Abstract
To better understand sexual orientation from an evolutionary perspective, we investigated whether, compared to heterosexual men, the fewer direct descendants of homosexual men could be counterbalanced by a larger number of other close biological relatives. We also investigated the extent to which three patterns generally studied separately––handedness, number of biological older brothers, and hair-whorl rotation pattern––correlated with each other, and for evidence of replication of previous findings on how each pattern related to sexual orientation. We surveyed at Gay Pride and general community festivals, analyzing data for 894 heterosexual men and 694 homosexual men, both groups predominantly (~80%) white/non-Hispanic. The Kinsey distribution of sexual orientation for men recruited from the general community festivals approximated previous population-based surveys. Compared to heterosexual men, homosexual men had both more relatives, especially paternal relatives, and more homosexual male relatives. We found that the familiality for male sexual orientation decreased with relatedness, i.e., when moving from first-degree to second-degree relatives. We also replicated the fraternal birth order effect. However, we found no significant correlations among handedness, hair whorl rotation pattern, and sexual orientation, and, contrary to some previous research, no evidence that male sexual orientation is transmitted predominantly through the maternal line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual orientation and attraction are important behavioral traits, exerting significant influence throughout life. Sexual attraction is a driving force for human mating, affecting several aspects of behavior and psychology, such as partner preference (Bailey, Gaulin, Agyei, & Gladue, 1994) or sexual arousal (Chivers, Rieger, Latty, & Bailey, 2004). Understanding what other traits correlate with sexual orientation can help advance knowledge of its development and expression.
Genetics: Familiality, Heritability, and Linkage
There is ample evidence from a number of family and twin studies that hereditary factors contribute to sexual orientation in men (for reviews, see Mustanski, Chivers, & Bailey, 2002; Sanders & Dawood, 2003). Family studies have shown homosexuality to be more common in biological relatives of homosexual men compared to relatives of heterosexual men, or compared to general population surveys (Bailey & Bell, 1993; Bailey & Pillard, 1991; Bailey et al., 1999; Hamer, Hu, Magnuson, Hu, & Pattatucci, 1993; Hershberger, 1997; Pillard & Weinrich, 1986); thus, it is familial or clusters in families. These studies find that brothers of homosexual probands (index subject in a family) are homosexual 7% to 22% of the time, with most findings around 10%. Differences among studies include ascertainment (advertisements, clinics, organizations, festivals), assessment method (interview, questionnaire), comparison group (heterosexual male probands versus general population survey rates), sample size, and whether the proband was the sole source of information about relatives (family history method). Those studies that corroborated the family history information from the homosexual proband directly with the proband’s relatives have found high rates of agreement (Bailey & Pillard, 1991; Hamer et al., 1993; Hershberger, 1997; Pillard & Weinrich, 1986). However, when Kirk et al. applied the family history method with general population (mostly heterosexual) probands using the co-twin as a corroborator in a twin study, lower accuracy was found (Kirk, Bailey, & Martin, 1999). These findings are consistent with homosexual probands more accurately reporting sexual orientation information for their family members compared to heterosexual probands, and are among the reasons some previous family studies have used general population survey rates as the comparison group. A previous finding of excess maternal transmission (relative to paternal) (Hamer et al., 1993) led to an initial focus on the X chromosome in linkage studies (see below) since a gene variant on the X chromosome influencing male sexual orientation would be one potential explanation of excess maternal transmission. The main finding from these family studies has been that male sexual orientation is significantly familial, though twin studies are required to assess if any of the familiality derives from hereditary contributions.
Twin studies consistently find higher concordances for sexual orientation for identical twins compared to same sex fraternal twins, though the exact concordances vary by study. Some studies individually report on male twins, while others combine information on male and female twins. Variation in twin study findings has been attributed to differences in ascertainment (opportunistic versus systematic), assessment (direct versus informant), and classification (e.g., assessment questions or cutoff used) of sexual orientation, methods of zygosity determination, and sample sizes. Some studies have assessed hundreds of pairs of twins (Alanko et al., 2009; Bailey, Dunne, & Martin, 2000; Hershberger, 1997; Kendler, Thornton, Gilman, & Kessler, 2000; Kirk, Bailey, Dunne, & Martin, 2000; Langstrom, Rahman, Carlstrom, & Lichtenstein, 2008), but many studies have assessed smaller numbers (Bailey & Pillard, 1991; Buhrich, Bailey, & Martin, 1991; Heston & Shields, 1968; Kallmann, 1952; King & McDonald, 1992; Whitam, Diamond, & Martin, 1993). Yet, while estimates vary, overall, genetic contributions have been estimated to account for up to about half of the variation in the trait of male sexual orientation based on larger studies using more recent methods (Alanko et al., 2009; Bailey et al., 2000; Kendler et al., 2000; Kirk et al., 2000; Langstrom et al., 2008), an estimate known as heritability. In any case, while the exact degree is unknown, the available data suggest substantial heritability of male sexual orientation.
