave 3: α = 0.92; Wave 4: α = 0.83. The three scores were skewed, (Wave 2: skewness: 4.15, kurtosis: 22.82; Wave 3: skewness: 4.67, kurtosis: 34.26; Wave 4: skewness: 2.87, kurtosis: 11.63), and each score was transformed (log10(x + 1)) (Wave 2: skewness: 0.99, kurtosis: 0.13; Wave 3: skewness: 0.59, kurtosis: −0.44; Wave 4: skewness: 0.49, kurtosis: −0.68).
During Waves 2–4, data were also collected on adolescent externalizing behavior using parent report on CBCL. Combining parent and self-report data would diminish possible reporter bias. However, because adolescents and parents completed two different measures, as well as no self-report data was collected during the first wave of assessment, parent reports on antisocial behavior were only included from the first wave of assessment.
Reliability of the antisocial behavior questionnaire
Test–retest reliability of the antisocial behavior questionnaire was evaluated by comparing responses of a subset of individuals who participated both in the questionnaire survey and in a telephone interview during the third wave of assessment: n = 72 (ages 16–17). On average, there were 2 months between the questionnaire being completed and returned and the telephone interview. The test–retest reliability was reasonable: r = 0.73.
Validity of the antisocial behavior questionnaire
The items in the antisocial behavior measure were initially derived from an instrument used in the project Delinquent Behavior among Young People in the Western World. This project compared self-reports of antisocial behavior in 14 studies in 13 different countries (Junger-Tas et al. 1994). The validity of the measure has been extensively addressed in each of these studies. Overall, the measure was reported to have good psychometric properties and moderate to high validity (see for example (Moffitt et al. 1994). The measure was later translated into Swedish, and transformed from interview- to questionnaire format (Ring 1999).
Attrition
Attrition was examined to determine whether subjects lost to follow-up in Waves 2–4 differed from responders at earlier waves, as it can bias the estimates in longitudinal analyses (Heath et al. 1998). No significant difference in antisocial behavior were found between responders and non-responders at Wave 2 or 3 compared with Wave 1 (Tuvblad et al. 2005; Tuvblad et al. 2006). At wave 4, when the twins were 19–20 years old, comparisons of responders and non-responders revealed that more boys than girls ceased to participate (OR (odds ratio) = 0.39; 95% CI: 0.32–0.48), and those scoring higher on the antisocial behavior score at Wave 3 were more likely to drop out at Wave 4 (OR = 1.46; 95% CI: 1.15–1.86).
We used two strategies to further explore attrition between Waves 3 and 4: (1) analyze complete cases, i.e., only analyze the twins that responded at all four time points, and (2) mean imputation (Taylor 2004). When we compared the pattern of results between these two set of analyses with the analyses of the full sample, they were found to be very similar, thus, only the results from the full sample are presented here.
Statistical analyses
Descriptive statistics
Data from four measurement occasions were used to calculate mean levels and standard deviations of antisocial behavior within each age group. As a preliminary assessment of the underlying sources of variance among the measures, comparisons were made among within-person correlations (i.e., phenotypic correlations among antisocial behavior Waves 1–4) and intraclass correlations (i.e., Twin-1 and -2 correlations within each Wave). The magnitude of the intraclass correlations provides important information about the genetic and environmental etiology of a given trait. For example, a dizygotic (DZ) intraclass correlation that is approximately half the value of the monozygotic (MZ) intraclass correlation indicates the presence of genetic effects, whereas a DZ intraclass correlation that is more than half a MZ intraclass correlation indicates the presence of both genetic and shared environmental effects.
Genetic analyses
Univariate models were fit to estimate the relative contributions of genetic (A) influences, genetic non-additivity (i.e., dominance or epistasis) (D) or shared environmental (C) influences, and non-shared environmental (E) influences to antisocial behavior within each wave. To test for sex differences, a model in which the magnitude of genetic and environmental effects were allowed to differ between males and females was compared to a model in which the estimates were constrained to be equal.
