Abstract
Sexual dysfunction related to treatment with selective serotonin reuptake inhibitors (SSRIs) is a common reason for discontinuation of otherwise effective antidepressant treatment regimens. Thus, identification of subjects at risk for this side effect remains a crucial challenge. After demonstrating task-related neural correlates of impaired sexual functioning under treatment with the SSRI paroxetine (Abler et al., 2011), we studied (1) if resting state brain function before treatment predicts subsequent development of treatment-related modulation of sexual function, and (2) which neural circuits relate to different aspects of the impairment. Effects of paroxetine and bupropion administration over 1 week on subjective sexual functioning were investigated in 17 healthy male volunteers in a placebo-controlled, randomized cross-over design using the Massachusetts General Hospital Sexual Function Questionnaire. Data from a 10 min eyes-closed resting state scan were used to analyze functional connectivity under placebo in previously identified brain regions, focussing on the sublenticular extended amygdala (SLEA), dopaminergic midbrain, and anterior cingulate cortex. Resting state functional connectivities of the pregenual anterior cingulate cortex (pgACC), midbrain, and insula to the SLEA sufficiently predicted the development of subjective SSRI-related decreased sexual functioning and distinguished vulnerable from resilient subjects. Furthermore, connectivity with the midbrain particularly predicted orgasm-related deficits, while connectivity with pgACC predicted sexual satisfaction. Linking SSRI-related subjective sexual functioning to pre-treatment resting state connectivities in cortico-subcortical network of sexual processing, we demonstrated the potential of novel, non-invasive and passive brain imaging techniques to guide therapeutic decisions and adjust treatment protocols in psychiatric disorders and sexual medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual dysfunction is a common side effect following treatment with selective serotonin reuptake inhibitors (SSRIs) and is frequently related to discontinuation and failure of treatment. Decreased sexual desire and interest in sexual activity along with erectile dysfunction are reported in as much as 60 % of the cases (Gregorian et al., 2002) with the highest rates of up to 64 % observed under paroxetine (Clayton et al., 2002), which is one of the frequently prescribed SSRIs. On the other hand, the same pharmacological effect is used advantageously in the treatment of premature ejaculation (Pryor et al., 2006). While these effects of SSRIs are well known, the underlying mechanisms and predictors of individual vulnerability are poorly understood. Such predictors of effects and side effects, like behavioral or neurobiological markers, are highly desirable as they would allow for individualized therapy protocols.
Regarding neurobiology, neuroimaging has revealed a broad network of brain regions that contribute to the different aspects of sexual functioning in men and women, including regions like the anterior cingulate cortex (ACC), lateral occipital cortex (LOC), insula, amygdala, hypothalamus, thalamus, and the ventral striatum (Abler, Grön, Hartmann, Metzger, & Walter, 2012; Arnow et al., 2002; Ferretti et al., 2005; Metzger et al., 2010; Moulier et al., 2006; Mouras et al., 2003; Redouté et al., 2000; Walter et al., 2008a; Walter, Stadler, Tempelmann, Speck, & Northoff, 2008b). These activations have been related to a multidimensional model of sexual functioning that assumes that sexual arousal—but also sexual dysfunction—is comprised of cognitive, motivational, autonomic, and emotional components that affect sexual appetite, performance, appraisal and orgasm (Redouté et al., 2000; Stoléru et al., 1999). This literature includes studies in men and women and has to be taken with caution when it comes to gender effects. However, at least Walter et al. (2008a) could show that neuronal effects in the above-mentioned regions were comparable when the same level of sexual arousal was achieved.
On the behavioral level, questionnaires like the Massachusetts General Hospital Sexual Function Questionnaire (MGH-SFQ; Labbate & Lare, 2001) have been developed to assess these different aspects separately. In task-related fMRI, however, differentiating these dimensions in one experiment would require very complex experimental paradigms. Furthermore, in a current study investigating task-induced fMRI signals during erotic video viewing, paroxetine altered the activation of relevant brain regions, however, task-related fMRI activations could not be identified as potential predictors of decreased sexual functioning under the SSRI paroxetine (Abler et al., 2011). We hypothesized that resting state fMRI (rsfMRI) is a promising alternative approach since it equally assesses synchronised brain activation in all relevant regions, independent of a specific task setting. The method of rsfMRI, as first described by Biswal, Yetkin, Haughton, and Hyde (1995), requires the subject to lie still with eyes closed in an MRI-scanner for 5–15 min and allows one to look at low-frequency BOLD fluctuations in the subject’s brain in the absence of any task-setting. Therefore, rsfMRI can be easily standardized and is less dependent on a subject’s task commitment, which is a prerequisite for investigating larger clinical samples (Fox & Greicius, 2010). While task fMRI shows changes in activation in those specific brain regions, which are assessed by the task and leaves out all other regions, rsfMRI equally investigates all covered brain regions at a time. Therefore it allows for the investigation of local behavior of certain regions of interest (ROI), but also global measures of complex network interaction between various brain regions over time.
Under task conditions, lower BOLD responses under paroxetine treatment are found, especially in the midbrain, the sublenticular extended amygdala (SLEA) and the posterior midcingulate cortex (pMCC) (Abler et al., 2011). As paroxetine is related to the clinical development of sexual dysfunction (Clayton et al., 2002), we hypothesized that especially the network showing altered effects of brain responses under this SSRI would mediate this side effect. While Abler et al. (2011) observed that decreased task-related activation did not correlate with measures of reduced sexual functioning, we hypothesized that network functional architecture as approachable in rsfMRI would be a better predictor of individual dispositions, due to the above mentioned advantages of rsfMRI. Such a relationship between altered cue reactivity and functional network integration has recently been found in psychiatric disorders, underlining the role of a baseline functional network architecture for focal activations (Fox & Greicius, 2010; Horn et al., 2010).
Therefore, high baseline connectivity in such a network susceptible to pharmacological modulation could be protective against alterations by paroxetine and consequently against development of impaired sexual function. Therefore our study aimed to assess the predictive potential of baseline functional connectivity (FC) in a network of brain regions related to altered sexual function identified previously (Abler et al., 2011) and including the SLEA, midbrain, and ACC.
Choosing this network in our search for potential predictors seemed reasonable not only because of the findings of Abler et al. (2011), but also because previous literature implicated this network in sexual functioning. Imaging studies in humans related the dopaminergic midbrain to orgasm (Georgiadis, Reinders, van der Graf, Paans, & Kortekaas, 2007) and the ACC, especially the pregenual ACC (pgACC) to the hedonic quality associated with sexual arousal (Walter et al., 2008a). Disease-dependent alterations of pgACC metabolism (Walter et al., 2009; Yüksel & Öngür, 2010) coincide with antipodal impairments related to sexual hypo- and also hyperfunctioning as observed in major depression or bipolar disorder (reviewed by Seidman & Roose, 2001). The pMCC has been associated with a cognitive component mediating sexual approach behavior (Redouté et al., 2000), but also with hypoactive sexual desire disorder (Stoléru et al., 2003).