Extending family and twin studies, linkage studies aim to map genomic regions linked to the studied trait in families. One research group found linkage to the Xq28 chromosome region in 40 pairs of homosexual brothers (Hamer et al., 1993) and replication thereof in another 33 such pairs (Hu et al., 1995), meaning this genomic region appeared to be shared more (via inheritance) than expected by chance in pairs of homosexual brothers. However, this finding was not replicated by other groups studying 54 such pairs from the U.S. (Sanders et al., 1998) or 52 such pairs from Canada (Rice, Anderson, Risch, & Ebers, 1999), or by the original research group in a full genome scan (i.e., including chromosome X and all the autosomes) with newly collected families (i.e., 73 additional pairs of homosexual brothers) (Mustanski et al., 2005). Moreover, while the original study indicated elevated maternal transmission of homosexuality (Hamer et al., 1993), another study has not found such a relationship (Bailey et al., 1999). In the latest linkage study, in the full set of 146 scanned pairs (40 + 33 = 73 previous pairs, plus 73 new pairs) of homosexual brothers, there was a suggestive linkage finding at chromosome 7q36 (Mustanski et al., 2005). However, this is expected to occur about once per genome scan just by chance (Lander & Kruglyak, 1995), and remains difficult to interpret further until additional samples are examined in a full genome scan.
The nature and mechanisms of genetic contributions to sexual orientation are currently unclear. Research findings seem most consistent with a complex model involving multiple contributing genes and important environmental (non-genetic) contributions, a setting where larger sample sizes are important to enable more reliable detection and replication of linkage (Altmuller, Palmer, Fischer, Scherb, & Wjst, 2001).
Fecundity and Family Size
While there appear to be significant genetic contributions to sexual orientation in men, homosexual men have fewer children than heterosexual men. This raises the Darwinian paradox (evolutionary conundrum) of male homosexuality in humans (Wilson, 1978). If male sexual orientation has a significant genetic component, and homosexual men reproduce less than heterosexual men and thereby have reduced reproductive fitness, then why do homosexual men constitute a significant minority of the male population (Laumann, Gagnon, Michael, & Michaels, 1994), persisting over time and cultures (Bell & Weinberg, 1978; Whitam, 1983)? One way to resolve the paradox is via balancing selection, where increased fecundity of close biological relatives of homosexual men offsets the homosexual men’s own reduced fecundity.
Several studies have produced findings consistent with the idea of enhanced reproduction among biological relatives of homosexual men. Research on a sample of 98 homosexual and 100 heterosexual Italian men found that maternal line relative classes of homosexual men (i.e., relatives sharing or potentially sharing their X chromosome with the proband, in this case referring to mothers, maternal aunts, and sons and daughters of maternal grandparents) had more offspring compared to those of heterosexual men (Camperio-Ciani, Corna, & Capiluppi, 2004). This same group recently replicated this finding in a new sample of 152 homosexual and 98 heterosexual Italian men for the same relative classes (Iemmola & Camperio Ciani, 2008). A contrasting study of 301 homosexual and 404 heterosexual white men from England found that the number of relatives in different categories (i.e., uncles, aunts, cousins, siblings, nephews, and nieces) was significantly larger for homosexual than for heterosexual men on the paternal side, but not on the maternal side (though maternal total had a trend level finding at p = .058) (King et al., 2005).