Next, to investigate the genetic and environmental etiology of antisocial behavior longitudinally, three multivariate models were tested: (I) Cholesky Decomposition Model, (II) an Independent Pathway Model and (III) a Common Pathway Model. These models were compared to a saturated model, in which the means and 8 × 8 matrices of covariances among the measures were estimated. A Cholesky model decomposes the individual variance of each variable, as well as the co-variances among the variables into genetic (A), shared environmental (C) and non-shared (E) environmental components. Cholesky models have the same number of factors in each of the A, C, and E components. The first genetic factor in A loads on all four (phenotypic) measures, the second genetic factor loads on all measures except the first measure and so on, until the last genetic factor only loads on the last measure; this same procedure repeats for the shared environmental (C) and non-shared (E) environmental components. In the Independent Pathway Model, genetic and environmental effects are of two types: common and time-specific. The model specifies common genetic (A), common shared environmental (C), and common non-shared environmental (E) factors that load on antisocial behavior at each of the four time points, as well as genetic and environmental effects that are specific (plus error) to each time point. In the Common Pathway Model, the common genetic (A), common shared environmental (C), and common non-shared environmental (E) factors are mediated through a shared latent factor that represents the variance in antisocial behavior shared among the four time points. The model estimates fewer parameters than the independent pathway model and therefore is more parsimonious. In addition to the genetic and environmental effects of the shared latent factor, parameters that are specific to each time point (As, Cs, and Es (plus error)) are also estimated (McArdle and Goldsmith 1990; Neale and Cardon 1992).
All models were fit in the structural equation program Mx (Neale et al. 2006), using a maximum likelihood estimation procedure for raw data. The raw maximum likelihood approach yields a goodness of fit index calculated as two times the log-likelihood of the data given the model (−2LL). The difference between the fit index of a full model and that of a submodel, in which parameters are fixed to be zero or constrained to be equal, follows a χ2 distribution with the difference in the number of estimated parameters as the degrees of freedom. A non-significant difference indicates that the constrained model fits the data no worse than the full model. The suitability of the models was also evaluated by comparing the model’s Akaike Information Criterion (AIC). The AIC represents the balance between model fit and the number of parameters (parsimony), with lower values (i.e., larger negative) of AIC indicating the more suitable model.
Results
Descriptive statistics
Table 1 presents means, standard deviations and number of participants across the four Waves. No significant mean or variance differences were found between twin-1 and -2, or across zygosity groups. Significant mean differences were found between males and females, with boys showing higher mean antisocial behavior scores at all ages (Wave 1: t = 4.91, df = 2094, p < 0.01; Wave 2: t = 6.30, df = 2108, p < 0.01; Wave 3: t = 6.08, df = 2115, p < 0.01; Wave 4: t = 4.47, df = 1633, p < 0.01).
Table 2 outlines the phenotypic correlations. There were moderate associations across the waves, with the exception of antisocial behavior measured at ages 8–9 years, indicating that antisocial behavior is somewhat stable during adolescence and early adulthood.
As can be seen in Table 3, all the MZ intraclass correlations were higher than the DZ intraclass correlations, suggesting that genetic influences are important for antisocial behavior. There was no evidence for genetic non-additivity (i.e., dominance or epistasis), as none of the MZ intraclass correlations exceeded twice the values of the DZ intraclass correlations for the same sex-twin pairs, apart from in girls at Wave 4. All the DZ intraclass correlations were greater than half the MZ intraclass correlations, suggesting shared environmental effects, again, apart from in girls at Wave 4.