Investigating altered baseline connectivity in this network as a potential predictor of SSRI-related decreases in sexual functioning, we chose the SLEA as our core seed region. The region is largely interconnected with subcortical regions, like the hypothalamus, the brainstem, encompassing the periaqueductal grey and the ventral tegmental area–identified as core regions of sexual processing (Walter et al., 2007). The SLEA has been identified as a brain area ideally suited to generate endocrine, autonomic, and somatomotor aspects of emotional and motivational states (Heimer, Harlan, Alheid, Garcia, & Olmos, 1997; Nieuwenhuys, 2008). Neuronal signatures were sought during an unmedicated resting state scan, which through their connectivity with the SLEA could reliably predict sexual side effects caused by SSRI treatment. In conclusion, we hypothesized, that FC of the SLEA during a baseline resting state scan towards brain regions with previously demonstrated SSRI effects, can predict sexual side effects under SSRI treatment.
Ultimately, such neuronal target connectivities could then be used as potential predictive biomarkers, assessable before the onset of medication with SSRIs. Since general placebo effects are known to significantly contribute to sexual dysfunction (Abler et al., 2011) and brain activation (Benedetti, Mayberg, Wager, Stohler, & Zubieta, 2005), our study targeted true drug effects controlled in a double-blind, placebo-controlled investigation (Meissner et al., 2011).
Method
Participants
A total of 23 healthy male, heterosexual subjects aged 23–34 years (M = 25.4, SD = 2.9, three left-handed) were included in the fMRI study. Participants were recruited by advertisement from local universities in the city of Ulm. Subjects interested in participating were sent a list of inclusion and exclusion criteria. None of the subjects that responded after that had to be excluded due to any of those criteria mentioned below. All were university students or had a university degree. Exclusion criteria consisted of psychiatric, neurological, and major medical diagnoses currently or in the past, any serious general medical condition, use of illegal drugs, excessive consumption of caffeine or alcohol, lifetime use of any psychopharmacological medication, including antidepressants, antipsychotics and medication against attention deficit hyperactivity disorder, any regular systemic medication, and any other medication within the week before the study. Only participants that reported to be sexually active at the time of the study were included. None of the participants reported a history of sexual dysfunction and none developed clinically relevant symptoms over the course of the study, which was both assessed by questionnaires and clinical interviews. Further sample characteristics are described in Abler et al. (2011).
Data from six subjects were excluded for technical reasons after scanning sessions took place: Two participants were excluded due to very low drug plasma levels (see Procedure), rendering intake doubtful. For one participant, a single blood sample was lost, rendering assessment of drug intake impossible. In three additional participants, MR-related technical problems in one of the scans led to exclusion from the study.
The study was approved by the local ethical review board of the University of Ulm. All volunteers gave written informed consent prior to the study in accordance with the Declaration of Helsinki.
Procedure
Participants took placebo (Plac) or antidepressant treatment with bupropion or paroxetine (Par) at routine clinical doses for seven days each in a counterbalanced order and were included in the fMRI study. Drug-filled gelatine capsules contained either pulverized mannitol (Plac), 150 mg of bupropion or 20 mg of paroxetine.
Functional network characteristics were assessed after each drug intake period using a rsfMRI setup scanned in the same session as the task-related fMRI scans described in Abler et al. (2011). Drug-intake was monitored by plasma-levels on the day of the scan. The randomized crossover design assured at least 14 days of washout time between treatments.
Measures
A German version (Reinecke, Schöps, & Hoyer, 2006) of the 5-item Massachusetts General Hospital Sexual Functioning Questionnaire (Labbate & Lare, 2001) was applied before medication and upon each treatment to assess basic sexual functioning and the modulation by medication. Versus an expert clinical interview, the German version has specificity of 0.90 and a sensitivity of 0.43 for detection of impaired sexual function. We modified the MGH-SFQ in terms of the assessed time-period from 4 to 1 week, fitting the purposes of our study (see supplementary text 1 for detailed description). The five subcategories encompassed (1) “sexual interest,” (2) “ability to gain sexual arousal,” (3) “ability to achieve orgasm,” (4) “ability to get and maintain an erection,” and (5) “sexual satisfaction.” Overall sexual function, as revealed by the difference in MGH-SFQ sum score between Par and Plac, formed the regressor for the second level analysis of fMRI data. Participants with an increase of the SFQ sum score of at least 5 points (average of at least one point in each item) were classified as participants with a clear vulnerability to impaired sexual function with SSRI. For our discriminant function analysis, we also investigated discriminative power of connectivities for absolute values of decreased sexual function as reported during Par irrespective of changes to Plac. Therefore participants with an MGH-SFQ sum score >14, were classified as participants with a clinically significant decrease in sexual functioning under Par (n = 7)—meaning they were at least minimally diminished in at least four of five items.
Given that the goal of the current analysis was to assess predictors of side effects under SSRI treatment, only fMRI data from Plac and behavioral data from Plac and Par, but not bupropion trials are presented here.
fMRI Settings and Data Acquisition
T1 anatomical volume images (1 × 1 × 1 mm3 voxels) and functional magnetic resonance images were acquired using a 3.0 Tesla Magnetom ALLEGRA Scanner (Siemens, Erlangen, Germany). Therefore 23 transversal slices were acquired with the following parameters: image size: 64 × 64 pixels, FOV: 192 mm, slice thickness: 3 mm, 0.75 mm gap, resulting in a voxel size of 3 × 3 × 3.75 mm3. This comparably high resolution was chosen to accommodate the anatomical extent of the subcortical target structures. Images were centered on basal structures of the brain to best fit the subcortical regions of interest (SLEA, midbrain, basal ganglia and prefrontal regions). In addition, all participants completed an fMRI trial including a 10 min eyes-closed resting state scan, therefore lying awake in the scanner with their eyes-closed, not asked to perform any specific task. In the meantime 300 EPI functional images were recorded during this resting state scan, using a T2*-sensitive gradient echo sequence (TR 1,500 ms, TE 35 ms, flip angle 90°), to measure changes in BOLD contrast.
fMRI and Behavioral Data Analysis
Resting state connectivity analyses were performed using DPARSF (Yan & Zang, 2010), which runs with the rsfMRI data analysis toolkit (REST) (Song et al., 2011) and SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). Preprocessing included slice timing, realignment and spatial normalization to 3 × 3 × 3 mm3 isovoxels to a standard template (Montreal Neurological Institute, MNI).
