Abstract
Previous research has linked the facial width-to-height ratio (FWHR) to a host of psychological and behavioral characteristics, primarily in men. In two studies, we examined novel links between FWHR and sex drive. In Study 1, a sample of 145 undergraduate students revealed that FWHR positively predicted sex drive. There were no significant FWHR × sex interactions, suggesting that FWHR is linked to sexuality among both men and women. Study 2 replicated and extended these findings in a sample of 314 students collected from a different Canadian city, which again demonstrated links between the FWHR and sex drive (also in both men and women), as well as sociosexuality and intended infidelity (men only). Internal meta-analytic results confirm the link between FWHR and sex drive among both men and women. These results suggest that FWHR may be an important morphological index of human sexuality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies have linked facial morphology to a variety of dispositional and behavioral characteristics. For instance, research on humans has found that the facial width-to-height ratio (FWHR) is positively correlated with men’s aggression (Carré & McCormick, 2008; Geniole, Denson, Dixson, Carré, & McCormick, 2015; Haselhuhn, Ormiston, & Wong, 2015; cf. Özener, 2012), unethical behavior (Geniole, Keyes, Carré, & McCormick, 2014; Haselhuhn & Wong, 2012), expression of prejudice (Hehman, Leitner, Deegan, & Gaertner, 2013), psychopathic traits (Anderi et al., 2016; Geniole et al., 2014), achievement drive (Lewis, Lefevre, & Bates, 2012), sacrifice toward the in-group (Stirrat & Perrett, 2012), as well as financial success and attractiveness as a short-term sexual partner (Valentine, Li, Penke, & Perrett, 2014). Together, these findings indicate that the FWHR, similar to other androgen-dependent masculinized craniofacial features and beardedness, may have been shaped by sexual selection as cues to underlying reproductively relevant characteristics (e.g., aggressiveness and social dominance) (e.g., Arnocky, Bird, & Perilloux, 2014; Dixson, Sulikowski, Gouda-Vossos, Rantala, & Brooks, 2016). Indeed, not only do wide-faced men exhibit these behavioral and psychological characteristics, but they are also perceived by naïve observers as being more socially dominant, untrustworthy, and aggressive compared to men with lower width-to-height ratios (Carré, McCormick, & Mondloch, 2009; Stirrat & Perrett, 2012; Valentine et al., 2014; see Geniole et al., 2015 for meta-analysis). In addition, recent evidence on non-human primates has found that the FWHR is positively correlated with assertiveness (Wilson et al., 2014) and dominance status (Lefevre et al., 2014), especially among low-ranking monkeys (Carré, 2014), a finding that is highly consistent with evidence in humans (Goetz et al., 2013).
Researchers have argued that the observed links between the FWHR and men’s dominant and aggressive attitudes and behavior may be a product of androgen exposure during critical periods of development. In support of this, sex differences in facial structure arise with the onset of puberty, ostensibly reflecting increased testosterone in males relative to females (Verdonck, Gaethofs, Carels, & de Zegher, 1999). Research measuring fetal androgens in samples of cord blood has found levels of androgens in utero, but not in adulthood, were positively associated with facial masculinity (but not FWHR) in men (Whitehouse et al., 2015). It has been proposed that links between FWHR and aggressive behavior may be due to the common influence of pubertal testosterone exposure on craniofacial growth and the organization of neural circuitry underlying aggression (Carré & McCormick, 2008).
Although some research in humans (Carré & McCormick, 2008; Weston, Friday, & Liò, 2007) and non-human primates (Lefevre et al., 2014) has reported that males have large FWHRs compared to females, other studies with larger samples have failed to confirm this sex difference (Lefevre et al., 2012; Özener, 2012) and meta-analytic evidence indicates only a relatively small sex difference in FWHR (d = .11, n = >10,000, Geniole et al., 2015), and that FWHR was linked to dominance behavior generally across both men and women. Lefevre, Lewis, Perrett, and Penke (2013) reported that individual differences in FWHR in a sample of adult men were positively correlated with variation in baseline testosterone concentrations, as well as with testosterone reactivity to a speed-dating paradigm. However, a more recent series of studies with a sample of men (n = 780) failed to find any evidence for a relation between adult baseline testosterone concentrations and FWHR, or testosterone reactivity following competition (Bird et al., 2016). Instead, recent data exploring testosterone and FWHR in a Bolivian hunter-gatherer population have shown positive links between male pubertal testosterone and FWHR (Hodges-Simeon, Hanson Sobraske, Samore, Gurven, & Gaulin, 2016). Although not described in the aforementioned published article, when the testosterone data are normalized (i.e., log transformed) as well as with appropriate age controls applied to the sample, FWHR clearly maps on to pubertal testosterone, with a moderate effect size (r partial = .28, p < .05) (see Welker, Bird, & Arnocky, 2016 and available online data from Hodges-Simeon et al., 2016). Thus, although support for a general sex difference in FWHR is relatively weak, there is some evidence that variation in testosterone concentrations at certain points in development may map onto within-sex variability in FWHR. The extent to which earlier exposure to androgens (e.g., prenatal) shapes variability in FWHR within men and women remains to be determined.
Notably, previous work linking FWHR to various behavioral outcomes have found that the effects held for men, but not women (Carré & McCormick, 2008; Geniole et al., 2014; Goetz et al., 2013; Haselhuhn & Wong, 2012). This does not preclude the possibility that FWHR is linked to other behavioral traits in women. Indeed, testosterone is a hormone that is related not only to dominance- and status-seeking behavior (see Carré, McCormick, & Hariri, 2011; Eisenegger, Haushofer, & Fehr, 2011 for reviews), but also to psychosexual stimulation, self-reported interest in sex (e.g., Anderson, Bancroft, & Wu, 1992), sexual fantasies, and sexual behavior (e.g., Bagatell, Heiman, River, & Bremner, 1994; Davidson, Camargo, & Smith, 1979; McCoy & Davidson, 1985). Hitherto, research on FWHR has focused solely on dominance and competition-related variables; other variables relevant to pubertal testosterone—chiefly, attitudes and orientations toward sexual activity—have yet to be considered. However, some recent research has extended inquiry of face shape into other areas of human sexuality such as sexual orientation. For instance, Skorska, Geniole, Vrysen, McCormick, and Bogaert (2015) found that facial masculinity was modestly associated with homosexuality in both women and men. Moreover, these facial cues provide perceptual validity to raters’ ability to detect sexuality in faces (González-Álvarez, 2017). The goal of the present study was to determine whether facial metrics, specifically FWHR, was linked to human sex drive (Study 1 and Study 2), along with indicators of pluralistic mating orientation via measures of sociosexuality and infidelity intentions (Study 2).