Univariate genetic analyses
The univariate model-fitting results within each Wave are presented in Table 4. Compared to the fit of the saturated model, a Cholesky AE model explained the data in the best and most parsimonious way at Waves 1 and 4, whereas ACE model was the best-fitting and most parsimonious model at Waves 2 and 3. Genetic and environmental influences on antisocial behavior could not be equated across sexes: 8–9 years (Δχ2 = 4.295, df = 2, p = 0.12, ∆AIC = 0.297), 13–14 years (Δχ2 = 6.570, df = 3, p = 0.09, ∆AIC = 0.570), 16–17 years (Δχ2 = 41.380, df = 3, p < 0.01, ∆AIC = 35.380), or 19–20 years (Δχ2 = 6.139, df = 2, p = 0.046, ∆AIC = 2.139).
Parameter estimates of the univariate models are presented in Table 5. Results from Wave 2, ages 13–14 years, and Wave 3, ages 16–17 years, have previously been reported (Tuvblad et al. 2005; Tuvblad et al. 2006). Overall, the results from the univariate genetic analyses indicate that genetic and non-shared environmental effects were most influential on antisocial behavior at Waves 1 and 4, in both males and females. During Waves 2 and 3, shared environment seemed to be more important for antisocial behavior than genetic effects in males. In contrast, the genetic factors were more influential on antisocial behavior in females.
Longitudinal genetic analyses
To investigate the genetic and environmental etiology of antisocial behavior longitudinally, a series of multivariate models were fitted (Model # 2–4 in Table 6). Specifically, a saturated model (Model # 1 in Table 6) was used as a baseline to which a Cholesky decomposition (Model # 2), an Independent Pathway (Model # 3), and a Common Pathway (Model # 4) were compared. Based on AIC criteria, the Common Pathway model provided the best balance of parsimony and fit. Both the Cholesky decomposition and the Independent Pathway model resulted in more positive AIC values, indicating that a common factor best account for the genetic and environmental covariance across time in antisocial behavior.
Next, we explored the extent to which the factor structure and underlying genetic and environmental etiology varied across sexes, using the full Common Pathway model (Model # 4) as our comparison model. The factor loadings could be constrained to be equal in males and females without a significant decrease in fit (Table 6: Model 4a, Δχ2 = 3.081; df = 4; p = 0.544), as could the common genetic and environmental influences (Table 6: Model 4b, Δχ2 = 5.730; df = 7; p = 0.572). However, equating the age-specific genetic and environmental effects across sexes resulted in a significant deterioration in fit compared to the full Common Pathway model (Table 6: Model 4c, Δχ2 = 60.102; df = 19; p < 0.01). This suggests that there are sex differences in the underlying genetic and environmental etiology of antisocial behavior, but that these are age-specific. Figure 1 displays standardized parameter estimates from this best fitting common pathway model.
Standardized parameter estimates from a common pathway model for antisocial behavior in males and females. Latent (unobserved) factors are depicted in circle: the A refer to additive genetic factors; the C to shared environmental factors, and the E to non-shared environmental factors. Oval denotes a latent underlying factor (i.e., persistent antisocial behavior). Observed variables are in rectangular, in this case: antisocial behavior ages 8–9 years, 13–14 years, 16–17 years, and 19–20 years. As (additive genetic): is residual variance specific to each time point, likewise for Cs (shared environment), and Es (non-shared environment). Estimates of the common latent factors (A, C, and E) as well as common factor loadings were the same for both males and females. Age-specific parameter estimates were different for males and females (presented in italic). Significant parameter estimates are presented in bold. aEstimates are the same for males and females
Squaring these standardized parameter estimates presented in Fig. 1 provides the relative contributions to the phenotypic variance. In the latent factor labeled Persistent Antisocial Behavior, 67% (p < 0.05) of the variance was due to genetic influences, 26% (p < .05) explained by shared environmental influences, and 7% (p < 0.05) due to non-shared environmental effects.
For males, the age specific variance was mainly due to non-shared environmental influences, which explained 27% (p < 0.05), 30% (p < 0.05), 34% (p < 0.05), and 50% (p < 0.05), of the variance in antisocial behavior in Waves 1–4, respectively. Unique genetic influences were found at ages 8–9 years, accounting for 64% (p < 0.05) of the variance, and at ages 13–14 years there were some shared environmental effects, 22% (p < 0.05).