An international (primarily European ancestry) study of a BBC internet sample of 87,798 men found that homosexual and bisexual men had more siblings than did heterosexual men (Blanchard & Lippa, 2007), a finding that is also consistent with balancing selection. This was also the case in a non-European sample where more siblings were found for 83 homosexual versus 114 heterosexual Samoan men (Vasey & VanderLaan, 2007). Another study from England of 147 homosexual (127 white and 20 non-white) and 155 heterosexual (102 white and 53 non-white) men found elevated maternal line fecundity for homosexuals for one relative class (aunts) in whites (Rahman et al., 2008). For non-whites, the study found elevated paternal line fecundity for heterosexuals for all tested classes (aunts, uncles, and grandparents) and for two (mothers and grandparents) of the four tested maternal line classes. These different white versus non-white patterns of fecundity suggest that ancestry may be important to take into account in these analyses. However, Rahman et al. noted the relatively small non-white proband sample size.
In general, the reviewed studies point to enhanced fecundity among biological relatives of homosexual men. However, the specific results vary across studies, in particular with regards to whether this arises from the maternal line, the paternal line, or both. Proposed reasons for these divergences include different probabilities of different classes of relatives sharing chromosome X with the proband (see Table 2 in Camperio-Ciani et al., 2004), differences in sample size, and different sampling methods (Iemmola & Camperio Ciani, 2008; Rahman et al., 2008).
In aggregate, the aforementioned studies suggest the possibility of one or more androphilic genetic variants, i.e., a genetic variant increasing sexual attraction towards men. When expressed in men, it could increase the likelihood they would be homosexual, but when expressed in women (i.e., female relatives of these men), it could increase the frequency of mating with men with a concomitant increase in the number of offspring––increased fecundity. Thus, such a gene would have more than one phenotypic effect, called pleiotropy, in this case depending on the sex of the person carrying the gene. In this balancing selection scenario, an androphilic genetic variant would be maintained in the population due to the increased fecundity of female relatives of homosexual men balancing the decreased direct reproductive fitness of homosexual men (Miller, 2000). We note that while such a gene might reside on the X chromosome, it could also reside on an autosome. We also note that while an androphilic genetic variant as described above could account for increases in maternal line fecundity, other explanations would be needed for increased paternal line fecundity or a general increase irrespective of line.
Birth Order
Besides heredity, another significant biological influence on male sexual orientation is the well established fraternal birth order effect––later born brothers from the same mother are more likely to be homosexual, with each older brother increasing a man’s chance of being homosexual by about a third (Blanchard, 2008; Blanchard & Bogaert, 1996; Jones & Blanchard, 1998). Only maternal biological older brothers contribute to this effect. Sisters, younger brothers, or older brothers who are not born from the same biological mother do not make a difference (Blanchard, 1997; Bogaert, 2006). Estimates for the proportion of homosexual men owing their sexual orientation to the fraternal birth order effect run from 15% to 29% (Blanchard, 2004; Blanchard & Bogaert, 2004; Cantor, Blanchard, Paterson, & Bogaert, 2002); these results are for the United States and Canada, and in cultures with more offspring per family these values could be higher.
A hypothesized mechanism for the fraternal birth order effect is the maternal immune hypothesis in which the mother progressively builds up an immunological response to male specific antigen(s), such as proteins encoded specifically on the Y chromosome (Blanchard & Klassen, 1997). The mother is exposed to these antigens, particularly during the mixing of her blood with the blood of her son during delivery, with each subsequent pregnancy increasing this exposure and hence the strength of the maternal immune response (e.g., more maternal antibodies). Later born sons are increasingly exposed to these antibodies or other aspects of the maternal immune response. According to this hypothesis, this may alter the development of brain region(s) relevant to the development of sexual orientation, thereby increasing the likelihood of being homosexual.
Handedness and Hair Whorl
Most studies have found that homosexual men are more likely than heterosexual men to be non-right handed (NRH). A number of studies, though, did not detect this pattern, leading to heterogeneity among studies (for reviews, see Blanchard, 2008; Lalumiere, Blanchard, & Zucker, 2000). Handedness studies use a variety of ways to assess hand preference, such as one task in isolation (e.g., writing, hammering, etc.) versus several scales of general handedness––especially the Edinburgh Inventory (Oldfield, 1971) or the Annett questionnaire (Annett, 1970)––and generally classify handedness as right handed (RH) versus NRH (Blanchard, Cantor, Bogaert, Breedlove, & Ellis, 2006; Blanchard & Lippa, 2007; Lalumiere et al., 2000). Recently, the fraternal birth order effect has been found to be substantial only in RH homosexual men (Blanchard, 2008; Blanchard et al., 2006; Blanchard & Lippa, 2007). In light of these findings, it is important to assay for both handedness and birth order.