The term “sex drive” refers to the strength of one’s sexual motivation (Baumeister, Catanese, & Vohs, 2001). Although the strength of men’s sex drive is typically found to be greater and less malleable than that of women (e.g., Baumeister, 2000), it is nevertheless clear that both sexes have evolved sexual desires which serve to promote mating and sexual behavior, and which ultimately have implications for an organism’s reproductive fitness (e.g., Massar & Buunk, 2009; Wallen, 1995). Much research has determined that sexual motives and behavior are modulated by testosterone in both men and women (see Davis & Tran, 2001; Isidori et al., 2005 for review). In men, for instance, low testosterone has been related to erectile dysfunction (Jannini et al., 1999), low libido and sex drive (Travison, Morley, Araujo, O’Donnell, & McKinlay, 2006), as well as less frequent masturbation and intercourse (Bagatell et al., 1994). Testosterone administration can increase both sexual desire and behavior frequency among men (e.g., Anderson et al., 1992; Kwan, Greenleaf, Mann, Crapo, & Davidson, 1983; Schiavi, White, Mandeli, & Levine, 1997; Snyder et al., 2016). Similarly, in women, low testosterone has been linked to various sexual desire disorders (see Davis & Tran, 2001 for review) and testosterone administration has been shown to be effective in increasing sex drive in women suffering from hypoactive sexual desire disorder (Kingsberg, 2007; Simon et al., 2005). van Anders, Hamilton, Schmidt, and Watson (2007) found that women’s testosterone levels were higher both pre- and post-sexual activity relative to a control activity.
Women’s testosterone levels have been found to be higher during the ovulatory versus follicular and luteal phases of their menstrual cycle (Schreiner-Engel, Schiavi, Smith, & White, 1981), and ovulatory testosterone levels have been shown to predict copulation frequency within married couples (Persky, Lief, Strauss, Miller, & O’Brien, 1978). However, Roney and Simmons (2013) found no significant effects of testosterone on the corresponding increases in sexual motivation when controlling for the effects of estradiol and progesterone. In a recent review of the literature, Cappelletti and Wallen (2016) suggest that supraphysiological (but not physiological) testosterone levels enhance the effectiveness of low-dose estrogen therapies for increasing women’s sexual desire, suggesting that the role of endogenous testosterone in modulating women’s sexual desire remains unclear.
Testosterone has similarly been implicated in both sociosexuality and romantic relationship dynamics. Across mammals, Sisk (2016) has argued that gonadal hormones organize sociosexual behavior during adolescence. Specific to humans, Edelstein, Chopik, and Kean (2011) found that partnered men and women who reported greater desire for uncommitted sexual activity had testosterone levels that were comparable to their unpartnered intrasexual counterparts. However, other research has shown that testosterone predicts a more unrestricted sociosexuality among men but not among oral contraceptive-using women (Puts et al., 2015). More circumstantial evidence has been observed via the 2D:4D ratio (potentially a marker for developmental testosterone concentrations) and men’s judgements of women’s faithfulness, such that women with more feminine finger-length ratios (i.e., putatively exposed to less prenatal androgens than those with masculine ratios) were rated by men as potentially being more sexually faithful. Men’s faithfulness ratings in turn mapped onto women’s actual scores on a measure of sociosexuality (DeLecce, Polheber, & Matchock, 2014).
Coinciding with a potential developmental influence of testosterone upon the formation of facial structures, the relation between testosterone and sex drive seems to also emerge during puberty in both boys and girls. For instance, longitudinal analyses of pubertal boys show an influence of testosterone upon boys’ transition to first intercourse and other aspects of sexual behavior and attitudes (Halpern, Udry, Campbell, Suchindran, & Mason, 1994). Moreover, in adolescent boys, intraindividual increases in salivary testosterone relate to increased sexual activity (Halpern, Udry, & Suchindran, 1998). For example, pubertal testosterone among boys has been linked to increased sexual fantasies and behavior (Campbell, Prossinger, & Mbzivo, 2005). Similarly, changes in testosterone throughout puberty predict the subsequent onset of sexual behavior in girls (Halpern, Udry, & Suchindran, 1997). Follicular testosterone has been linked to adolescent girls’ increased likelihood of having masturbated, having masturbated in the past month, and thinking about sex (Udry, Talbert, & Morris, 1986).
Study 1
Given that FWHR has been associated with a variety of androgen-mediated behavioral and personality characteristics, we predicted that FWHR would be positively correlated with sex drive (Hypothesis 1). Further, given that testosterone plays a significant role in the sex drive and behavior of both men and women, we predicted that associations between FWHR and sex drive would be similar in men and women (Hypothesis 2). We further anticipated these effects to remain consistent after controlling for additional facial metrics that may be associated with pubertal testosterone (Hodges-Simeon et al., 2016): lower face/face height, cheekbone prominence, face width/lower face height.
Method
Participants
A total of 145 heterosexual male (n = 69; 48%) and female (n = 76; 52%) students who were currently in romantic relationships (M age = 22 years, SD = 3.62) completed questionnaires pertaining to their interpersonal and sexual behavior, and then provided a facial photograph. Recruitment took place at a mid-sized Canadian university via recruitment stations located in common areas (e.g., lobbies, cafeterias). Participants were largely of Caucasian descent (82%). Three cases with missing self-reported sex drive data were subsequently removed from analysis.
Measures
Facial Measurement
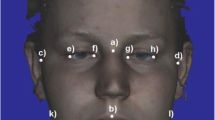
Facial photographs were taken using standardized distance and lighting and against a neutral backdrop with a .3 Megapixel Dell digital web-camera with Advanced Light sensitivity and a resolution of 640 × 480. ImageJ (NIH open-source software) was then used by two independent raters to measure facial width-to-height ratio (FWHR), or the bi-zygomatic width of the face (left and right zygion or the most lateral point of the zygomatic arch) divided by the height of the upper face (i.e., the distance between the upper lip and brow) (see Weston et al., 2007). Following Hodges-Simeon et al. (2016), raters also measured three metrics that were of secondary interest to the present study, including face width/lower face height (FWHR-lower) (bi-zygomatic width divided by the height of the lower face), cheekbone prominence (bi-zygomatic width divided by the width of the face at the corners of the mouth), and lower face/face height (height of the lower face divided by the full face height). Intraclass correlation showed that raters’ FWHR (R = .96), face width/lower face height (R = .85), cheekbone prominence (R = .92), and lower face/face height (R = .85) measurements were highly internally consistent so the average of the measurements for each face was computed. Examination of scores by sex revealed no difference between men’s (M = 1.6, SD = .12) and women’s (M = 1.60, SD = .10) FWHR, t(140) < 1, d = .09. However, results showed sex differences in men’s (M = 1.08, SD = .07) and women’s (M = 1.11, SD = .06) width/lower face height (FWHR-lower), t(140) = −2.87, p = .005, d = .46; men’s (M = 1.13, SD = .06) and women’s (M = 1.17, SD = .07) cheekbone prominence, t(140) = −2.96, p = .004, d = .61; and men’s (M = .64, SD = .03) and women’s (M = .62, SD = .03) lower face/face height ratio, t(140) = 4.04, p < .001, d = .66.