For females, age-specific variance was mainly explained by genetic and non-shared environmental influences. Age-specific genetic effects (As) explained 71% (p < 0.05) and 17% (p < 0.05) of the variance in antisocial behavior at ages 8–9 and 13–14 years, respectively. Age-specific non-shared environmental influences (Es) accounted for 21% (p < 0.05), 24% (p < 0.05), 19% (p < 0.05) and 37% (p < 0.05) of the variance in antisocial behavior in Waves 1–4, respectively. As in males, some age-specific shared environmental influences (Cs) at ages 13–14 years were found, explaining 12% of the variance in antisocial behavior at that age.
The breakdown of common and age-specific influences on antisocial behavior based on the factor loadings presented in Fig. 1 is summarized in Table 7. For both males and females, the total variance at ages 8–9 years was mainly explained by age-specific genetic effects, with lesser contributions of shared and non-shared environment.
For males, at ages 13–14 years the genetic, shared and non-shared environmental influences approximately explained a third each. At ages 16–17 years, genetic effects were more important than shared environmental influences. At ages 19–20 years, approximately half of the total variance in antisocial behavior was explained by the non-shared environment. Genetic and shared environmental contributions to the variance were of equal importance.
In females, genetic effects explained approximately half of the variance of the antisocial behavior within each time point. Shared and non-shared environmental influences were of equal importance at ages 16–17 and 19–20 years. At ages 19–20 years, shared environmental effects were less important. The estimates presented in Table 7 slightly differ from the univariate results (Table 5) because the data analysed in multivariate models includes additional information on covariances between the variables, both within-person and across-twins.
Discussion
In the present study we examined the genetic and environmental etiology of antisocial behavior measured at four time points from childhood to early adulthood. We found that the covariance in antisocial behavior could be explained by a common latent factor defined as persistent antisocial behavior. This latent persistent antisocial behavior factor had the same structure in males and females. A common genetic influence accounted for 67% of the total variance in this latent factor, the shared environment explained 26%, and the remaining 7% was due to the non-shared environment. Age-specific genetic and environmental effects differed across sexes.
Our finding that a common latent factor explained the covariance in antisocial behavior across development and that the variance in this latent factor was due to genetic as well as shared environmental influences may be interpreted in support of Moffitt’s developmental taxonomy of antisocial behavior. According to the theory, persistent antisocial behavior is associated with inherited (genetic) neuropsychological impairments and family adversity (Moffitt 1993). Early neuropsychological deficits are thought to result in traits such as poor cognitive functioning, emotional reactivity, and hyperactivity/impulsivity, which are all risk factors for antisocial behavior (Lahey et al. 2003). Further, common adverse family experiences are likely to be expressed as shared environmental effects in our model. Previous research examining the relationship between parenting style and antisocial behavior also emphasize the importance of parenting characteristics in the development of antisocial behavior. Specifically, harsh and inconsistent parenting is strongly associated with the development of antisocial behavior (Farrington et al 1996; Farrington and Loeber 2000; Moffitt et al. 2001).
Results from the current study suggested that the development of persistent antisocial behavior was primarily influenced by genetic factors, explaining 67% of the total variance. Similar findings were reported in a recent longitudinal study, in which common genetic influences were found to account for the variance in antisocial behavior from childhood to adulthood (Silberg et al. 2007). Common genetic influences were also found to be important for the development of antisocial behavior in a study using retrospective data. However, common genetic influences were only important during adolescence and adulthood, and did not overlap with antisocial behavior prior to the age of 15 years (Jacobson et al. 2002). Differences in data collection procedures (i.e., prospective versus retrospective data) as well as inclusion of females in the present report may explain the discrepancies between these studies. Also, a common factor model was not tested in the previous studies, making the comparison of their results somewhat limited.