To address the issue of individually differing partial brain coverage as a result of the high spatial resolution and low TR, all normalized brains were masked with a group specific zero mask, corrected for all voxels contained in each participant’s volume, to calculate the following steps—like global mean regression—on equal volumes. Linear trends were removed and data were filtered for frequencies between 0.01 and 0.08 Hz. Motion correction, global mean regression as well as white matter signal and cerebrospinal fluid signal were regressed out and treated as regressors of no interest. Spatial smoothing of 8 mm was applied to the functional data. As seed regions for FC analysis, four seed ROIs were chosen according to the previous study by Abler et al. (2011). In this investigation, these four regions had been identified to show the strongest interaction effect of drug treatment and erotic stimulation. The effect was even greater for left lateralized ROIs which let us focus on left-sided regions as seeds for FC analyses. Seed regions provide a single time course for each participant, which is used to calculate whole brain FC of each other voxel in the brain with this region of interest. FC is calculated based on the degree of modeled temporal correlations of the seed time course and each voxels time course (Biswal et al., 1995). Seed regions were specified as follows: (1) SLEA (5 mm sphere at x: −22, y: 0, z: −10), (2) midbrain (5 mm sphere at x: −4, y: −24, z: −14), (3) pgACC (10 mm sphere at x: −6, y: 44, z: 6) and (4) pMCC (10 mm sphere at x: 10, y: 24, z: 36) (Fig. 1).
Seed regions of interest. Four regions of interest were chosen, representing a predefined network of sexual dysfunction according to Abler et al. (2011) in the midbrain (mb), the sublenticular extended amygdala (SLEA), the pregenual anterior cingulate cortex (pgACC) and the posterior midcingulate cortex (pMCC)
For each participant, whole brain FC was calculated for each region of interest, resulting in five participant-specific three-dimensional FC maps. For peaks centered in white matter (pgACC and pMCC), ROIs were positioned in the adjacent grey matter, taking only the grey matter voxels within the sphere into account for the creation of the ROI, using the “create ROI” function offered by MRIcron (http://www.mricron.com).
Second level statistics across all participants were performed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). A simple regression model was used to predict sexual function under Par from FC of ROIs of interest under Plac. Therefore, on the basis of whole brain FC maps for each ROI, a simple regression model was performed, with individual changes in sexual function under Par compared to Plac as one single regressor. Thresholds were set to a significance level of p < .001 adding a cluster level correction of k > 10 voxels for cortical and subcortical regions. Single subject ROI FC values were extracted using MarsBar (http://marsbar.sourceforge.net).
A second analysis was performed to relate the different dimensions of sexual function to connectivities of specific target regions of the SLEA, as revealed by the former regression analysis. Therefore, the correlations with those three sub-items of the five-item MGH-SGQ, which significantly changed under Par compared to Plac, and FC of the SLEA under Plac were analysed.
For the SLEA, a specific role for sexual functioning as well as a specific change under SSRI treatment has been proposed by previous studies (Abler et al., 2011; Walter et al., 2008a). Thus, the significant target connectivities derived from the main regression analysis were taken to a secondary discriminant function analysis to test for a prediction of treatment related side effects based on connectivities during Plac treatment.
For the analysis of behavioral data, paired t tests and correlation analyses were performed using SPSS 15 to identify differences between Plac and Par trials as well as intercorrelations between trials. As drug administration itself had an impact on participants’ reported decrease in sexual functioning (Abler et al., 2011), Par was compared to Plac instead of baseline sexual functioning, to exclude such placebo effects.
Results
Behavioral Testing: MGH-SFQ
As expected under treatment with an SSRI, paired testing revealed significantly lower scores of sexual function according to the MGH-SFQ sum score under Par compared to Plac (p = .019, t = −2.61, df = 16, Fig. 2). The post hoc paired analysis of subcategories showed that this effect of Par was mainly based on differences in the sub-categories “ability to gain sexual arousal” (category 2, p = .041, t = −2.21, df = 16), “ability to achieve orgasm” (category 3, p = .014, t = −2.74, df = 16) and “overall sexual satisfaction” (category 5, p = .052, t = −2.09, df = 16). The paired t-test for differences of MGH-SFQ sum scores between Plac and pre-treatment baseline condition revealed no significant difference between baseline and placebo sum score (p > .05) in the presented sample.
5-Item Massachusetts General Hospital Sexual Function Questionnaire (MGH-SFQ) sum score (left) and subcategories (right) revealing an increased subjective sexual dysfunction under paroxetine (Par) compared to placebo (Plac, + p < .10, * p < .05). SFQ1 “sexual interest”; SFQ2 “ability to gain sexual arousal”; SFQ3 “ability to achieve orgasm”; SFQ4 “ability to get and maintain an erection”; SFQ5 “overall sexual satisfaction”. Minimal score (sum/per question): 5/1 = increased sexual interest as compared to normal; “average” score (sum/per question): 10/2 = no change in sexual functioning compared to before; scores >10/2: decreased sexual functioning; maximal score (sum/per question): 30/5 = no sexual interest
Interrelations of the subscores were calculated at a Bonferroni corrected statistical threshold of p = .001 (target threshold p < .05, 45 comparisons for five subscales and two treatment conditions). Subjective overall sexual satisfaction (item 5) under Plac was highly correlated with sub-items 1–3 (p < .001, r > .75) but not with item 4. Sexual interest (item 1) was further correlated with the ability to achieve orgasm (item 3, p < .001, r = .74). During Par treatment, only sub-items 1 and 2 were correlated with each other. No significant correlation was found between subscores of sexual function under Par and the subscores under Plac.
Changes in sexual functioning related to markedly increased SFQ values (≥5 points in the sum score) under Par in 7 of the participants, while scores remained largely unchanged or even slightly improved (≤3 points in the sum score) in 10 of the participants.
Prediction of Decreased Sexual Function Under Paroxetine by Baseline Functional Connectivity
Potential predictors for the individual disposition to decreased sexual function under Par treatment were then evaluated in terms of baseline resting state behavior.
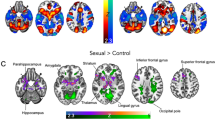
Using the SLEA ROI from the previous study as a first seed in the FC analysis, results confirmed our hypothesis of the SLEA as a core node in the network (Fig. 3a–c). Significant positive correlations were found for the FC between SLEA and LOC, as well as with the bilateral fusiform gyrus (FusG, Table 1). Additionally, a significant negative correlation of FC between SLEA and midbrain was found and for FC between SLEA and pgACC (Table 2) as well as ventrolateral prefrontal cortex (vlPFC). Thus, the connectivity analysis confirmed a potentially crucial role for impaired sexual function of brain regions previously identified to be related to paroxetine effects. Here, healthy participants with stronger changes and thus low scores for subjective sexual function (overall change in MGH-SFQ ≥5) during Par treatment tended to show higher FC under placebo between the midbrain, the LOC and the SLEA (see also Fig. 3b) and lower FC between pgACC, vlPFC and SLEA.
a Target regions with highest correlation of functional connectivity (FC) with the sublenticular extended amygdala (SLEA) and the change of scores in the sexual functioning questionnaire under paroxetine compared to placebo. The highest negative correlation was found with the individual FC between SLEA and midbrain (blue), and between SLEA and pgACC (pregenual anterior cingulate cortex, green), whereas the highest positive correlation of this regressor was observed with the FC between SLEA and LOC (lateral occipital cortex, red). b FC of SLEA under the placebo condition for groups of low (left) and high sexual dysfunction (right) with midbrain (blue), pgACC (green), LOC (red). c Plots of FC between SLEA and target regions (peak values) (Color figure online)
Analysis of Subcategories
To further characterize specific roles of connectivities with the SLEA we calculated correlations of whole brain FC with those three MGH-SFQ subcategories with significant changes under Par as revealed in the initial analysis of the behavioral data (Fig. 2). Impairments in the ability to gain sexual arousal (item 2) under Par were related to significantly reduced baseline connectivities between SLEA and midbrain as well as between SLEA and anterior insula. Reduced baseline FC between SLEA and midbrain further accounted specifically for increased difficulties to achieve orgasm (item 3), as indicated by a significant negative regression (Fig. 4). In contrast, overall sexual satisfaction (item 5) was related to connectivities between SLEA and pgACC. Here, the negative correlation indicated that participants with smaller FCs under Plac were to show stronger impairments in this category under Par.