Sex Drive
The Sex drive Questionnaire (SDQ; Ostovich & Sabini, 2004) was used to measure the strength of participants’ sex drive. The SDQ consists of the following four items: (1) How often do you experience sexual desire? (2) How often do you orgasm in the average month? (3) How many times do you masturbate in the average month? (4) How would you compare your level of sex drive with that of the average person of your gender and age? Response options used either 6- or 7-point Likert-type scales. Scores were then z-transformed due to the varying response scale options. Previous studies have shown the measure to exhibit acceptable internal consistency (e.g., Penke & Asendorpf, 2008). In the present study, the SDQ showed good internal consistency (α = .78). Previous research has shown the SDQ to be conceptually distinct from measures of sociosexuality (Ostovich & Sabini, 2004).
Statistical Analyses
Multiple ordinary least squares regression analyses were conducted using the PROCESS macro for SPSS (Hayes, 2013). Variables were mean-centered (for continuous variables FWHR and sex drive) and dummy-coded (for dichotomous variable sex). FWHR, sex (as the moderating variable), and their interaction were calculated with sex drive entered as the dependent variable. Following the guidelines outlined by Hayes (n.d.), we present unstandardized regression coefficients with data represented visually in Fig. 1.
Results
FWHR and Sex Drive
We examined the relation between FWHR and sex drive with participant sex entered as a moderator variable. Regression analysis indicated that participant sex predicted sex drive, b = −.35, SE = .06, t(138) = −6.33, p < .001, partial-r = −.47, such that men reported higher sex drive than women. Also, results showed that FWHR was positively related to sex drive, b = 1.40, SE = .51, t(138) = 2.73, p = .007, partial-r = .23 (see Fig. 1).Footnote 1 The FWHR × sex interaction was not statistically significant, b = .17, SE = .51, t(138) = .34, p = .73, partial-r = .03, indicating that the relation between FWHR and sex drive scores was similar in men and women. Indeed, bivariate correlations indicated that FWHR was positively correlated with sex drive in men (r = .22, p = .077) and women (r = .24, p = .041).
Supplementary Analyses with Other Facial Metrics
None of the other facial metrics (lower face/face height, cheekbone prominence, face width/lower face height) predicted variability in sex drive (p values ranged from .48 to .60), nor did any of these facial metrics interact with participant sex to predict variability in sex drive (p values ranged from .42 to .92). Finally, we also entered all four facial metrics in the same regression model (with participant sex) to examine the extent to which FWHR would remain a significant predictor of sex drive. Results indicated that FWHR remained a significant predictor of sex drive, b = 1.71, SE = .66, t(135) = 2.60, p = .01, partial-r = .22. None of the other facial metrics predicted significant variability in sex drive (p values between .40 and .93).
Study 2
In a second study, we examined whether the link between FWHR and sex drive observed in Study 1 was replicable. We also examined additional variables that are conceptually distinct, yet related to sex drive: sociosexuality and intended infidelity. Sociosexual orientation is considered a trait-based orientation toward sexuality that ranges between restricted and unrestricted. A restricted orientation entails general discomfort with the concept of sex without love or commitment, whereas an unrestricted orientation entails comfort with casual sex. Ostovich and Sabini (2004) showed that sociosexual orientation is related to, yet conceptually distinct from, sex drive. For instance, in predicting lifetime number of sex partners, sociosexuality but not sex drive, emerges as a significant predictor. This is intuitive given that one can be high in sex drive yet simultaneously monogamous to alone partner. Whereas sociosexuality refers to the degree to which an individual subscribes to “casual” sex, it does not explicitly capture another related, yet distinct variable: extra-pair mating (i.e., having sex with someone outside of an established pair-bond). Interestingly, previous research has linked pluralistic mating to testosterone in both men and women (van Anders, Hamilton, & Watson, 2007). Thus, in Study 2 we also included a measure of anticipated infidelity. It was expected that FWHR would relate positively to each of these variables. As with Study 1, we examined potential sex difference across all three outcomes.
Method
Participants
As a part of a larger study, 314 participants (43% men; M age = 20 years, SD = 2.33) completed questionnaires pertaining to their interpersonal and sexual behavior, and provided a facial photograph. This sample size was sufficiently powered (power > .95) to detect an effect size of r = .21 with alpha set at .05 (two-tailed), as the smallest effect found in Study 1. Recruitment took place at a small Canadian university and college that was approximately 350 km in distance from the institution where Study 1 took place. Participants were recruited via recruitment stations located in common areas and via the university online research participation system, and were compensated with either partial course credit or $5 CAD for their time. Participants were largely of Caucasian descent (91%). Participant sexual orientation was determined using the following item: Which of the following best describes your sexual orientation?, with response options being “heterosexual, lesbian/gay, bisexual, or other”. Seven participants reported homosexual orientation, 6 reported bisexual orientation, and 9 reported other sexual orientation.
Measures
Facial Measures
Facial photographs were taken using a 16 megapixel Nikon Cool Pix L830 digital camera using standardized distance and lighting and against a neutral backdrop. ImageJ (NIH open-source software) was then used by two independent raters to measure FWHR, lower face/face height, cheekbone prominence, and face width/lower face height. Intraclass correlation showed that raters’ FWHR (R = .91), face width/lower face height (R = .90), cheekbone prominence (R = .79), and lower face/face height (R = .81) measurements were highly internally consistent so the average of the measurements for each face was computed. Three participants had FWHR scores greater than 3 SDs from the mean, and were thus removed prior to performing the main analyses. Examination of scores by sex revealed no significant difference between men’s (M = 1.84, SD = .14) and women’s (M = 1.83, SD = .13) FWHR, t(312) < 1, d = .07. However, results showed sex differences in men’s (M = 1.17, SD = .07) and women’s (M = 1.24, SD = .07) width/lower face height (FWHR-lower), t(312) = −8.20, p < .001, d = 1.00; men’s (M = 1.12, SD = .06) and women’s (M = 1.15, SD = .05) cheekbone prominence, t(312) = −5.19, p < .001, d = .54; and men’s (M = .61, SD = .03) and women’s (M = .59, SD = .02) lower face/face height ratio, t(312) = 6.60, p < .001, d = .78.
Sex Drive
As in Study 1, the SDQ was used to measure participants’ sex drive. The measure showed good internal consistency in the present sample (α = .85).