A different line of research examined the relationship of handedness with hair whorl rotation pattern (Klar, 2003). By surreptitiously inspecting people’s heads at public venues, Klar found that in the mostly RH general public, the counter-clockwise (CCW) hair whorl rotation pattern prevalence was 8.4%. However, in a directly assessed sample of NRH individuals, the prevalence of CCW hair whorl rotation pattern was 44.9%. Furthermore, out of an independently assessed sample of 23 CCW individuals, 12 were RH and 11 were NRH. Klar proposed that a single gene causes this pattern by affecting brain and body symmetry development, and that the handedness and hair whorl rotation pattern of people with two recessive r (for random) alleles are determined randomly and independently.
Subsequently, in a study of men in Wales (N = 173), a handedness and hair whorl association was detected with a higher rate of CCW in the NRH group (54% of 48 NRH) than the RH group (13% of 125 RH) (Beaton & Mellor, 2007). However, a substantially larger study, in a predominantly (90%) male German military sample (N = 1,212), found no significant association between handedness and hair whorl (Jansen et al., 2007). Furthermore, Jansen et al. pointed out two older reviews that also found no association between handedness and hair whorl (Collins, 1977; Newman, 1934). Another study completed after these reviews was also negative (Annett, 1985). Discrepancies between studies may be in large part due to direct versus surreptitious examination of the hair whorl, and assessed versus inferred handedness, issues addressed in the current study.
Regarding sexual orientation and hair whorl patterns, a sample of 272 presumably mostly homosexual men (heads surreptitiously observed at several visits to a predominantly gay beach) was compared to a sample of 190 presumably mostly heterosexual males (heads surreptitiously observed at multiple public venues such as shopping malls). Casual (at a distance) head inspection showed 29.8% CCW whorls in the former and 8.2% in the latter, representing a 3.6-fold excess of CCW hair whorl rotation pattern in presumably homosexual men compared to unselected (mostly heterosexual) men (Klar, 2004). Klar then speculated that the sexual orientation of men possessing the r/r genotype is randomly determined like handedness and hair whorl, and men possessing at least one dominant allele (R/R or R/r) for the gene would be RH, have a CW whorl, and be heterosexual, but men possessing the r/r genotype would randomly express the above phenotypes (handedness, hair whorl, and sexual orientation).
A new study of homosexual (N = 100) and heterosexual (N = 100) men recruited via targeted sampling (90% RH and 79% white) in London revealed no significant differences in hair whorl rotation (overall CCW 16%, assessed blindly with respect to sexual orientation and handedness, via digital photographs) by sexual orientation, and no relationship between hair whorl rotation and handedness (Rahman, Clarke, & Morera, 2009). We are aware of no other publications as of yet attempting to replicate the reported association of hair whorl pattern with sexual orientation, though other studies are underway; however, due to the media prominence of the initial report despite that report’s methodological shortcomings described above, we included hair whorl examination in our study in order to rigorously evaluate the previous claim.
The Present Study
In our study, we investigated several factors that have been related to sexual orientation. While primarily focusing on family size, we also included assessment of handedness, hair whorl rotation pattern, and variables related to family composition (e.g., number of older brothers, familiality). To our knowledge, no previous study has included all of these factors on the same sample. We hypothesized homosexual men to have larger family sizes than heterosexual men, through one or both lines (maternal or paternal). We sought to further replicate the fraternal birth order effect, at least for RH men, and the familiality of sexual orientation. We also hypothesized that NRH men have a higher incidence of CCW hair whorl than RH men, and homosexual men have a higher incidence of CCW hair whorl rotation pattern than heterosexual men.