In addition to a strong genetic influence, the shared environment was also important for antisocial behavior that persists from childhood to early adulthood, accounting for 26% of the total variance. This finding differs from the results reported in Silberg et al. (2007), where the shared environment was shown to be of minimal importance for persistent antisocial behavior. The authors discussed their findings in light of possible gene–environment interaction (G × E) effects. If G × E is influential for the development of persistent antisocial behavior, shared environmental contributions would be confounded in the estimate of genetic effects. Effects of G × E may also be present in the current study, as suggested by substantial genetic contributions to the latent factor of the persistent antisocial behavior. Clearly, inconsistent findings regarding the etiology of persistent antisocial behavior warrants further research, in which gene-environment interplay is taken into account.
Previous cross-sectional studies have reported that non-shared environmental effects explain around 50% of the variance in antisocial behavior (Rhee and Waldman 2002). In the current study, these effects accounted for only 7%, whereas familial effects (i.e., genetic and shared environmental effects), explained more than 90% of the variance in persistent antisocial behavior in both males and females. The most likely explanation for the difference between the moderate familial influence reported in cross-sectional studies and the large familial influence found in the current study is the reduced measurement error when the four time points are considered together in multivariate analyses, and that true non-shared environmental effects are likely to be time-specific.
No significant sex differences were found in the etiology of the latent persistent antisocial behavior factor. Likewise, genetic and shared environmental influences on antisocial behavior were reported to be similar in males and females in another longitudinal study (Jacobson et al. 2002). These findings together suggest that Moffitt’s theory of life-course-persistent antisocial behavior can be applied not only to males, but also to females. As in males (Lyons et al. 1995; Taylor et al. 2000), persistent forms of antisocial behavior in females seem to develop primarily due to inherited factors.
The pattern of age-specific genetic and environmental influences was to some extent supportive of adolescence-limited antisocial behavior (DiLalla and Gottesman 1989; Moffitt 1993). Age-specific shared environmental effects were important at ages 13–14 years in both males and females. These shared environmental effects are likely to reflect the influence of delinquent peers (Farrington and Loeber 2000; Silberg et al. 2007). However, the shared environmental effects could also include family related factors, such as parenting style (Narusyte et al. 2007), or other types of shared environmental experiences such as, for example, residing in a disadvantaged neighborhood (Sampson et al. 1997). At ages 16–17 and 19–20 years shared environmental effects were not significant and the age-specific variance of antisocial behavior was primarily explained by the non-shared environment (including measurement error) (Table 7, Fig. 1). This would suggest that the influence of common environmental experiences for the development of antisocial behavior is less important at later ages. However, socializing with deviant peers may still be influential through affecting each twin individually, which would be expressed as non-shared environment.
The magnitude of age-specific genetic and environmental effects was found to differ across sexes. As shown in Table 7, age-specific genetic effects were more important in females than in males. This suggests that genetic liability for antisocial behavior may be higher among females, as compared to males. Results of adoptions studies also show that genetic risk for criminal outcome in adult years tends to be higher for female offenders than for male offenders (Baker et al. 1989). Despite the fact that the prevalence of antisocial behavior generally is lower in females, affected female probands usually have more affected relatives than male probands (Cloninger and Gottesman 1987; Rhee and Waldman 2002). Our finding of a stronger genetic influence for antisocial behavior in females than in males may also be compared to a more recent study, which investigated all convictions for violent crimes in Sweden between 1973 and 2004. In the study, the risk of violent convictions among relatives of violent individuals was compared to relatives of matched, non-violent controls, using a nested case–control design. Interestingly, familial risk (genetic and shared environmental factors) was stronger among females, in individuals in higher socio-economic settings, and for early onset interpersonal violence (Frisell et al. 2011). It should also be kept in mind, that the majority of research on antisocial behavior has tended to focus on males only, therefore more research including females examining the genetic and environmental etiology of antisocial behavior is needed.