Sociosexual Orientation
Participants also completed the Revised Sociosexual Orientation Inventory (SOI-R; Penke & Asendorpf, 2008). A high score on this measure indicates a more unrestricted sociosexuality, whereas a low score indicates a more restricted sociosexuality (Penke & Asendorpf, 2008). The measure is comprised of three subscales that can be averaged together. The Behavior subscale consisted of three items scored on a 9-point Likert-type scale ranging from 1 = “0 times” to 9 = “20 or more times.” An example item was “With how many different partners have you had sex within the past 12 months?” The Attitude subscale consisted of three questions utilizing a 9-point response scale anchored at 1 = strongly disagree and 9 = strongly agree: “Sex without love is ok”, “I can imagine myself being comfortable and enjoying ‘casual’ sex with different partners,” and “I do not want to have sex with a person until I am sure that we will have a long-term, serious relationship” (reverse scored). Similarly, the Desire subscale was anchored at 1 = never and 9 = at least once a day, and consisted of the following three items: “How often do you have fantasies about having sex with someone you are not in a committed romantic relationship with?”, “How often do you experience sexual arousal when you are in contact with someone you are not in a committed romantic relationship with?”, and “In everyday life, how often do you have spontaneous fantasies about having sex with someone you have just met?” The revised measure has previously shown good internal consistency in large samples, as well as good discriminant validity (being higher in males relative to females), and is predictive of future sexual behavior, such as number of sex partners (Penke & Asendorpf, 2008). All items were averaged to create a composite SOI score. The measure showed acceptable internal consistency (α = .75).
Anticipated Infidelity
Participants also completed a modified version of the Susceptibility to Infidelity questionnaire (Goetz & Causey, 2009). This measure employed two items meant to capture the participants’ likelihood of being sexually unfaithful to their current partner or future romantic partner: (1) “How likely do you think it is that you will in the future have sexual intercourse with someone other than your partner?” and (2) “Please indicate your agreement or disagreement with the following statement: “I will probably be sexually unfaithful to my partner.” Responses were made on a 7-point Likert-type scale anchored at 1 = Not at all likely/Completely disagree, and 7 = Extremely likely/Completely agree. The items showed good inter-correlation, r(313) = .19, p = .001.
Results
FWHR and Sex Drive
We first examined the relation between FWHR and sex drive. Consistent with results from Study 1, sex was a strong predictor of sex drive, b = −.42, SE = .04, t(310) = −10.27, p < .001, partial-r = −.50, such that men reported higher sex drive scores relative to women. Also, results showed that FWHR was positively correlated with sex drive, b = .77, SE = .30, t(311) = 2.56, p = .011, partial-r = .14Footnote 2 (see Fig. 2). The FWHR × sex interaction was not statistically significant, b = −.25, SE = .30, t(310) = −.85, p = .40, partial-r = −.05, suggesting that FWHR related to increased sex drive, regardless of sex. Bivariate correlations indicated that FWHR was positively correlated with sex drive in men (r = .26, p = .003) and women (r = .09, p = .24), although the relation among women did not approach statistical significance.
FWHR and Sociosexual Orientation
We next examined the relation between FWHR and sociosexuality. Results showed that sex predicted sociosexuality, b = −.37, SE = .05, t(310) = −7.86, p < .001, partial-r = −.41, such that men reported a higher (i.e., more unrestricted) sociosexual orientation relative to women. FWHR did not predict sociosexuality, b = .23, SE = .35, t(310) = .66, p = .51, partial-r = .04. However, there was a participant sex x FWHR interaction, b = −.71, SE = .34, t(310) = −2.07, p = .039, partial-r = −.12. Simple slopes analysis showed that FWHR predicted sociosexuality among men (b = 1.04, SE = .50, t(310) = 2.09, p = .038) but not women (b = −.39, SE = .47, t(310) = −.81, p = .42) (see Fig. 3).
FWHR and Intention to Commit Infidelity
We then examined the relation between FWHR and intention to commit infidelity. Results showed that sex predicted intention to commit infidelity, b = −.14, SE = .05, t(309) = −2.94, p = .004, partial-r = −.17, such that men reported a greater infidelity intention relative to women. Also, FWHR was positively correlated with intended infidelity, b = .88, SE = .36, t(309) = 2.43, p = .016, partial-r = .14 (see Fig. 4). The participant sex x FWHR interaction was not significant, b = −.59, SE = .36, t(309) = −1.64, p = .102, partial-r = −.09. Although the interaction was not statistically significant, bivariate correlations indicated that FWHR was positively correlated with intended infidelity in men (r = .25, p = .003), but not women (r = .06, p = .45).
Supplementary Analyses with Other Facial Metrics
Sex Drive
Cheekbone prominence and face width/lower face height did not predict variability in sex drive (p values ranged from .07 to .24) and did not interact with participant sex to predict sex drive (p values ranged from .60 to .94). Lower face/face height positively predicted sex drive, b = 3.74, SE = 1.77, t(310) = 2.11, p = .035, partial-r = .13. Lower face/face height did not interact with participant sex to predict sex drive, b = 2.40, SE = 1.74, t(310) = 1.38, p = .17, partial-r = .08. Finally, we entered all four facial metrics in the same regression model (with participant sex) to examine the extent to which FWHR would remain a significant predictor of sex drive. Results indicated that FWHR remained a significant predictor of sex drive, b = 1.05, SE = .39, t(308) = 2.71, p = .007, partial-r = .15. None of the other facial metrics predicted significant variability in sex drive (p values between .07 and .71).
Sociosexual Orientation
Face width/lower face height was negatively associated with sociosexuality, b = −1.43, SE = .63, t(310) = −2.28, p = .02, partial-r = −.13. Thus, a more masculinized face width/lower face height predicted higher sociosexuality. There was no participant sex x face width/lower face height interaction, b = −.52, SE = .64, t(310) = −.82, p = .41, partial-r = −.05. Cheekbone prominence was also negatively associated with sociosexuality, b = −1.95, SE = .86, t(310) = −2.26, p = .025, partial-r = −.13. Thus, a more masculinized cheekbone prominence predicted higher sociosexuality. Also, cheekbone prominence interacted with participant sex to predict sociosexuality, b = 1.77, SE = .85, t(310) = 2.08, p = .039, partial-r = .12. Simple slopes analysis indicated a negative association between cheekbone prominence in men (b = −3.97, SE = 1.21, t(310) = −3.30, p = .001), but not women (b = −.42, SE = 1.21, t(310) = −.35, p = .73). Lower face/face height was positively associated with sociosexuality, b = 5.27, SE = 2.03, t(310) = 2.60, p = .01, partial-r = .15. Thus, a more masculinized lower face/face height predicted higher sociosexuality. There was no participant sex x lower face/face height interaction, b = 2.76, SE = 1.99, t(310) = 1.39, p = .17, partial-r = 08.
Finally, we entered all four facial metrics in the same regression model (with participant sex) to examine whether any of the predictors would explain unique variability in sociosexuality. Results indicated that none of the facial metrics significantly predicted sociosexuality (p values ranged from .10 to .44). Furthermore, when all two-way interactions were included in the regression model, none of them emerged as significant predictors (p values ranged from .13 to .82).
Anticipated Infidelity
None of the other facial metrics (lower face/face height, cheekbone prominence, face width/lower face height) predicted variability in intention to commit infidelity (p values range from .15 to .72), nor did any of these facial metrics interact with participant sex to predict intention to commit infidelity (p values ranged from .18 to .79).