Method
Participants
We recruited men (N = 1,694), 18 years of age or older, by staffing a research booth at eight general community festivals (N = 978; mostly heterosexual men) in the Chicago metropolitan area and 16 Gay Pride (or other gay themed) festivals (N = 716; mostly homosexual men) throughout the United States (Chicago, Dallas, Durham, Fort Lauderdale, Palm Springs, Philadelphia, Phoenix, San Diego, and San Francisco) and Canada (Ottawa and Vancouver) in 2006 and 2007. Both types of festivals were held in parks or cordoned-off streets with food, music, multiple vendor booths, and many attendees. We excluded men (N = 12) not answering one or more of the sexual attraction, fantasy, or identity questions, and the men (N = 94, 77.7% of whom were recruited at Gay Pride festivals) falling in the bisexual area, leaving us with a sample of heterosexual (N = 894) and homosexual (N = 694) men for further analyses (total N = 1,588). The homosexual and heterosexual samples were similar to each other demographically on distributions of ethnicity and race (U.S. census categories), education, employment status, current occupation, highest level of education achieved, and best approximation of current marital status (including “common law” marriages, civil union, and gay marriage within the married category) (Table 1). However, the homosexual probands were significantly older (M = 40.4 years) than the heterosexual sample (M = 34.2 years). The main reason was that one of the Gay Pride festival locations was Palm Springs, California (accounting for 18.3% of such festival participants, with M = 46.4 years), which is known to have an older population due in large part to being a famous retirement destination. This aspect was also reflected in the proportion of retirees––higher in the homosexual sample (Table 1), again largely due to the Palm Springs location. In any case, due to the age difference, we statistically controlled for proband age in the family sizes analyses. Our total analyzed samples of heterosexual and homosexual men were primarily white/non-Hispanic, and showed a non-significant trend for the heterosexual group including more white/non-Hispanic men (727/894 = 81%, and 540/694 = 78%, respectively; χ2 = 2.98, df = 1, p = .08 for group difference). We also queried for seven European ancestries for each proband’s parents, and found them to be very similarly distributed in the homosexual versus heterosexual proband groups. We found these similarities despite the homosexual sample deriving largely from a wider geographical region (Gay Pride festivals) than the heterosexual sample, which derived largely from the Chicago metropolitan area (general community festivals). Listed in overall rank order for the white/non-Hispanic men, the endorsed parental ancestries were Anglo-Saxon (37%), West European (31%), East European/Slavic (10%), Northern European (9%), Ashkenazi Jew (6%), Mediterranean (4%), and Russian (3%). Our study was IRB (Institutional Review Board) approved at NorthShore University HealthSystem and Northwestern University with a waiver of written informed consent, since it was an anonymous study.
Measures
We administered an approximately 5 min long, anonymous questionnaire with questions on sexual orientation, handedness, and demographics. Additional measures included a pedigree drawn by a researcher at the festival with input from the participant, and a direct observation of the hair whorl by the researcher. We verified a high accuracy rate of data entry and cleaning via double-entering entire questionnaires (including pedigree coding) for a randomly chosen 5% of the entire sample, where we found that the initial entries were correct 99.3% of the time.
Sexual orientation (attraction, fantasy, behavior, and identity) was assessed using the Kinsey scale (Kinsey, Pomeroy, & Martin, 1948), i.e., the 7-point scale ranging from 0 for exclusively heterosexual to six for exclusively homosexual. Responses to the four questions strongly correlated with each other (r ≥ .96 for each pair of measures), similar to previously reported correlations (e.g., r = .92 for attraction and fantasy in a twin registry sample in (Bailey et al., 2000)). The correlations of the three other questions (attraction, fantasy, and identity) with the behavior question were slightly lower (r = .96) than the correlations of these three measures with each other (r = .98), and a small number (N = 20) of the young participants had not had any sexual activity yet. Thus, we classified orientation as heterosexual if the mean score of the three items (attraction, fantasy, and identity) was ≤1, and as homosexual when ≥5. The distributions of these mean Kinsey score bins are shown in Fig. 1 separately for the participants recruited at general community festivals and Gay Pride festivals. The bimodal distribution pattern (dipping close to 0% in the bisexual ranges) for our general community festival sample (Fig. 1) was very similar to previously reported patterns in the general population (Laumann et al., 1994) and twin registry samples (Bailey et al., 2000). However, our general community festival sample included 3% to 4% more homosexual men probably because two of the eight general community festivals were in close proximity to Chicago’s primary homosexual male neighborhoods (Gates & Ost, 2004). These two festivals had 2.4 times as many homosexual participants as the other general community festivals and accounted for 61.4% of the homosexual participants from general community festivals. Not surprisingly, most (89.3%) of the general community festival sample was heterosexual, and most (86.4%) of the Gay Pride festival sample was homosexual (Fig. 1). We excluded the 5.5% of the entire sample falling in the bisexual range of mean Kinsey scores from further analyses to enable comparisons with previous studies comparing only homosexual versus heterosexual samples.
