Abstract
We tested whether telomere length is altered in the brains of patients diagnosed with major depression (MD), bipolar disorder (BD) and schizophrenia (SZ) by measuring mean telomere length (mTL) with real-time PCR. The samples are cerebellar gray matter from 46 SZ, 46 BP, and 15 MD patients, and 48 healthy controls. We found no difference in mTL between SZ and controls, BD and controls, MD and controls, or all cases and controls; no correlation between mTL and age was observed, either. This suggests that brain gray matter is unlikely to be related to the telomere length shortening reported in blood of psychiatric patients. White matter deserves further investigation as it has been reported to have a different mTL dynamic from gray matter. Since mTL has been reported to be a heritable quantitative trait, we also carried out genome-wide mapping of genetic factors for mTL, treating mTL as a quantitative trait. No association survived correction of multiple testing for the number of SNPs studied. The previously reported rs2630578 (BICD1) association was not replicated. This suggests that telomere length of cerebellar gray matter is determined by multiple loci with “weak effects.”
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Telomeres are DNA–protein complexes located at the ends of chromosomes involved in preventing chromosome fusion and maintaining genome stability (Blackburn 2000). Telomeres comprise a variable number of repeats of the sequence TTAGGG (Blackburn 1991; Zakian 1995) and their lengths decline with cell division (Lansdorp 2008). Oxidative stress has been proposed to be an important modulator of telomere length shortening (von Zglinicki 2002).
Telomere shortening in psychiatric diseases
Telomere shortening has been reported in psychological stress and psychiatric disorders, including schizophrenia (SZ), bipolar disorder (BD) and major depression (MD) (Epel et al. 2004; Kao et al. 2008; Lung et al. 2007; Simon et al. 2006). However, the previous telomere studies of psychiatric diseases all measured mean telomere length (mTL) of DNA from leukocytes in blood, not from the more relevant brain tissue.
Prefrontal and limbic system dysfunction are known to be strongly involved in the development of SZ, BD, and MD (Barch 2005; Maletic et al. 2007; McIntosh et al. 2008; Miklowitz and Johnson 2006). Also, telomere length has been shown to vary among tissues (Prowse and Greider 1995; Wang et al. 2005). Though cerebellum is not thought to be the most relevant brain region for psychiatric disease, studies have shown that the cerebellum may play a role in BD, SZ, and MD (Andreasen and Pierson 2008; Konarski et al. 2005; Schmahmann et al. 2007). Most importantly, cerebellum has very similar cellular composition, development and aging dynamics as other brain regions, in contrast to blood.
Therefore, we have tested whether telomere length is altered in the cerebellum of psychiatric patients with MD, BD, and SZ. The facts that oxidative status in the cerebellum, cerebral cortex, hippocampus, and peripheral leucocytes is associated with anxiety levels in mice (Rammal et al. 2008), and that oxidative status is also thought to be related to telomere length (von Zglinicki 2002), support a prediction that psychiatric disease status and mTL are associated.
Genetics of telomere length
BD, SZ, and MD all have underlying genetic factors (Alaerts and Del-Favero 2009; Shyn et al. 2009). If telomere shortening is related to psychiatric disease, discovery of genetic factors that determine telomere length or shortening would be important for understanding the genetics of these diseases.
The mTL of most somatic cells is negatively correlated with age, although it varies greatly among individuals, even those in the same age group (Andrew et al. 2006; Okuda et al. 2000; Vasa-Nicotera et al. 2005; Vaziri et al. 1994; Vaziri et al. 1993). Estimates of the heritability of mTL in leucocytes vary: past twin and family studies have placed it around 78–84% (Jeanclos et al. 2000; Slagboom et al. 1994), while a more recent, large, female twin study found a heritability of 36% after adjusting for batch effects, with a large common environmental effect of 49% (Andrew et al. 2006).
Data from yeast suggest that telomere length variation is associated with polymorphisms at a large number of loci (Gatbonton et al. 2006). In humans, although a genetic association mapping study of two candidate genes failed to generate a positive result (Zhang et al. 2007), linkage analysis has mapped telomere regulatory regions to two different chromosomal regions (chromosome 14q23.2 and chromosome 12p11.2) in two leukocyte studies (Andrew et al. 2006; Vasa-Nicotera et al. 2005). The latter group further found that single nucleotide polymorphism (SNP) rs2630578 in BICD1 was associated with telomere length (Mangino et al. 2008).