Finally, we also entered all four facial metrics in the same regression model (with participant sex) to examine the extent to which FWHR would remain a significant predictor of intention to commit infidelity. Results indicated that FWHR remained a significant predictor of intention to commit infidelity, b = 1.22, SE = .48, t(307) = 2.56, p = .011, partial-r = .14. None of the other facial metrics predicted significant variability in intention to commit infidelity (p values between .11 and .63).
Internal Meta-analysis
To boost statistical power and reach greater precision for estimation (Cumming, 2013), an internal meta-analysis was conducted for the outcome variable sex drive across both samples, yielding a total sample of 458. In order to account for potential differences across samples, measures of FWHR and sex drive were first standardized within their respective samples, and sex remained dummy-coded at M = −1 and F = +1. Moderated regression analysis was conducted to test the relation between FWHR and sex drive, as well as their interaction with sex. Results revealed main effects for both sex, b = −.51, SE = .04, t(452) = −12.67, p < .001, partial-r = −.51 and FWHR, b = .15, SE = .04, t(452) = 3.63, p < .001, partial-r = .17. There was no significant FWHR × sex interaction, b = −.02, SE = .04, t(452) = −.56, p = .58, partial-r = −.03, suggesting that FWHR predicted sex drive among both men and women (see Fig. 5). Bivariate correlations indicated that FWHR was positively correlated with sex drive in men (r = .24, p = .001) and women (r = .12, p = .050).
Discussion
Previous studies have linked FWHR to aggressive and dominant behavior (e.g., Carré & McCormick, 2008). The present research extended this line of inquiry by identifying links between FWHR and human sex drive (Study 1 and 2), sociosexuality (Study 2), and intended infidelity (Study 2). Taken together, this extension of the study of behavioral correlates of facial morphology provides evidence in support of the hypothesis that larger FWHR may function as a biomarker of sex drive. Moreover, this research provides the first evidence implicating FWHR in relation with women’s sexual psychology.
Recent research suggests that FWHR may be related to circulating testosterone. For instance, in a study of adult men, Lefevre et al. (2013) reported that individual differences in the FWHR were positively correlated with baseline testosterone and with testosterone reactivity to a speed-dating paradigm. However, a more recent analysis with a much larger sample detected no significant relation between men’s testosterone concentrations, or testosterone reactivity following competition, and their FWHR’s (Bird et al., 2016). Nevertheless, male pubertal testosterone may be linked to FWHR. Given that sexual motives and behavior in humans are in part modulated by hormones (especially testosterone; Davis & Tran, 2001) and that pubertal testosterone is linked to later sexual motives and behavior (e.g., Edelstein et al., 2011), it was expected that FWHR would correspond with sex drive. Consistent with previous findings, men in the present studies reported significantly higher sex drive compared to women (see Baumeister et al., 2001 for review). Results further indicated that FWHR positively predicted participants’ self-reported sex drive, independent of biological sex. That is, the predictive relation between FWHR and sex drive held for men and women (in Study 1, Study 2, and internal meta-analysis with normalized and combined sampling). Beyond Study 1, Study 2 showed that FWHR also predicted a more unrestricted sociosexual orientation and higher intention to commit infidelity. Previous research has linked high testosterone in men to a lower likelihood of being in a monogamous relationship (van Anders & Watson, 2006). Men in polygynous relationships have higher testosterone than men in monogamous relationships (Gray, 2003), and self-report a more unrestricted sociosexual orientation (Edelstein et al., 2011). To the extent that craniofacial masculinization may be driven at least in part by testosterone, the findings of the present study suggest that FWHR may serve as a novel marker of human sexual psychology.
Limitations and Future Directions
The present research was limited by its focus on a relatively narrow age range typical of studies on university students. This sample was chosen given that early adulthood represents a period of elevated sexual interest in men and women (e.g., Arnett, 2000). Future research would benefit from exploring whether these effects can be detected in adolescence, and whether they remain throughout adulthood. Given that the mating dynamics of university students often differ from those of later adulthood, it would be interesting to determine whether these results are replicable in long-term marriage relationships among older adults. It would also be interesting for future research to examine if these results are replicable across different populations, including more ethnically diverse samples, and among individuals of either homosexual or bisexual orientation. The present study employed a relatively restricted measure of infidelity intentions comprised of only two items that did not show particularly strong inter-item correlation. Although results were consistent with the overall pattern of findings among other study variables, we recommend that future research employ a more comprehensive measure of infidelity intentions and behavior. Our results were robust across samples, however future work might control for other variables that may influence sex drive such as conservative beliefs, sexual passivity, emotions of sadness and shame related to sexual activity, and degree of dyadic cohesion (Carvalho & Nobre, 2010, 2011). Future research would also benefit from examining a broader constellation of sexual motives and behavior, including actual sexual behavior (e.g., number of lifetime sex partners, number of casual sex partners, sexual openness, and sexual risk-taking).
Finally, the link between women’s FWHR and sex drive is novel in that most studies of FWHR have focussed primarily on this facial metric as a correlate for male (but not female) psychological and behavioral functioning—probably due to some evidence linking FWHR to male-typical sex hormones. Although the present research identified links between FWHR and sex drive irrespective of sex, it is nevertheless noteworthy that at the bivariate level, FWHR was more strongly correlated with sex drive among men relative to women. Thus, further examinations of this relation among women and of the potential mechanisms underlying this relation are necessary. Recent research shows that progesterone may function in part to dull women’s sex drive (for instance, from mid-cycle to the luteal phase during women’s menstrual cycles; Roney & Simmons, 2013). Interestingly, facial adiposity (which ostensibly would increase the facial width-to-height ratio) relates negatively to trait progesterone in women (Tinlin et al., 2013), suggesting that wide-faced women’s exhibition of a higher sex drive relative to women with narrower faces may be driven, in part, by hormonal processes that are functionally distinct from those potentially underlying the FWHR-sex drive link in men. Future research might explore whether the positive FWHR-sex drive link might be mediated by trait progesterone levels in women. Finally, future research should consider the interactive effects of organizational (e.g., 2D:4D ratio; FWHR) and activational (current testosterone levels) hormones on sex drive.
Conclusion
The present research was the first to link the human FWHR to sex drive. These findings extend the field’s understanding of FWHR as a morphological index of psychology and behavior, which to this point has focused on traits that can be considered primarily masculine in nature, such as aggression (e.g., Carré & McCormick, 2008), psychopathy (Geniole et al., 2014), and even the achievement drive of US presidents (Lewis et al., 2012). Researchers have typically attributed these findings to testosterone, which may also be positively correlated with the FWHR during developmental periods that are also complicit in forming adult sexual attitudes and desires. By examining sex drive as a factor known to be positively influenced by androgens in both men and women (Davis & Tran, 2001), the present study is the first to establish that the FWHR might influence factors that are androgen driven in both sexes. Results also provide novel insight into FWHR as a morphological predictor of men’s sociosexuality and infidelity intentions, which seem to correspond with extant research linking other indicators of masculinity in males (such as grip strength, shoulder-to-hip ratio) to sociosexuality (e.g., Gallup, White, & Gallup, 2007). Taken together, this research is the first to link a novel facial metric (FWHR) to adult sexual psychology.