Regression analysis of sexual functioning questionnaire (MGH-SFQ) subcategories. Negative correlations (left, blue box): item 2 (red, “ability to gain sexual arousal”) was negatively correlated with functional connectivity (FC) between sublenticular extended amygdala (SLEA) and midbrain as well as FC between SLEA and anterior insula. Item 3 (blue, “ability to achieve orgasm”) was also negatively correlated with FC between SLEA and midbrain, item 5 (yellow, “overall sexual satisfaction”) with FC between SLEA and pregenual anterior cingulate cortex (pgACC). Positive correlation (right, red box): A positive correlation was found between item 3 (blue) and FC between SLEA and hippocampus, as well as FC between SLEA and fusiform gyrus (FusG). Item 5 (yellow) was positively correlated with FC between SLEA and posterior midcingulate cortex (pMCC) (Color figure online)
Of note, the analysis of subcategories replicated and specified the importance of the target structures initially identified by the main analysis using the sum MGH-SFQ score. Only one region, the anterior insula, which was not revealed by the main analysis, was added by the negative correlation of subscores. Additionally two regions were revealed by positive regressions, indicating that (1) participants with higher baseline FC between SLEA and hippocampus developed stronger impairments during orgasms (item 3) and (2) connectivities between SLEA and pMCC predicted reduced overall sexual satisfaction (item 5). Higher connectivities between SLEA and FusG were revealed to be associated not only to the sum MGH-SFQ score but particularly to the subscore of orgasm related impairments (Fig. 4).
Brain Based Prediction of Paroxetine-Related Modulation of Sexual Function
Discriminative function analyses were then conducted to identify whether the individual connectivities of the SLEA with the three main regions, midbrain, pgACC and LOC under Plac, rather predict the absolute ratings of subjective sexual function under Par or the specific worsening as compared to Plac. Connectivity strengths predicting genuine treatment effects were expected to relate to greater impairment in sexual function under Plac rather than to the absolute values. The stepwise discriminative analysis to differentiate between subjects with high from those with low ratings of sexual function under Par, irrespective of the status under Plac, revealed an optimized model of discriminance based on one factor: the FC of SLEA with LOC (eigenvalue of discriminative function = 1.28, canonical correlation = 0.74, Wilks’s Lambda = 0.43, χ 2 = 11.95, p = .001, df = 1).
The stepwise discriminative analysis to differentiate between subjects with high and low changes in sexual function from Plac to Par on the same regions revealed an optimal model of discriminance based on the FC between SLEA and midbrain under Plac (eigenvalue of discriminative function = 0.37, canonical correlation = 0.52, Wilks’s Lambda = 0.72, χ 2 = 4.58, p = .032, df = 1). The two functional connectivities under Plac thus predict different though overlapping aspects of Par-related decreases in sexual functioning (Fig. 5a). The best discrimination between subjects with high or low changes in sexual functioning under Par compared to Plac was reached with a compound measure of the product of the two connectivities at baseline (Fig. 5b). Receiver operator characteristics of the three individual connectivities with the SLEA (midbrain, pgACC, LOC) and the compound measure confirmed the superiority of the latter (Fig. 5c).
a Connectivity measures under placebo (Plac) conditions can be used to separate subjects with great (red) from those with little (green) changes in sexual functioning and thus side effects under paroxetine (Par). b A quite clear separation of the two groups is evident when taking the product of functional connectivity (FC) between the sublenticular extended amygdala (SLEA) and midbrain and FC between SLEA and lateral occipital cortex (LOC). c Receiver operator characteristics of the individual connectivities between SLEA and midbrain (blue), pregenual anterior cingulate cortex (pgACC, green) and LOC (red) testing for prediction of the development of sexual dysfunction under Par as compared to Plac. The figure shows that all three individual connectivities have a high discriminative power, with the positive connections to pgACC (area under the curve, AUC 0.903, p = .005) and midbrain (AUC 0.917, p = .004) having slightly higher sensitivity for the prediction of the SSRI-induced sexual dysfunction than connectivities to LOC (AUC 0.875, p = .009). The compound measure (FCSLEA-midbrain × FCSLEA-LOC) however outperforms the individual connectivities in identifying subjects with high SSRI induced sexual dysfunction (AUC 0.931, p = .003, dotted line) (Color figure online)
Confirmation of SLEA-Based Network
To control for the reliability of the network found when using the SLEA ROI as the seed region, that revealed pgACC, pMCC and midbrain as target regions, we tested on networks related to seeds derived from the other three predefined ROIs comprising these regions. Again, we used the decrease in sexual function under paroxetine as a regressor to now test connectivities with midbrain, pgACC, and pMCC for their predictive value. Replicating the findings using the SLEA seed, participants with high connectivities between midbrain and SLEA exhibited a lower tendency to develop paroxetine-related impairment of sexual function. Additionally, the vlPFC as part of the negatively correlated network was confirmed with higher connectivity between midbrain and vlPFC indicating a lower vulnerability towards developing this side effect (Table 1). Regarding the positively correlated network, analyses with the midbrain as a seed as well as with the pgACC as a seed confirmed the role of the FusG already suggested from the analysis using the SLEA seed (Table 2). The negative correlation with the FC between pgACC and bilateral hippocampus (p < .001, k > 10, Table 1) supports the involvement of the region identified as part of the network first in the context of analyses of subcategories of sexual function (Fig. 4). Correlations of FC with the change of overall sexual function between pMCC as seed and both, FusG and vlPFC strengthen the assumption of an involvement of the latter regions in the network.
A complete overview on all predictive connectivities, including uncorrected clusters, is listed in supplementary Table 1.