Limitations of the present study
The majority of the variance in antisocial behavior at ages 8–9 years was accounted by age-specific effects. There are several possible explanations for this. First, self-report data on antisocial behavior was not collected when the twins were 8–9 years old. Therefore, different raters were used: caregiver reports on their twin’s antisocial behavior at ages 8–9 years and youth self-reports at ages 13–14, 16–17, and 19–20 years. Cross-informant information may endanger reliable assessment of the continuity of risk factors since rater-specific influences may confound the association between early (parent-report based) and later (self-report based) assessments. In addition, agreement between caregiver and child ratings are usually reported to be rather low, as was the case in the present study (Achenbach et al. 1987; Youngstrom et al. 2000). Second, low correlations between antisocial behaviors assessed when the twins were 8–9 years old and when assessed at later time points may also be explained by the use of different measures to evaluate antisocial behavior at the different ages (i.e., externalizing measure from CBCL at ages 8–9 years versus a self-report delinquency measure at subsequent time points). There were also a longer time period between Wave 1 (8–9 years) and Wave 2 (13–14 years) compared with tighter time intervals between later waves. Finally, a higher prevalence of antisocial behavior in adolescence as compared to childhood could also contribute to the low correlations. Regardless, it is important to note that it was possible to identify a common latent factor, suggesting that the covariance among behavior problems at different ages is not only due to reporter artifact.
A second limitation concerns attrition. Between Wave 3 and Wave 4, the response rate went down from 82 to 51%. During the fourth assessment, the twins were 19–20 years old, a time when many youths are off to college, or for other reasons have moved away from home. We used two strategies (analyze complete cases and mean imputation) to explore attrition between these waves. Even though we found that the pattern of results between these two sets of analyses and the results from the analyses using the full sample were very similar, it is possible that those dropping out include a disproportionate percentage with psychopathology. Thus, it is not certain that all of the results are generalizable to individuals with the most extreme externalizing behaviors.
Another limitation may be assortative mating. Assortative mating in the parent generation acts to increase the resemblance between dizygotic twins, and thereby bias shared environmental estimates upward and additive genetic effects downward. A significant correlation between spouses for a particular trait is often interpreted as assortative mating (Maes et al. 1998). The few studies on antisocial behavior that have controlled for the effects of assortative mating conclude that assortative mating accounts for a modest part of antisocial behavior (Maes et al. 2007; Taylor et al. 2000), for an exception see (Krueger et al. 1998). In the present study, caregivers were asked to retrospectively report their antisocial behavior during their early twenties. A correlation of 0.15 (p < 0.01) was found between mothers’ and fathers’ antisocial behavior, indicating a small effect of assortative mating in the current sample. The estimates of shared environment may therefore be slightly biased upward. However, the limited number of parents responding at Wave 4 should also be kept in mind.
Finally, crucial to the twin design is the equal environment assumption (EEA). If this assumption is violated, higher correlations among MZ twins may be due to environmental factors, rather than genetic factors, and the heritability estimate is overestimated. One way to test the validity of the EEA is to examine whether a trait of interest is influenced by perceived versus assigned zygosity. We included the effect of perceived zygosity as a “specified” familial environment in a univariate ACE model (Kendler et al. 1993). The effect of perceived zygosity on antisocial behavior was negligible and could be omitted without any significant loss in data fit (p > 0.35). The EEA seems therefore to hold for antisocial behavior problems in our sample.
Conclusions
Understanding the genetic and environmental causes of antisocial behavior is an important priority for crime prevention. The current study found substantial genetic and shared environmental factors together with a more limited non-shared environmental influence on antisocial behavior that persists from childhood to emerging adulthood, in both males and females. The results of our study make an important step towards the understanding of genetic and environmental etiology of antisocial behavior that persists across development.