Mean attraction, fantasy, and identity: Distribution of bins for the mean of the Kinsey scale items of sexual attraction, fantasy, and identity for 1,694 men recruited from general community festivals (N = 978) and Gay Pride festivals (N = 716), as their percentage of their festival category. Combining men from both types of festivals yielded a sample of 53% heterosexual (0 ≤ M ≤ 1), 41% homosexual (5 ≤ M ≤ 6), and 6% bisexual (1 < M < 5) men, with the latter group excluded from further analyses
We classified participants as RH if they used the right hand for at least 70% of a list of 10 common tasks (writing, throwing a ball, cutting with scissors, using a knife, using a screwdriver, using a hammer, combing hair, brushing teeth, using chopsticks, and removing a splinter from the foot) per self report, and otherwise classified participants as NRH. These 10 handedness items were similar to items from the Edinburgh Inventory (Oldfield, 1971), which has been shown to produce familial genetic handedness data (e.g., Longstreth, 1980), and were specifically from a relatively standard version of the Edinburgh Inventory previously employed (Geschwind, Miller, DeCarli, & Carmelli, 2002). The pedigree charting interview elicited the number and sexes of parents, parents’ siblings, grandparents, and siblings (including half siblings), as well the number of offspring from each of these categories. The pedigree also yielded the birth order and sex of siblings, and the sexual orientation (as known by the participant, with options of homosexual, bisexual, heterosexual, and unknown) of their biological relatives. For a subset (N = 70), two raters independently scored the hair whorls (single whorl, CW versus CCW) and we found a high level of inter-rater reliability via the kappa test (κ = .97).
Procedure
At the festivals, we used banners inviting research participation and asked men passing by our booth to consider volunteering for the anonymous questionnaire. Interested participants were told what the study entailed and were asked to complete the questionnaire. We then drew and annotated the pedigree with the participant’s input. A small number of participants had totally missing pedigrees due to being adopted or to declining that portion of the study, but these were equally distributed in the heterosexual (2.3%) and homosexual (2.4%) samples. Some participants had half-missing pedigrees, i.e., only paternal or maternal side missing, commonly due to a participant having no knowledge of one of his parents or in other cases due to a parent having been adopted. This proportion did not vary between the heterosexual and homosexual samples (1.7% in each); in both samples, there were more and equivalent rates of paternal (1.4%) than maternal (0.3%) sides missing, consistent with higher rates of uncertain paternity than uncertain maternity. Finally, we scored the hair whorl by direct examination. Only single hair whorls that were clearly CW or CCW were scored and analyzed, which included 82% of the participants; 18% of the men did not have a hair whorl scored due to reasons of too little hair (e.g., bald), too much hair (obscured), more than one whorl, or declining to complete that portion of the study.
To compare family sizes of homosexual and heterosexual men, we generally used logistic regression analyses due to its appropriateness and to enable comparison to a previous study (King et al., 2005). For all these analyses, we included age as a predicting covariable to control for the older age of homosexual probands. In other words, for these analyses, both a certain type of relative (e.g., “older brothers”) and age were used simultaneously to predict sexual orientation. The calculated chi-square values indicated whether the difference between a model without a given predictor and with a given predictor was significant. For additional birth order analyses, we used the z-approximation to the binomial test, with the expected sibling sex ratio of 0.515 as the test value. For the majority of the other analyses, including analyses of familiality, handedness, and hair whorl rotation pattern, χ2 tests were performed.