Discussion
Summary of Results
We could verify our hypothesis that the individual disposition to develop decreased subjective sexual functioning under SSRIs could be predicted by baseline resting state connectivity within a network previously related to physiological (Walter et al., 2008a) and particularly SSRI-related altered sexual processing (Abler et al., 2011). With the extended amygdala as a core node, the network predicting alterations on the behavioral level comprised of the ACC, midbrain, and LOC. Significantly altered subdimensions of sexual functioning were linked to specific aspects of the network. Low midbrain-SLEA connectivities were related to the development of decreased ability to achieve orgasm, while reduced sexual arousal was predicted by the baseline connectivity of the SLEA with the anterior insula. Decreased individual pgACC-SLEA connectivities predicted low overall sexual satisfaction. Moreover, individual characteristics of SLEA connectivities to midbrain, pgACC and LOC were shown to reliably identify participants with greater vulnerability towards SSRI-related decreases of sexual functioning.
A Network of Sexual Functioning explored by Resting State fMRI
The network suggested to predict the development of decreased sexual function under SSRIs by its connectivity strengths converges with previously reported neuronal effects of actual paroxetine treatment with altered brain activations in SLEA, midbrain and ACC during processing of visual erotic stimuli (Abler et al., 2011). Furthermore, the brain regions with the predictive interconnections have been reliably related to the processing of sexual stimuli before. ACC activations, especially in the pgACC were recently discussed to code the valence component of sexual arousal during visual erotic stimulation (Walter et al., 2008a). The SLEA as extended part of the amygdala has been previously described in studies investigating sexual functioning (Ferretti et al., 2005; Hamann, Herman, Nolan, & Wallen, 2004; Karama et al., 2002; Redouté et al., 2000). In general, the amygdala has been shown to code the emotional component of erotic stimuli (Walter et al., 2008a). In this sense, it is rather active during the appetitive phase of sexual arousal, whereas a characteristic deactivation has been described during the consumatory and orgastic phase (Georgiadis & Holstege, 2005; Holstege et al., 2003).
The midbrain has been less consistently reported in imaging studies of erotic stimulation in humans. However, using PET, Bocher et al. (2001) found that midbrain activation was related to interindividual differences of subjective sexual arousal. Strongest activations in this area were found for participants with the highest scores for subjective sensation of erection. Furthermore, a recent study by Georgiadis et al. (2007) reported rCBF increases in the midbrain consistently over several data sets related to male ejaculation.
It is of note, however, that the rsfMRI data presented here are not related to explicit erotic stimulation. Rather, we investigated measures of the interregional crosstalk at rest. Greatest connectivity between brain regions of a network consistently linked to sexual processing were found in participants with lowest changes in sexual functioning under SSRI treatment. We therefore suggest interpreting strong interconnections at baseline as protective against the development of sexual dysfunction under the influence of serotonergic agents. The demonstrated interconnections converge with well described ascending pathways from the serotonergic raphe nuclei to the forebrain including the amygdala and prefrontal cortex (Alex & Pehek, 2007; Azmitia & Segal, 1978; Hensler, 2006) as well as with the predescribed modulation of dopaminergic midbrain activity by serotonin. Brain imaging in humans investigating tryptophan depletion linked amygdala-cingulate connectivity to the modulation of serotonergic principles (von dem Hagen, Passamonti, Nutland, Sambrook, & Calder, 2011). Resting state connectivities in humans between amygdala and medial prefrontal cortex, i.e., in a network previously described to process emotional valence and associated arousal of erotic stimuli (Walter et al., 2008a) were recently shown to be modulated under the SSRI citalopram (McCabe, Jones, Nikolopoulou, Wathen, & Luqmani, 2011).
While the link between greater positive interconnections in this network at baseline and greater protection against external modulatory influences is intuitive, the finding of greater negative interconnections as a protective factor, as for SLEA and LOC, is less easily interpreted. Activation of the LOC was previously described in studies using visual sexual stimuli (Arnow et al., 2002; Bocher et al., 2001; Ferretti et al., 2005; Moulier et al., 2006; Mouras et al., 2003; Ponseti et al., 2006; Walter et al., 2008a) and has been interpreted in terms of the overlap of this area with the extrastriate body area, which specifically codes for the perception of human body parts (Downing, Jiang, Shuman, & Kanwisher, 2001). Although the exact neuroscientific meaning of anticorrelations (i.e., negative correlations) is still under debate, “negative connectivity” has been established in a number of neuropsychiatric disorders (Castellanos et al., 2008) and its reduction was interpreted as a symptom of reduced functional flexibility (Horn et al., 2010).
Subdimensions of Sexual Function
The relation of subdimensions of sexual function and neural network components suggested connectivities with the midbrain as a state marker for orgasm-related impairments and connectivities with the pgACC for sexual satisfaction. This duality is mirrored by previous functional interpretations of the two structures in imaging studies of sexual processing. Most of these either investigated mild sexual arousal, commonly related to visual erotic stimulation or the effects of actually achieved orgasm on brain function. Midbrain activation was found in particular in those studies that focus on orgasm itself (Georgiadis et al., 2007) and less consistently during passive viewing paradigms.
The pgACC, in contrast, has been implicated in coding the valence-dependent emotional component of sexual arousal (Walter et al., 2008a), which may be best mirrored by the SFQ item on overall sexual satisfaction in our experiment. In the previous study, activation of the pgACC was specifically linked to an interaction of reported pleasantness and sexual arousal. These interpretations are in line with the suggested role of this portion of the rostral ACC in the generation and maintenance of a subjective value in the context of emotional experience in healthy and diseased populations (Mayberg, 1997; Phan, Wager, Taylor, & Liberzon, 2004). Furthermore, anhedonia, or the inability to experience pleasure related to a normally preferred stimulus or context, was found to be specifically related to a dysfunction of the pregenual portion of the ACC and adjacent medial prefrontal cortex in depressed patients (Keedwell, Andrew, Williams, Brammer, & Phillips, 2005; Walter et al., 2009). Therefore, the link between SLEA and pgACC connectivity and overall subjective sexual dissatisfaction as revealed by our analysis can be taken as a plausible marker of vulnerability.
While connectivities of SLEA and midbrain seemed to predict consummatory aspects of sexual functioning, impairments in achieving sexual arousal were predicted by the connectivity between SLEA and the anterior insula that was thus related to more appetitive aspects. This interpretation is underlined by the finding that the activation of the anterior insula precedes, but does not coincide, with penile responses under erotic stimulation by a 20 s lag (Mouras et al., 2003). The region has been hypothesized to mediate the awareness of bodily feelings, perceptions, and vegetative states (reviewed by Craig, 2011). Consequently, a reduced appetitive sexual arousal might be associated with reduced insula connectivity, since reduced activation in this region was also observed for anticipation of affective stimuli after SSRI administration (Simmons, Arce, Lovero, Stein, & Paulus, 2009).