Notes
FWHR remained a significant predictor of sex drive when ethnicity (dichotomized as Caucasian vs. non-Caucasian) was included as a covariate, b = 1.32, SE = .53, t(137) = 2.49, p = .014, partial-r = .21 and when BMI was included as a covariate, b = 1.43, SE = .57, t(136) = 2.52, p = .013, partial-r = .21.
FWHR remained a significant predictor of sex drive when ethnicity (dichotomized as Caucasian vs. non-Caucasian) was included as a covariate, b = .79, SE = .30, t(309) = 2.64, p = .009, partial-r = .15. Unfortunately, body mass index was not collected in Study 2, and thus we could not control for this variable. When the sample was restricted to include only heterosexual participants, results were not meaningfully different from those presented for the full sample.
References
Anderi, C., Hahn, T., Schmidt, A., Moldenhauer, H., Notebaert, K., Clément, C. C., & Windmann, S. (2016). Facial width-to-height ratio predicts psychopathic traits in males. Personality and Individual Differences, 88, 99–101. doi:10.1016/j.paid.2015.08.057.
Anderson, R. A., Bancroft, J., & Wu, F. C. (1992). The effects of exogenous testosterone on sexuality and mood of normal men. Journal of Clinical Endocrinology and Metabolism, 75, 1503–1507.
Arnett, J. J. (2000). Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist, 55, 469–480. doi:10.1037/0003-066X.55.5.469.
Arnocky, S., Bird, B. M., & Perilloux, C. (2014). An evolutionary perspective on characteristics of physical attractiveness in humans. In A. Rennolds (Ed.), Psychology of interpersonal perception and relationships (pp. 115–155). New York, NY: NOVA Publishers.
Bagatell, C. J., Heiman, J. R., River, J. E., & Bremner, W. J. (1994). Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. Journal of Clinical Endocrinology and Metabolism, 78, 711–716. doi:10.1210/jc.78.3.711.
Baumeister, R. F. (2000). Gender differences in erotic plasticity: The female sex drive as socially flexible and responsive. Psychological Bulletin, 126, 347–374. doi:10.1037/0033-2909.126.3.347.
Baumeister, R. F., Catanese, K. R., & Vohs, K. D. (2001). Is there a gender difference in strength of sex drive? Theoretical views, conceptual distinctions, and a review of relevant evidence. Personality and Social Psychology Review, 5, 242–273. doi:10.1207/S15327957PSPR0503_5.
Bird, B. M., Cid, V., Geniole, S. N., Welker, K. M., Zilioli, S., Maestripieri, D., … Carré, J. M. (2016). Does facial width-to-height ratio map onto variability in men’s testosterone concentrations? Evolution and Human Behavior, 37, 392–398. doi:10.1016/j.evolhumbehav.2016.03.004.
Campbell, B. C., Prossinger, H., & Mbzivo, M. (2005). Timing of pubertal maturation and the onset of sexual behavior among Zimbabwe school boys. Archives of Sexual Behavior, 34, 505–516. doi:10.1007/s10508-005-6276-7.
Cappelletti, M., & Wallen, K. (2016). Increasing women’s sexual desire: The comparative effectiveness of estrogens and androgens. Hormones and Behavior, 78, 178–193. doi:10.1016/j.yhbeh.2015.11.003.
Carré, J. M. (2014). Social status, facial structure, and assertiveness in brown capuchin monkeys. Frontiers in Psychology, 5, 567. doi:10.3389/fpsyg.2014.00567.
Carré, J. M., & McCormick, C. M. (2008). In your face: Facial metrics predict aggressive behaviour in the laboratory and in varsity and professional hockey players. Proceedings of the Royal Society B: Biological Sciences, 275, 2651–2656. doi:10.1098/rspb.2008.0873.
Carré, J. M., McCormick, C. M., & Hariri, A. R. (2011). The social neuroendocrinology of human aggression. Psychoneuroendocrinology, 36, 935–944. doi:10.1016/j.psyneuen.2011.02.001.
Carré, J. M., McCormick, C. M., & Mondloch, C. J. (2009). Facial structure is a reliable cue of aggressive behavior. Psychological Science, 20, 1194–1198. doi:10.1111/j.1467-9280.2009.02423.x.
Carvalho, J., & Nobre, P. (2010). Predictors of women’s sexual desire: The role of psychopathology, cognitive-emotional determinants, relationship dimensions, and medical factors. Journal of Sexual Medicine, 7, 928–937. doi:10.1111/j.1743-6109.2009.01568.x.
Carvalho, J., & Nobre, P. (2011). Predictors of men’s sexual desire: The role of psychological, cognitive-emotional, relational, and medical factors. Journal of Sex Research, 48, 254–262. doi:10.1080/00224491003605475.
Cumming, G. (2013). Understanding the new statistics: Effect sizes, confidence intervals, and meta-analysis. New York: Routledge.
Davidson, J. M., Camargo, C. A., & Smith, E. R. (1979). Effects of androgen on sexual behavior in hypogonadal men. Journal of Clinical Endocrinology and Metabolism, 48, 955–958. doi:10.1210/jcem-48-6-955.
Davis, S. R., & Tran, J. (2001). Testosterone influences libido and well being in women. Trends in Endocrinology and Metabolism, 12, 33–37. doi:10.1016/S1043-2760(00)00333-7.
DeLecce, T. L., Polheber, J. P., & Matchock, R. L. (2014). Sociosexual orientation and 2D:4D ratios in women: Relationship to men’s desirability ratings as a long-term pair bond. Archives of Sexual Behavior, 43, 319–327. doi:10.1007/s10508-013-0201-2.
Dixson, B. J. W., Sulikowski, D., Gouda-Vossos, A., Rantala, M. J., & Brooks, R. C. (2016). The masculinity paradox: Facial masculinity and beardedness interact to determine women’s ratings of men’s facial attractiveness. Journal of Evolutionary Biology, 29, 2311–2320. doi:10.1111/jeb.12958.
Edelstein, R. S., Chopik, W. J., & Kean, E. L. (2011). Sociosexuality moderates the association between testosterone and relationship status in men and women. Hormones and Behavior, 60, 248–255. doi:10.1016/j.yhbeh.2011.05.007.
Eisenegger, C., Haushofer, J., & Fehr, E. (2011). The role of testosterone in social interaction. Trends in Cognitive Sciences, 15, 263–271. doi:10.1016/j.tics.2011.04.008.