To test whether there is a genetic variant(s) with strong effect that determines telomere length in brain, we have carried out a genome-wide SNP-based association mapping of regulatory regions of mTL in human brain. Previous studies have only examined mTL in leukocytes; we have used brain tissue since telomere length and its determinants could differ between brain and blood, and brain is biologically more relevant to psychiatric diseases.
Materials and methods
We studied genomic DNA extracted from 155 brain samples (gray matter of cerebellums) from individuals of European ancestry from the Stanley Medical Research Institute (SMRI). The samples are from persons with SZ (n = 46), BD (n = 46), MD (n = 15), and from healthy controls (n = 48). We measured mTL by quantitative PCR (Brouilette et al. 2007; Cawthon 2002) with some modifications (see supplementary methods). The amplification data were analyzed using SDS software (version 2.1, Applied Biosystems). The relative T/S ratio was calculated as previously described (Cawthon 2002). We genotyped subjects using the Affymetrix Genome-Wide Human SNP Array 5.0 (Affymetrix, Santa Clara, CA); the SNP genotyping was performed by David W. Craig and Margot Redman at the Translational Genomic Research Institute (TGEN). There were 240,576 common SNPs of sufficiently high quality (MAF > 0.1, HWE p-value > 0.001) to include in the subsequent mapping analysis. Average call rate was 99.9%.
Analysis of covariance (ANCOVA) was employed to test the significance of telomere length differences between SZ (or BD or MD or all cases) and controls. Telomere length (T/S ratio) was the dependent variable, and disease status (case or control), age, and sex were covariates.
For genome-wide association analysis of SNP genotypes with mTL, linear regression was carried out using PLINK (Purcell et al. 2007) with mTL as the dependent variable. Since the ANCOVA showed that age and disease status did not have an effect on mTL in this study, these factors were excluded in the linear regression. Data on lifetime antipsychotic use were analyzed as quantitative covariates. Smoking and alcohol use were analyzed as binary covariates.
Results
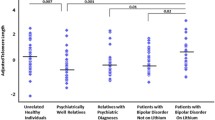
We observed no difference in mTL between SZ and controls, BD and controls, MD and controls, or all cases and controls (all p-values > 0.1; see Fig. 1; Table 1). Also, age, gender, medication and drug use had no effect on mTL (all p-values greater than 0.05, data not shown).
Telomere length in cerebellar gray matter. The x-axis shows four groups of individuals. BP represents bipolar patients, SZ represents schizophrenia patients, controls represent healthy individuals and Dep represents patients with depression. The y-axis shows telomere length. For each box plot, the top bar is the maximum observation, the lower bar is the minimum observation, the top of the box is the upper or third quartile, the bottom of box is the lower or first quartile, the middle bar is the median value and circles are possible outliers
In the mTL quantitative trait locus (QTL) genome-wide mapping analysis, SNP rs12519633 on chromosome 5 showed the most significant association, with a p-value of 4.31E-06. The top three most significant associations are shown in Table 2, but the permutated p-values are not significant after correcting for the number of SNPs tested, since all are greater than 0.05.
Discussion
In the present study, we investigated the correlation of mTL in brains with disease status for SZ, BD, or MD for the first time. No difference of mTL in the cerebellar gray matter was found in SZ, BD, MD, or all cases compared to controls. The mTL in MD was nonsignificantly shorter than in BD, SZ, and controls.