Prediction of Decreased Sexual Function
Highly sensitive and specific receiver operator characteristics confirmed the strong predictive value of brain connectivity at rest for the main connectivities, namely SLEA to midbrain, pgACC, and LOC. While connectivities between SLEA and midbrain were indeed particularly predictive for the actual change in sexual function from Plac to Par as shown by the discriminative function analyses, connectivity between SLEA and LOC better discriminated the absolute achieved degree of decreased sexual function under Par, independent of baseline scores of sexual function. SSRI-related change and absolute value represent different outcome criteria regarding sexual function. Our results suggest that baseline brain activation could be predictive for both criteria of the side effect. Such predictors, if replicated in larger and clinical samples, could be highly relevant in clinical settings. Singling out patients at particular risk and assigning them to alternative treatments could increase adherence to treatments, especially in young male patients, in which medication-related impairments of sexual function is a particularly common reason for discontinuation.
Limitations
A limitation of our study is that our findings were observed in healthy participants, not patients. Direct inferences about the relationship of connectivites and occurrence of side effects in depressed populations can only be drawn with caution, especially since the network investigated, including pgACC, SLEA and midbrain, seems to be functionally altered in depression, even in the absence of SSRI treatment (Veer et al., 2011). However, our findings have implications for the use of SSRIs in otherwise healthy participants with premature ejaculation (Pryor et al., 2006). In this case, resting state brain activity could help predicting participants with particularly good response to SSRIs.
Another potential limitation regards the midbrain effects. These might be biased by the choice of the SSRI used, given that among commonly used ones, paroxetine was shown to be the one with the highest serotonin transporter occupancy, especially within the midbrain (Meyer et al., 2004). While paroxetine is not the most selective SSRI, it was chosen for this study because it is known to cause sexual side effects most frequently among SSRIs (Clayton et al., 2002) and is one of the frequently prescribed SSRIs in clinical use. A different SSRI might have resulted in a slightly different distribution of effects.
It is well known, that antidepressants have different side effects in men and women (Clayton, Keller, & McGarvey, 2006). While sexual side effects with SSRIs are described for both genders, there is evidence, that those might be more frequent in men while genders also differ in extent of these effects (Montejo-Gonzáles et al., 1997). Therefore, we carried out our study in a homogeneous sample of only males, in order to eliminate potential gender differences.
The MGH-SFQ is limited to a certain number of self-rated items to assess sexual functioning. More detailed self-reported questionnaires or other objective measures like penile tumescence to visual erotic stimulation (Ferretti et al., 2005; Mouras et al., 2003) might have resulted in a more detailed description of the impairment of sexual functioning. Due to the resting state condition itself, such direct effects of visual erotic stimulation cannot be addressed by this study.
Performing pharmacological fMRI in healthy participants is an established method to look for drug-effects independent of disease interactions (Scheidegger et al., 2012); however, direct implications for clinical populations have to be treated with caution. In the same view, the use of an SSRI for 1 week might not be sufficiently long enough to exert treatment effects in patients. Changes in sexual functioning—especially concerning premature ejaculation—have been reported after a single dose of SSRIs (Patel & Hellstrom, 2009) and affected libido and sexual arousal after a continuous intake of 8 days (Dunn, Arakawa, Greist, & Clayton, 2007). However, in healthy participants, 1 week is sufficient to cause not only neuronal changes, but also side effects (Abler et al., 2011; Graf, Abler, Hartmann, Metzger, & Walter, 2012). Therefore the specific question of short- and long-term effects, as described for SSRIs, has to be addressed in future studies.
These data link changes in sexual functioning to baseline connectivity. Therefore it would also be of interest to link those changes in sexual functioning to changes in connectivity between the two sessions instead of baseline connectivity. However, to avoid circularity within statistical inference (Kriegeskorte, Lindquist, Nichols, Poldrack, & Vul, 2010), this question should be addressed to an independent sample.
In the current study, we reported both positive and negative connectivity and changes in such (like less negative FC between SLEA and LOC in the group with low sexual functioning under Par). However, such positive and negative connectivities cannot be taken as absolute values or—especially for negative FC—as measures of true negative interregional connectivity. They are mainly introduced by certain preprocessing steps like global mean regression (Fox, Zhang, Snyder, & Raichle, 2009; Weissenbacher et al., 2009), but have also been shown independent of certain preprocessing strategies (Chai, Castanón, Ongür, & Whitfield-Gabrieli, 2012). Future implementations of geometrical estimates of the global signal may therefore be of interest to follow up on these specific connectivities between SLEA and LOC.
Conclusions
Our study demonstrated the predictive potential of connectivities of the SLEA especially with midbrain, pgACC, LOC, and insula, for the development of SSRI-related decreases in sexual functioning. Greater overall development of impaired sexual function compared to placebo was predicted by low baseline connectivities. While all these connectivities showed a high predictive potential towards absolute ratings of sexual function, the connectivity with midbrain best predicted the decrease in sexual function as compared to under placebo. A compound measure of resting-state brain connectivities under placebo allowed for errorless distinction of participants with high or low vulnerability to SSRI-related impairments of sexual functioning. While larger studies in an independent sample are needed to confirm the overall importance of our findings, we demonstrated the potential application of imaging techniques to the understanding and prediction of SSRI treatment side effects.
References
Abler, B., Grön, G., Hartmann, A., Metzger, C., & Walter, M. (2012). Modulation of frontostriatal interaction aligns with reduced primary reward processing under serotonergic drugs. Journal of Neuroscience, 32, 1329–1335. doi:10.1523/JNEUROSCI.5826-11.2012.
Abler, B., Seeringer, A., Hartmann, A., Grön, G., Metzger, C., Walter, M., et al. (2011). Neural correlates of antidepressant-related sexual dysfunction: A placebo-controlled FMRI study on healthy males under subchronic paroxetine and bupropion. Neuropsychopharmacology, 36, 1837–1847. doi:10.1038/npp.2011.66.
Alex, K. D., & Pehek, E. A. (2007). Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacology & Therapeutics, 113, 296–320. doi:10.1016/j.pharmthera.2006.08.004.
Arnow, B. A., Desmond, J. E., Banner, L. L., Glover, G. H., Solomon, A., Polan, M. L., … Atlas, S. W. (2002). Brain activation and sexual arousal in healthy, heterosexual males. Brain: A Journal of Neurology, 125, 1014–1023. doi:10.1093/brain/awf108.
Azmitia, E. C., & Segal, M. (1978). An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. Journal of Comparative Neurology, 179, 641–668. doi:10.1002/cne.901790311.
Benedetti, F., Mayberg, H. S., Wager, T. D., Stohler, C. S., & Zubieta, J. K. (2005). Neurobiological mechanisms of the placebo effect. Journal of Neuroscience, 25, 10390–10402. doi:10.1523/JNEUROSCI.3458-05.2005.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–541. doi:10.3410/f.714597885.790202808.
Bocher, M., Chisin, R., Parag, Y., Freedman, N., Meir Weil, Y., Lester, H., … Bonne, O. (2001). Cerebral activation associated with sexual arousal in response to a pornographic clip: A 15O-H2O PET study in heterosexual men. NeuroImage, 14, 105–117. doi:10.1006/nimg.2001.0794.