References
Achenbach TM (1991) Manual for the child behavior checklist/4–18 and 1991 profile. University of Vermont, Department of Psychiatry, Burlington, VT
Achenbach TM, Rescorla LA (2000) Manual for ASEBA school-age forms & profiles. University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT
Achenbach TM, McConaughy SH, Howell CT (1987) Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull 101(2):213–232
Arseneault L, Moffitt TE, Caspi A, Taylor A, Rijsdijk FV, Jaffee SR et al (2003) Strong genetic effects on cross-situational antisocial behaviour among 5-year-old children according to mothers, teachers, examiner-observers, and twins’ self-reports. J Child Psychol Psychiatry 44(6):832–848
Baker LA, Mack W, Moffitt TE, Mednick S (1989) Sex differences in property crime in a Danish adoption cohort. Behav Genet 19(3):355–370
Bartels M, van den Oord EJ, Hudziak JJ, Rietveld MJ, van Beijsterveldt CE, Boomsma DI (2004) Genetic and environmental mechanisms underlying stability and change in problem behaviors at ages 3, 7, 10, and 12. Dev Psychol 40(5):852–867
Burt SA, McGue M, Carter LA, Iacono WG (2007) The different origins of stability and change in antisocial personality disorder symptoms. Psychol Med 37(1):27–38
Cloninger CR, Gottesman I (1987) Genetic and environmental factors in antisocial behavior disorders. In: Mednick S, Moffitt TE, Stack SA (eds) The causes of crime: new biological approaches. Cambridge University Press, New York, pp 96–100
DiLalla LF, Gottesman I (1989) Heterogeneity of causes for delinquency and criminality: lifespan perspectives. Dev Psychopathol 1:339–349
Farrington DP, Loeber R (2000) Epidemiology of juvenile violence. Child Adolesc Psychiatr Clin North Am 9:733–748
Farrington DP, Barnes GC, Lambert S (1996) The concentration of offending in families. Legal Criminol Psychol 1:47–63
Frisell T, Lichtenstein P, Låneström N (2011) Violent crime runs in families: a total population study of 12.5 million individuals. Psychol Med 41(1):97–105
Heath AC, Madden PA, Martin NG (1998) Assessing the effects of cooperation bias and attrition in behavioral genetic research using data-weighting. Behav Genet 28(6):415–427
Jacobson KC, Prescott CA, Kendler KS (2002) Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev Psychopathol 14(2):395–416
Junger-Tas J, Terlouw G-J, Klein MW (1994) Delinquent behavior among young people in the western world. Kugler, Amsterdam
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1993) A test of the equal-environment assumption in twin studies of psychiatric illness. Behav Genet 23(1):21–27
Krueger RF, Moffitt TE, Caspi A, Bleske A, Silva PA (1998) Assortative mating for antisocial behavior: developmental and methodological implications. Behav Genet 28(3):173–186
Lahey BB, Moffitt TE, Caspi A (eds) (2003) Causes of conduct disorder and juvenile delinquency. Guilford Press, New York
Lichtenstein P, De faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL (2002) The Swedish twin registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med 252(3):184–205
Lichtenstein P, Tuvblad C, Larsson H, Carlstrom E (2007) The Swedish twin study of child and adolescent development: the TCHAD-study. Twin Res Hum Genet 10(1):67–73
Lyons MJ, True WR, Eisen SA, Goldberg J, Meyer JM, Faraone SV et al (1995) Differential heritability of adult and juvenile antisocial traits. Arch Gen Psychiatry 52(11):906–915
Maes HH, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL et al (1998) Assortative mating for major psychiatric diagnoses in two population-based samples. Psychol Med 28(6):1389–1401
Maes HH, Silberg JL, Neale MC, Eaves LJ (2007) Genetic and cultural transmission of antisocial behavior: an extended twin parent model. Twin Res Hum Genet 10(1):136–150
McArdle JJ, Goldsmith HH (1990) Alternative common factor models for multivariate biometric analyses. Behav Genet 20(5):569–608
Moffitt TE (1993) Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychol Rev 100(4):674–701