Significant telomere reductions have been reported in leukocytes and lymphocytes of MD, BD, and SZ. Decreased telomere length in MD (measured by Southern blot) has been reported in white blood cells when comparing 253 patients to 411 controls (Lung et al. 2007) (age MD = 44.48 ± 15.18, controls = 45.32 ± 13.85). A significant difference in mTL (measured by Southern blot) was also reported in a lymphocyte study of 44 combined mood disorders (15 MD and 15 BD with anxiety and 14 BD without anxiety) and 44 controls (p-value = 0.002) (Simon et al. 2006). Mean telomere length (measured by RT–PCR) was shortened in lymphocytes of 31 SZ (age 38.77 ± 10.2) compared to 41 controls (25.85 ± 7.43) (p = 0.002) (Kao et al. 2008). Kao et al. also replicated their finding in another age-matched male SZ-control study (26 controls and 33 SZ, age 33.19 ± 8.81, 36.24 ± 8.99, respectively).
Our findings should be most comparable to those of Kao et al., since our mTL RT–PCR measurement methods were very similar. We did not detect a correlation between affection status and mTL, while Kao et al. did. The likely explanation for the discrepancy is that we used brain tissue instead of blood. Previous studies have shown that telomere length varies among tissues (Prowse and Greider 1995; Wang et al. 2005). Thomas et al. observed a significant age effect on mTL in white blood cells but not in buccal cells (Thomas et al. 2008).
Due to the lack of mitosis of neurons and slow replacement of glial cells, telomere length is considered to be stable in adult brain. However, Nakamura and colleagues found that telomeres in the cerebral gray matter were longer than those in the cerebral white matter, and observed no difference in telomere length between infants and individuals aged 60–69 years (Nakamura et al. 2007). We also found that telomere length is not significantly correlated with aging in the cerebellar gray matter of individuals under 70 years of age.
Though we were not able to study prefrontal cortex or limbic system regions due to sample availability problems, the findings of Nakamura et al. suggest those and other brain regions, particularly cerebral or cerebellar white matter of the brain, should be explored further. Studies of cerebellar and cerebral white matter of psychiatric disease patients for age- and disease-status-related telomere erosion and the correlation of telomere length between brain white matter and blood cells may also be important.
Although telomere length in cerebellar gray matter was age-independent and stable, it still showed inter-individual variability (mean ± SD (standard deviation) in our control samples (1.055 ± 0.251)). The SD in our samples was comparable to control group A in the study by Kao et al. (2008), despite the difference in mean (control group A, 1.51 ± 0.33; control group B, 1.51 ± 0.62).
SNP rs2630578 in BICD1 was previously reported to be associated with telomere length in leukocytes (Mangino et al. 2008). We did not observe any association from this region, including rs2630578 (p = 0.245).
The genetic factor underlying mTL is evident, but our negative genome-wide QTL mapping results were negative, suggesting that multiple “weak effect” genes may exist that contribute to telomere length variation in human, similar to many other complex phenotypes. This is consistent with the yeast genome-wide linkage findings (Gatbonton et al. 2006). A large sample study would be needed to detect those “weak effect” genes controlling telomere length, including candidate genes such as DDX11 and Rtel that have been suggested by previous linkage studies in human or other animal models (Ding et al. 2004; Vasa-Nicotera et al. 2005).
The mTLs measured in most studies, including ours, are actually the outcomes of the combined effects of two factors: at-birth initial telomere length and telomere erosion or restoration rate. Both factors could have independent genetic and environmental influences. As we only measured mTL at one point in time, it is impossible to differentiate between these two factors. A longitudinal study would be ideal for dissecting these two factors for studies of the genetics of telomere length. Brain is not a feasible target of such a study in humans, but an appropriate animal model would be an excellent alternative.