Castellanos, F. X., Margulies, D. S., Kelly, C., Uddin, L. Q., Ghaffari, M., Kirsch, … Milham, M. P. (2008). Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry, 63, 332–337. doi:10.1016/j.biopsych.2007.06.025.
Chai, X. J., Castanón, A. N., Ongür, D., & Whitfield-Gabrieli, S. (2012). Anticorrelations in resting state networks without global signal regression. NeuroImage, 59, 1420–1428. doi:10.1016/j.neuroimage.2011.08.048.
Clayton, A., Keller, A., & McGarvey, E. L. (2006). Burden of phase-specific sexual dysfunction with SSRIs. Journal of Affective Disorders, 91, 27–32. doi:10.1016/j.jad.2005.12.007.
Clayton, A. H., Pradko, J. F., Croft, H. A., Montano, C. B., Leadbetter, R. A., Bolden-Watson, C., … Metz, A. (2002). Prevalence of sexual dysfunction among newer antidepressants. Journal of Clinical Psychiatry, 63, 357–366.
Craig, A. D. B. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225, 72–82. doi:10.1111/j.1749-6632.2011.05990.x.
Downing, P. E., Jiang, Y., Shuman, M., & Kanwisher, N. (2001). A cortical area selective for visual processing of the human body. Science, 293, 2470–2473. doi:10.1126/science.1063414.
Dunn, J. A., Arakawa, R., Greist, J. H., & Clayton, A. H. (2007). Assessing the onset of antidepressant-induced sexual dysfunction using interactive voice response technology. Journal of Clinical Psychiatry, 68, 525–532.
Ferretti, A., Caulo, M., Del Gratta, C., Di Matteo, R., Merla, A., Montorsi, F., … Romani, G. L. (2005). Dynamics of male sexual arousal: Distinct components of brain activation revealed by fMRI. NeuroImage, 26, 1086–1096. doi:10.1016/j.neuroimage.2005.03.025.
Fox, M. D., & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience, 4, 19. doi:10.3389/fnsys.2010.00019.
Fox, M. D., Zhang, D., Snyder, A. Z., & Raichle, M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101, 3270–3283. doi:10.1152/jn.90777.2008.
Georgiadis, J. R., & Holstege, G. (2005). Human brain activation during sexual stimulation of the penis. Journal of Comparative Neurology, 493, 33–38. doi:10.1002/cne.20735.
Georgiadis, J. R., Reinders, A. A. T. S., van der Graaf, F. H. C. E., Paans, A. M. J., & Kortekaas, R. (2007). Brain activation during human male ejaculation revisited. NeuroReport, 18, 553–557. doi:10.1097/WNR.0b013e3280b10bfe.
Graf, H., Abler, B., Hartmann, A., Metzger, C. D., & Walter, M. (2012). Modulation of attention network activation under antidepressant agents in healthy subjects. International Journal of Neuropsychopharmacology. doi:10.1017/S1461145712001368.
Gregorian, R. S., Golden, K. A., Bahce, A., Goodman, C., Kwong, W. J., & Khan, Z. M. (2002). Antidepressant-induced sexual dysfunction. Annals of Pharmacotherapy, 36, 1577–1589. doi:10.1345/aph.1A195.
Hamann, S., Herman, R. A., Nolan, C. L., & Wallen, K. (2004). Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience, 7, 411–416. doi:10.1038/nn1208.
Heimer, L., Harlan, R. E., Alheid, G. F., Garcia, M. M., & Olmos, J. d. (1997). Substantia innominata: A notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience, 76, 957–1006. doi:10.1016/S0306-4522(96)00405-8.
Hensler, J. G. (2006). Serotonergic modulation of the limbic system. Neuroscience and Biobehavioral Reviews, 30, 203–214. doi:10.1016/j.neubiorev.2005.06.007.
Holstege, G., Georgiadis, J. R., Paans, A. M. J., Meiners, L. C., van der Graaf, F. H. C. E., & Reinders, A. A. T. S. (2003). Brain activation during human male ejaculation. Journal of Neuroscience, 23, 9185–9193.
Horn, D. I., Yu, C., Steiner, J., Buchmann, J., Kaufmann, J., Osoba, A., Eckert, U., … Walter, M. (2010). Glutamatergic and resting-state functional connectivity correlates of severity in major depression-the role of pregenual anterior cingulate cortex and anterior insula. Frontiers in Systems Neuroscience, 4. doi:10.3389/fnsys.2010.00033.
Karama, S., Lecours, A. R., Leroux, J.-M., Bourgouin, P., Beaudoin, G., Joubert, S., et al. (2002). Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping, 16, 1–13. doi:10.1002/hbm.10014.
Keedwell, P. A., Andrew, C., Williams, S. C. R., Brammer, M. J., & Phillips, M. L. (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58, 843–853. doi:10.1016/j.biopsych.2005.05.019.
Kriegeskorte, N., Lindquist, M. A., Nichols, T. E., Poldrack, R. A., & Vul, E. (2010). Everything you never wanted to know about circular analysis, but were afraid to ask. Journal of Cerebral Blood Flow and Metabolism, 30, 1551–1557. doi:10.1038/jcbfm.2010.86.
Labbate, L. A., & Lare, S. B. (2001). Sexual dysfunction in male psychiatric outpatients: Validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychotherapy and Psychosomatics, 70, 221–225. doi:10.1159/000056257.
Mayberg, H. S. (1997). Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences, 9, 471–481. http://neuro.psychiatryonline.org/data/Journals/NP/3847/471.pdf.
McCabe, C., Jones, Q., Nikolopoulou, A., Wathen, C., & Luqmani, R. (2011). Pulmonary–renal syndromes: An update for respiratory physicians. Respiratory Medicine, 105, 1413–1421. doi:10.1016/j.rmed.2011.05.012.
Meissner, K., Bingel, U., Colloca, L., Wager, T. D., Watson, A., & Flaten, M. A. (2011). The placebo effect: Advances from different methodological approaches. Journal of Neuroscience, 31, 16117–16124. doi:10.1523/JNEUROSCI.4099-11.2011.
Metzger, C. D., Eckert, U., Steiner, J., Sartorius, A., Buchmann, J. E., Stadler, J., … Walter, M. (2010). High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Frontiers in Neuroanatomy, 4, 138. doi:10.3389/fnana.2010.00138.
Meyer, J. H., Wilson, A. A., Sagrati, S., Hussey, D., Carella, A., Potter, W. Z., … Houle, S. (2004). Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: An [11C]DASB positron emission tomography study. American Journal of Psychiatry, 161, 826–835. doi:10.1176/appi.ajp.161.5.826.
Montejo-Gonzáles, A. L., Llorca, G., Izquierdo, J. A., Ledesma, A., Bousono, M., Calcedo, A., … Vicens, E. (1997). SSRI-induced sexual dysfunction: Fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. Journal of Sex & Marital Therapy, 23, 176–194. doi:10.1080/00926239708403923.