Gallup, A. C., White, D. D., & Gallup, D. D. (2007). Handgrip strength predicts sexual behavior, body morphology, and aggression in male college students. Evolution and Human Behavior, 28, 423–429. doi:10.1016/j.evolhumbehav.2007.07.001.
Geniole, S. N., Denson, T. F., Dixson, B. J., Carré, J. M., & McCormick, C. M. (2015). Evidence from meta-analyses of the facial width-to-height ratio as an evolved cue of threat. PLoS ONE, 10, e0132726. doi:10.1371/journal.pone.0132726.
Geniole, S. N., Keyes, A. E., Carré, J. M., & McCormick, C. M. (2014). Fearless dominance mediates the relationship between the facial width-to-height ratio and cheating. Personality and Individual Differences, 57, 59–64. doi:10.1016/j.paid.2013.09.023.
Goetz, A. T., & Causey, K. (2009). Sex differences in perceptions of infidelity: Men often assume the worst. Evolutionary Psychology. doi:10.1177/147470490900700208.
Goetz, S. M., Shattuck, K. S., Miller, R. M., Campbell, J. A., Lozoya, E., Weisfeld, G. E., & Carré, J. M. (2013). Social status moderates the relationship between facial structure and aggression. Psychological Science, 24, 2329–2334. doi:10.1177/0956797613493294.
González-Álvarez, J. (2017). Perception of sexual orientation from facial structure: A study with artificial face models. Archives of Sexual Behavior, 46, 1251–1260. doi:10.1007/s10508-016-0929-6.
Gray, P. B. (2003). Marriage, parenting, and testosterone variation among Kenyan Swahili men. American Journal of Physical Anthropology, 122, 279–286. doi:10.1002/ajpa.10293.
Halpern, C. T., Udry, J. R., Campbell, B., Suchindran, C., & Mason, G. A. (1994). Testosterone and religiosity as predictors of sexual attitudes and activity among adolescent males: A biosocial model. Journal of Biosocial Science, 26, 217–234. doi:10.1017/S0021932000021258.
Halpern, C. T., Udry, J. R., & Suchindran, C. (1997). Testosterone predicts initiation of coitus in adolescent females. Psychosomatic Medicine, 59, 161–171. doi:10.1097/00006842-199703000-00008.
Halpern, C. T., Udry, J. R., & Suchindran, C. (1998). Monthly measures of salivary testosterone predict sexual activity in adolescent males. Archives of Sexual Behavior, 27, 445–465. doi:10.1023/A:1018700529128.
Haselhuhn, M. P., Ormiston, M. E., & Wong, E. M. (2015). Men’s facial width-to-height ratio predicts aggression: A meta-analysis. PLoS ONE, 10, e0122637. doi:10.1371/journal.pone.0122637.
Haselhuhn, M. P., & Wong, E. M. (2012). Bad to the bone: Facial structure predicts unethical behaviour. Proceedings of the Royal Society of London B: Biological Sciences, 279, 571–576. doi:10.1098/rspb.2011.1193.
Hayes, A. F. (2013). Introduction to mediation, moderation, and conditional process analysis. New York, NY: Guilford.
Hayes, A. F. (n.d.). Macro and script rules and frequently asked questions. Retrieved July 5, 2016 from http://www.afhayes.com/macrofaq.html.
Hehman, E., Leitner, J. B., Deegan, M. P., & Gaertner, S. L. (2013). Facial structure is indicative of explicit support for prejudicial beliefs. Psychological Science, 24, 289–296. doi:10.1177/0956797612451467.
Hodges-Simeon, C. R., Hanson Sobraske, K. N., Samore, T., Gurven, M., & Gaulin, S. J. C. (2016). Facial width-to-height ratio (fWHR) is not associated with adolescent testosterone levels. PLoS ONE, 11, e0153083. doi:10.1371/journal.pone.0153083.
Isidori, A. M., Giannetta, E., Gianfrilli, D., Greco, E. A., Bonifacio, V., Aversa, A., … Lenzi, A. (2005). Effects of testosterone on sexual function in men: Results of a meta-analysis. Clinical Endocrinology, 63, 381–394. doi:10.1111/j.1365-2265.2005.02350.x.
Jannini, E. A., Screponi, E., Carosa, E., Pepe, M., Giudice, F. L., Trimarchi, F., et al. (1999). Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosterone. International Journal of Andrology, 22, 385–392. doi:10.1046/j.1365-2605.1999.00196.x.
Kingsberg, S. (2007). Testosterone treatment for hypoactive sexual desire disorder in postmenopausal women. Journal of Sexual Medicine, 4, 227–234. doi:10.1111/j.1743-6109.2007.00449.x.
Kwan, M., Greenleaf, W. J., Mann, J., Crapo, L., & Davidson, J. M. (1983). The nature of androgen action on male sexuality: A combined laboratory-self-report study on hypogonadal men. Journal of Clinical Endocrinology and Metabolism, 57, 557–562. doi:10.1210/jcem-57-3-557.
Lefevre, C. E., Lewis, G. J., Bates, T. C., Dzhelyova, M., Coetzee, V., Deary, I. J., & Perrett, D. I. (2012). No evidence for sexual dimorphism of facial width-to-height ratio in four large adult samples. Evolution and Human Behavior, 33, 623–627. doi:10.1016/j.evolhumbehav.2012.03.002.
Lefevre, C. E., Lewis, G. J., Perrett, D. I., & Penke, L. (2013). Telling facial metrics: Facial width is associated with testosterone levels in men. Evolution and Human Behavior, 34, 273–279. doi:10.1016/j.evolhumbehav.2013.03.005.
Lefevre, C. E., Wilson, V. A. D., Morton, F. B., Brosnan, S. F., Paukner, A., & Bates, T. C. (2014). Facial width-to-height ratio relates to alpha status and assertive personality in capuchin monkeys. PLoS ONE, 9, e93369. doi:10.1371/journal.pone.0093369.
Lewis, G. J., Lefevre, C. E., & Bates, T. C. (2012). Facial width-to-height ratio predicts achievement drive in US presidents. Personality and Individual Differences, 52, 855–857. doi:10.1016/j.paid.2011.12.030.
Massar, K., & Buunk, A. P. (2009). The effect of a subliminally primed context on intrasexual competition depends on individual differences in sex drive. Journal of Research in Personality, 43, 691–694. doi:10.1016/j.jrp.2009.02.004.
McCoy, N. L., & Davidson, J. M. (1985). A longitudinal study of the effects of menopause on sexuality. Maturitas, 7, 203–210. doi:10.1016/0378-5122(85)90041-6.
Ostovich, J. M., & Sabini, J. (2004). How are sociosexuality, sex drive, and lifetime number of sexual partners related? Personality and Social Psychology Bulletin, 30, 1255–1266. doi:10.1177/0146167204264754.
Özener, B. (2012). Facial width-to-height ratio in a Turkish population is not sexually dimorphic and is unrelated to aggressive behavior. Evolution and Human Behavior, 33, 169–173. doi:10.1016/j.evolhumbehav.2011.08.001.