References
Alaerts M, Del-Favero J (2009) Searching genetic risk factors for schizophrenia and bipolar disorder: learn from the past and back to the future. Hum Mutat 30:1139–1152
Andreasen NC, Pierson R (2008) The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88
Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD (2006) Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet 78:480–486
Barch DM (2005) The cognitive neuroscience of schizophrenia. Annu Rev Clin Psychol 1:321–353
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Blackburn EH (2000) Telomere states and cell fates. Nature 408:53–56
Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ (2007) Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 369:107–114
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Ding H, Schertzer M, Wu X, Gertsenstein M, Selig S, Kammori M, Pourvali R, Poon S, Vulto I, Chavez E, Tam PP, Nagy A, Lansdorp PM (2004) Regulation of murine telomere length by Rtel: an essential gene encoding a helicase-like protein. Cell 117:873–886
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM (2004) Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 101:17312–17315
Gatbonton T, Imbesi M, Nelson M, Akey JM, Ruderfer DM, Kruglyak L, Simon JA, Bedalov A (2006) Telomere length as a quantitative trait: genome-wide survey and genetic mapping of telomere length-control genes in yeast. PLoS Genet 2:e35
Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A (2000) Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension 36:195–200
Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Porton B (2008) Rapid telomere erosion in schizophrenia. Mol Psychiatry 13:118–119
Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH (2005) Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci 30:178–186
Lansdorp PM (2008) Telomeres, stem cells, and hematology. Blood 111:1759–1766
Lung FW, Chen NC, Shu BC (2007) Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet 17:195–199
Maletic V, Robinson M, Oakes T, Iyengar S, Ball SG, Russell J (2007) Neurobiology of depression: an integrated view of key findings. Int J Clin Pract 61:2030–2040
Mangino M, Brouilette S, Braund P, Tirmizi N, Vasa-Nicotera M, Thompson JR, Samani NJ (2008) A regulatory SNP of the BICD1 gene contributes to telomere length variation in humans. Hum Mol Genet 17:2518–2523
McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM (2008) Prefrontal function and activation in bipolar disorder and schizophrenia. Am J Psychiatry 165:378–384
Miklowitz DJ, Johnson SL (2006) The psychopathology and treatment of bipolar disorder. Annu Rev Clin Psychol 2:199–235
Nakamura K, Takubo K, Izumiyama-Shimomura N, Sawabe M, Arai T, Kishimoto H, Fujiwara M, Kato M, Oshimura M, Ishii A, Ishikawa N (2007) Telomeric DNA length in cerebral gray and white matter is associated with longevity in individuals aged 70 years or older. Exp Gerontol 42:944–950
Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A (2000) Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis 152:391–398
Prowse KR, Greider CW (1995) Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA 92:4818–4822
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Rammal H, Bouayed J, Younos C, Soulimani R (2008) Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun 22:1156–1159
Schmahmann JD, Weilburg JB, Sherman JC (2007) The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum 6:254–267
Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, Garriock HA, Yokoyama JS, McGrath PJ, Peters EJ, Scheftner WA, Coryell W, Lawson WB, Jancic D, Gejman PV, Sanders AR, Holmans P, Slager SL, Levinson DF, Hamilton SP (2009) Novel loci for major depression identified by genome-wide association study of sequenced treatment alternatives to relieve depression and meta-analysis of three studies. Mol Psychiatry (Advanced Online Publication)
Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK (2006) Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry 60:432–435
Slagboom PE, Droog S, Boomsma DI (1994) Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet 55:876–882
Thomas P, O’ Callaghan NJ, Fenech M (2008) Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer’s disease. Mech Ageing Dev 129:183–190
Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson JR, Mason A, Bodycote CL, Raleigh SM, Louis E, Samani NJ (2005) Mapping of a major locus that determines telomere length in humans. Am J Hum Genet 76:147–151
Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley CB (1993) Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet 52:661–667
Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM (1994) Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA 91:9857–9860
Von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wang JC, Warner JK, Erdmann N, Lansdorp PM, Harrington L, Dick JE (2005) Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc Natl Acad Sci USA 102:14398–14403
Zakian VA (1995) Telomeres: beginning to understand the end. Science 270:1601–1607
Zhang F, Kato BS, Gardner JP, Kimura M, Spector TD, Ahmadi KR (2007) Lack of association between leukocyte telomere length and genetic variants in two ageing-related candidate genes. Mech Ageing Dev 128:415–422
Acknowledgments
This study was supported by funds from the Brain Research Foundation and NIH MH080425 (to C. Liu). We acknowledge Ms. Kay Grennan for her language editing. We thank the families of the individuals involved in this study. The SMRI, its collaborators, and Drs. M. Elashoff, F. Torrey and M. Webster generously gave us access to their sample collections.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by William Kremen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Cheng, L., Craig, D.W. et al. Cerebellar Telomere Length and Psychiatric Disorders. Behav Genet 40, 250–254 (2010). https://doi.org/10.1007/s10519-010-9338-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10519-010-9338-0