Moulier, V., Mouras, H., Pélégrini-Issac, M., Glutron, D., Rouxel, R., Grandjean, B., … Stoléru, S. (2006). Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. NeuroImage, 33, 689–699. doi:10.1016/j.neuroimage.2006.06.037.
Mouras, H., Stoléru, S., Bittoun, J., Glutron, D., Pélégrini-Issac, M., Paradis, A.-L., et al. (2003). Brain processing of visual sexual stimuli in healthy men: A functional magnetic resonance imaging study. NeuroImage, 20, 855–869. doi:10.1016/S1053-8119(03)00408-7.
Nieuwenhuys, R. (2008). The human central nervous system (4th ed.). Berlin: Springer.
Patel, K., & Hellstrom, W. J. (2009). Central regulation of ejaculation and the therapeutic role of serotonergic agents in premature ejaculation. Current Opinion in Investigational Drugs, 10, 681–690.
Phan, K. L., Wager, T. D., Taylor, S. F., & Liberzon, I. (2004). Functional neuroimaging studies of human emotions. CNS Spectrums, 9, 258–266.
Ponseti, J., Bosinski, H. A., Wolff, S., Peller, M., Jansen, O., Mehdorn, H. M., … Siebner, H. R. (2006). A functional endophenotype for sexual orientation in humans. NeuroImage, 33, 825–833. doi:10.1016/j.neuroimage.2006.08.002.
Pryor, J. L., Althof, S. E., Steidle, C., Rosen, R. C., Hellstrom, W. J. G., Shabsigh, R., … Kell, S. (2006). Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: An integrated analysis of two double-blind, randomised controlled trials. Lancet, 368, 929–937. doi:10.1016/S0140-6736(06)69373-2.
Redouté, J., Stoléru, S., Grégoire, M. C., Costes, N., Cinotti, L., Lavenne, F., … Pujol, J. F. (2000). Brain processing of visual sexual stimuli in human males. Human Brain Mapping, 11, 162–177. doi:10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A.
Reinecke, A., Schöps, D., & Hoyer, J. (2006). Sexuelle Dysfunktionen bei Patienten einer verhaltenstherapeutischen Hochschulambulanz: Häufigkeit, Erkennen, Behandlung [Sexual dysfunction in patients at a university outpatient department specialized for behavioral therapy: Quantity, detection, treatment]. Verhaltenstherapie, 16, 166–172.
Scheidegger, M., Walter, M., Lehmann, M., Metzger, C. D., Grimm, S., Boeker, H., … Seifritz, E. (2012). Ketamine decreases resting state functional connectivity in healthy subjects: Implications for antidepressant drug action. PLoS ONE, 7, e44799. doi:10.1371/journal.pone.0044799.
Seidman, S. N., & Roose, S. P. (2001). Sexual dysfunction and depression. Current Psychiatry Reports, 3, 202–208.
Simmons, A. N., Arce, E., Lovero, K. L., Stein, M. B., & Paulus, M. P. (2009). Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. Journal of Neuropsychopharmacology, 12, 1009–1020. doi:10.1017/S1461145709990149.
Song, X.-W., Dong, Z.-Y., Long, X.-Y., Li, S.-F., Zuo, X.-N., Zhu, C.-Z., … Zang, Y.-F. (2011). REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PloS ONE, 6, e25031. doi:10.1371/journal.pone.0025031.
Stoléru, S., Grégoire, M. C., Gérard, D., Decety, J., Lafarge, E., Cinotti, L., … Comar, D. (1999). Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of Sexual Behavior, 28, 1–21.
Stoléru, S., Redouté, J., Costes, N., Lavenne, F., Le Bars, D., Dechaud, H., … Pujol, J. F. (2003). Brain processing of visual sexual stimuli in men with hypoactive sexual desire disorder. Psychiatry Research, 124, 67–86. doi:10.1016/S0925-4927(03)00068-4.
Veer, I. M., Oei, N. Y. L., Spinhoven, P., van Buchem, M. A., Elzinga, B. M., & Rombouts, S. A. R. B. (2011). Beyond acute social stress: Increased functional connectivity between amygdala and cortical midline structures. NeuroImage, 57, 1534–1541. doi:10.1016/j.neuroimage.2011.05.074.
von dem Hagen, E. A. H., Passamonti, L., Nutland, S., Sambrook, J., & Calder, A. J. (2011). The serotonin transporter gene polymorphism and the effect of baseline on amygdala response to emotional faces. Neuropsychologia, 49, 674–680. doi:10.1016/j.neuropsychologia.2010.12.013.
Walter, M., Bermpohl, F., Mouras, H., Schiltz, K., Tempelmann, C., Rotte, M., … Northoff, G. (2008a). Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. NeuroImage, 40, 1482–1494. doi:10.1016/j.neuroimage.2008.01.040.
Walter, M., Henning, A., Grimm, S., Schulte, R. F., Beck, J., Dydak, U., … Northoff, G. (2009). The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Archives of General Psychiatry, 66, 478–486. doi:10.1001/archgenpsychiatry.2009.39.
Walter, M., Stadler, J., Tempelmann, C., Speck, O., & Northoff, G. (2008b). High resolution fMRI of subcortical regions during visual erotic stimulation at 7 T. Magnetic Resonance in Medicine, 21, 103–111. doi:10.1007/s10334-007-0103-1.
Walter, M., Witzel, J., Wiebking, C., Gubka, U., Rotte, M., Schiltz, K., … Northoff, G. (2007). Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biological Psychiatry, 62, 698–701. doi:10.1016/j.biopsych.2006.10.018.
Weissenbacher, A., Kasess, C., Gerstl, F., Lanzenberger, R., Moser, E., & Windischberger, C. (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage, 47, 1408–1416. doi:10.1016/j.neuroimage.2009.05.005.
Yan, C., & Zang, Y. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience, 4, 13. doi:10.3389/fnsys.2010.00013.
Yüksel, C., & Öngür, D. (2010). Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry, 68, 785–794. doi:10.1016/j.biopsych.2010.06.016.
Acknowledgments
Coraline D. Metzger and Martin Walter contributed equally to this publication. This work was supported by the German Research Foundation (DFG), Collaborative Research Centre 779 (SFB 779) (CDM, MW). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. We thank Prof. Dr. C. Hiemke and his staff at the University of Mainz (Germany), Department of Psychiatry and Psychotherapy, for measurements of drug blood serum levels. In addition, we thank Henrik Dobrowolny, Otto-von-Guericke University Magdeburg (Germany), Department of Psychiatry and Psychotherapy, for his skillful assistance and Marie José van Tol, Leibniz Institute for Neurobiology Magdeburg (Germany), for her help with manuscript correction.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Metzger, C.D., Walter, M., Graf, H. et al. SSRI-Related Modulation of Sexual Functioning is Predicted by Pre-treatment Resting State Functional Connectivity in Healthy Men. Arch Sex Behav 42, 935–947 (2013). https://doi.org/10.1007/s10508-013-0103-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-013-0103-3