Moffitt TE, Silva PA, Lynam DR, Henry B (1994) Self-reported delinquency at age 18: New Zealand’s Dunedin multidisciplinary health and development study. In: Junger-Tas J, Terlouw G-J, Klein MW (eds) Delinquent behavior among young people in the western world. Kugler Publications, Amsterdam
Moffitt TE, Caspi A, Rutter M, Silva PA (2001) Sex differences in antisocial behaviour: conduct disorder, delinquency and violence in the Dunedin longitudinal study. Cambridge University Press, Cambridge
Narusyte J, Andershed AK, Neiderhiser JM, Lichtenstein P (2007) Aggression as a mediator of genetic contributions to the association between negative parent-child relationships and adolescent antisocial behavior. Eur Child Adolesc Psychiatry 16(2):128–137
Neale MC, Cardon LR (1992) Methodology for genetic studies of twins and families. Kluwer Academic Publisher, Dordrecht, The Netherlands
Neale MC, Boker SM, Xie G, Maes HH (2006) Mx: statistical modelling, 7th edn. Department of Psychiatry, Medical College of Virginia, Richmond, VA
Rhee SH, Waldman ID (2002) Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull 128(3):490–529
Ring J (1999) Hem och skola, kamrater och brott [Home and school, peers and delinquency]. Kriminologiska institutionen, Stockholms universitet, Stockholm
Sampson RJ, Raudenbush SW, Earls F (1997) Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 277(5328):918–924
Silberg JL, Rutter M, Tracy K, Maes HH, Eaves L (2007) Etiological heterogeneity in the development of antisocial behavior: the Virginia twin study of adolescent behavioral development and the young adult follow-up. Psychol Med 37(8):1193–1202
Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP et al (1997) Modeling genetic and environmental influences in the etiology of conduct disorder: a study of 2,682 adult twin pairs. J Abnorm Psychol 106(2):266–279
Taylor A (2004) The consequences of selective participation on behavioral-genetic findings: evidence from simulated and real data. Twin Res 7(5):485–504
Taylor J, Iacono WG, McGue M (2000a) Evidence for a genetic etiology of early-onset delinquency. J Abnorm Psychol 109(4):634–643
Taylor J, McGue M, Iacono WG (2000b) Sex differences, assortative mating, and cultural transmission effects on adolescent delinquency: a twin family study. J Child Psychol Psychiatry 41(4):433–440
Tuvblad C, Eley TC, Lichtenstein P (2005) The development of antisocial behaviour from childhood to adolescence. A longitudinal twin study. Eur Child Adolesc Psychiatry 14(4):216–225
Tuvblad C, Grann M, Lichtenstein P (2006) Heritability for adolescent antisocial behavior differs with socioeconomic status: gene-environment interaction. J Child Psychol Psychiatry 47(7):734–743
van Beijsterveldt CE, Bartels M, Hudziak JJ, Boomsma DI (2003) Causes of stability of aggression from early childhood to adolescence: a longitudinal genetic analysis in Dutch twins. Behav Genet 33(5):591–605
van der Valk JC, van den Oord EJ, Verhulst FC, Boomsma DI (2003) Genetic and environmental contributions to stability and change in children’s internalizing and externalizing problems. J Am Acad Child Adolesc Psychiatry 42(10):1212–1220
Youngstrom E, Loeber R, Stouthamer-Loeber M (2000) Patterns and correlates of agreement between parent, teacher, and male adolescent ratings of externalizing and internalizing problems. J Consult Clin Psychol 68(6):1038–1050
Acknowledgment
This study was funded by the Swedish Council for Working Life and Social Research (project 2004-0383) and the Swedish Research Council (2004-1415).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Irwin Waldman.
Rights and permissions
About this article
Cite this article
Tuvblad, C., Narusyte, J., Grann, M. et al. The Genetic and Environmental Etiology of Antisocial Behavior from Childhood to Emerging Adulthood. Behav Genet 41, 629–640 (2011). https://doi.org/10.1007/s10519-011-9463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-011-9463-4