Penke, L., & Asendorpf, J. B. (2008). Beyond global sociosexual orientations: A more differentiated look at sociosexuality and its effects on courtship and romantic relationships. Journal of Personality and Social Psychology, 95, 1113–1135. doi:10.1037/0022-3514.95.5.1113.
Persky, H., Lief, H. I., Strauss, D., Miller, W. R., & O’Brien, C. P. (1978). Plasma testosterone level and sexual behavior of couples. Archives of Sexual Behavior, 7, 157–173. doi:10.1007/BF01542376.
Puts, D. A., Pope, L. E., Hill, A. K., Cardenas, R. A., Welling, L. L., Wheatley, J. R., & Breedlove, S. M. (2015). Fulfilling desire: Evidence for negative feedback between men’s testosterone, sociosexual psychology, and sexual partner number. Hormones and Behavior, 70, 14–21. doi:10.1016/j.yhbeh.2015.01.006.
Roney, J. R., & Simmons, Z. L. (2013). Hormonal predictors of sexual motivation in natural menstrual cycles. Hormones and Behavior, 63, 636–645. doi:10.1016/j.yhbeh.2013.02.013.
Schiavi, R. C., White, D., Mandeli, J., & Levine, A. C. (1997). Effects of testosterone administration on sexual behavior and mood in men with erectile dysfunction. Archives of Sexual Behavior, 26, 231–241. doi:10.1023/A:1024518730222.
Schreiner-Engel, P., Schiavi, R. C., Smith, H., & White, D. (1981). Sexual arousability and the menstrual cycle. Psychosomatic Medicine, 43, 199–214. doi:10.1097/00006842-198106000-00002.
Simon, J., Braunstein, G., Nachtigall, L., Utian, W., Katz, M., Miller, S., et al. (2005). Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. Journal of Clinical Endocrinology and Metabolism, 90, 5226–5233. doi:10.1210/jc.2004-1747.
Sisk, C. L. (2016). Hormone-dependent adolescent organization of socio-sexual behaviors in mammals. Current Opinion in Neurobiology, 38, 63–68. doi:10.1016/j.conb.2016.02.004.
Skorska, M. N., Geniole, S. N., Vrysen, B. M., McCormick, C. M., & Bogaert, A. F. (2015). Facial structure predicts sexual orientation in both men and women. Archives of Sexual Behavior, 44, 1377–1394. doi:10.1007/s10508-014-0454-4.
Snyder, P. J., Bhasin, S., Cunningham, G. R., Matsumoto, A. M., Stephens-Shields, A. J., Cauley, J. A., … Ellenberg, S. S. (2016). Effects of testosterone treatment in older men. New England Journal of Medicine, 374, 611–624. doi:10.1056/NEJMoa1506119.
Stirrat, M., & Perrett, D. I. (2012). Face structure predicts cooperation: Men with wider faces are more generous to their in-group when out-group competition is salient. Psychological Science, 23, 718–722. doi:10.1177/0956797611435133.
Tinlin, R. M., Watkins, C. D., Welling, L. L., DeBruine, L. M., Al-Dujaili, E. A., & Jones, B. C. (2013). Perceived facial adiposity conveys information about women’s health. British Journal of Psychology, 104, 235–248. doi:10.1111/j.2044-8295.2012.02117.x.
Travison, T. G., Morley, J. E., Araujo, A. B., O’Donnell, A. B., & McKinlay, J. B. (2006). The relationship between libido and testosterone levels in aging men. Journal of Clinical Endocrinology and Metabolism, 91, 2509–2513. doi:10.1210/jc.2005-2508.
Udry, J. R., Talbert, L. M., & Morris, N. M. (1986). Biosocial foundations for adolescent female sexuality. Demography, 23, 217–230.
Valentine, K. A., Li, N. P., Penke, L., & Perrett, D. I. (2014). Judging a man by the width of his face: The role of facial ratios and dominance in mate choice at speed-dating events. Psychological Science, 25, 806–811. doi:10.1177/0956797613511823.
van Anders, S. M., Hamilton, L. D., Schmidt, N., & Watson, N. V. (2007). Associations between testosterone secretion and sexual activity in women. Hormones and Behavior, 51, 477–482. doi:10.1016/j.yhbeh.2007.01.003.
van Anders, S. M., Hamilton, L. D., & Watson, N. V. (2007). Multiple partners are associated with higher testosterone in North American men and women. Hormones and Behavior, 51, 454–459. doi:10.1016/j.yhbeh.2007.01.002.
van Anders, S. M., & Watson, N. V. (2006). Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: Cross-sectional and longitudinal data. Psychoneuroendocrinology, 31, 715–723. doi:10.1016/j.psyneuen.2006.01.008.
Verdonck, A., Gaethofs, M., Carels, C., & de Zegher, F. (1999). Effects of low-dose testosterone treatment on craniofacial growth in boys with delayed puberty. European Journal of Orthodontics, 21, 137–143. doi:10.1093/ejo/21.2.137.
Wallen, K. (1995). The evolution of female sexual desire. In P. Abramson & S. Pinkerton (Eds.), Sexual nature, sexual culture (pp. 57–79). Chicago, IL: University of Chicago Press.
Welker, K. M., Bird, B. M., & Arnocky, S. (2016). Commentary: Facial width-to-height ratio is not associated with adolescent testosterone levels. Frontiers in Evolutionary Psychology and Neuroscience, 7, 1745. doi:10.3389/fpsyg.2016.01745.
Weston, E. M., Friday, A. E., & Liò, P. (2007). Biometric evidence that sexual selection has shaped the hominin face. PLoS ONE, 2, e710. doi:10.1371/journal.pone.0000710.
Whitehouse, A. J., Gilani, S. Z., Shafait, F., Mian, A., Tan, D. W., Maybery, M. T., … Eastwood, P. (2015). Prenatal testosterone exposure is related to sexually dimorphic facial morphology in adulthood. Proceedings of the Royal Society B: Biological Sciences, 282, 20151351.
Wilson, V., Lefevre, C. E., Morton, F. B., Brosnan, S. F., Paukner, A., & Bates, T. C. (2014). Personality and facial morphology: Links to assertiveness and neuroticism in capuchins (Sapajus [Cebus] apella). Personality and Individual Differences, 58, 89–94. doi:10.1016/j.paid.2013.10.008.
Acknowledgements
Funding support came from the Canadian Institutes of Health Research, Canada Research Chairs Program (Grant Nos. #950-203794; #950-229048).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included each study.
Rights and permissions
About this article
Cite this article
Arnocky, S., Carré, J.M., Bird, B.M. et al. The Facial Width-to-Height Ratio Predicts Sex Drive, Sociosexuality, and Intended Infidelity. Arch Sex Behav 47, 1375–1385 (2018). https://doi.org/10.1007/s10508-017-1070-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-017-1070-x