Results
Familiality
As seen in Table 2, the rates of homosexuality in male relatives were consistently higher for homosexual probands than for heterosexual probands. We found first-degree male relatives (sons, fathers, and brothers) of homosexual probands to have higher rates (106/1,740 = 6.1%) of homosexuality than their second-degree relatives (nephews, uncles, and grandfathers) (98/4,867 = 2.0%) (χ2 = 71.25, df = 1, p < .0001). Interestingly, the only third-degree male relatives (male first cousins) of homosexual probands, for whom we had data, showed an intermediate rate of homosexuality (136/3,094 = 4.5%)––lower than the first-degree male relatives (χ2 = 6.15, df = 1, p = .019), but higher than the second-degree relatives (χ2 = 39.13, df = 1, p < .0001). The enrichment for male homosexual relatives of male homosexual probands (versus male heterosexual probands) was higher for first-degree relatives (6.1%/1.3% = 4.7 fold) than for the other two classes, which were similar to each other––second-degree relatives (2.0%/0.8% = 2.5 fold) and third-degree relatives (4.5%/1.7% = 2.6 fold). The most distant and eldest generation of male relatives of the proband we assayed (grandfathers) had the lowest rate of reported homosexuality (6/1,329 = 0.5% for homosexual probands; 0/1,728 = 0% for heterosexual probands). By comparing the rate of homosexual brothers of homosexual probands to those of heterosexual probands (9.0% vs. 2.3%), we generated an estimate of λsibs (the relative rate of homosexuality for a male sibling of a homosexual male, compared to the general population, using the rate in brothers of heterosexual probands as a proxy for the general population) in our sample of 3.9.
We found that sisters of homosexual probands were more likely to be homosexual (65/917 = 7.1%) than sisters of heterosexual probands (6/902 = 0.7%) (χ2 = 50.02, df = 1, p < .0001). The latter proportion did not significantly differ (Fisher’s exact test, p = .2) from prevalence estimates of female homosexuality in the general population (e.g., 0.3% of women with only homosexual attraction in Table 8.3B in Laumann et al., 1994). Furthermore, sisters of homosexual probands were about as likely to be homosexual (65/917 = 7.1%) as brothers of homosexual probands (90/999 = 9.0%) (χ2 = 2.37, df = 1, p = .12).
Of relevance to the possibility of maternal inheritance being more prominent than paternal, we found no significant difference (χ2 = 0.97, df = 1, p = .3) between the rate (31/1,120 = 2.8%) of homosexuality in paternal uncles (who do not share their X chromosome with the proband) of homosexual probands versus the rate (38/1,087 = 3.5%) of homosexuality in maternal uncles (who might share their X chromosome with the proband). Another analysis followed from the idea that if maternal inheritance is much more prevalent relative to paternal inheritance, then father to son transmission is expected to be rare. However, we found that this was not the case: homosexual probands reported having homosexual fathers 1.6% (11 out of 675) of the time and homosexual sons 7.6% (5 out of 66) of the time, though the latter group was a less confident estimate due to the sample size. A further prediction of increased maternal inheritance is that male first cousins descended from maternal aunts of homosexual probands would have a higher rate of homosexuality than the other male first cousins (due to the latter group not sharing the X chromosome with the proband). However, we found no significant difference between the rates of homosexuality for these two groups: first cousins via maternal aunts being 42/852 = 4.9%, and for other first cousins being 94/2,197 = 4.3% (χ2 = 0.61, df = 1, p = .4). Thus, in general, our results did not suggest that maternal inheritance was more prominent than paternal inheritance for male sexual orientation.
Logistic regression analyses controlling for the total number of male relatives on the maternal and paternal line, respectively, showed that, compared to heterosexual male probands, homosexual male probands had more homosexual male relatives both on the maternal line (χ2 = 38.36, p < .0001, OR = 2.53; 95% CI = 1.90–3.40), and the paternal line (χ2 = 53.37, p < .0001, OR = 3.24; 95% CI = 2.38–4.49). An additional within-subject comparison suggested that the increased number of male homosexual relatives of homosexual probands was not significantly different between the maternal and paternal line (χ2 = 1.39, p = .24, OR = 0.87, 95% CI = 0.69–1.09). Thus, homosexual male probands had more homosexual male relatives than heterosexual male probands, and they were distributed approximately equally on both the maternal and paternal lines. These results also failed to support the importance of the maternal over the paternal line for male sexual orientation.
Family Size
Table 3 shows the mean sizes of various relative classes for homosexual versus heterosexual male probands. After controlling for age, we found that homosexual men had more relatives than heterosexual men in several relative classes––significantly so for paternal aunts and uncles, siblings (younger sisters, older brothers, and older sisters), and nieces and nephews, and at a trend level for paternal complete generation and paternal total (Table 3). Homosexual male probands had more nieces and nephews (M = 3.7) than heterosexual male probands (M = 2.1) to a substantial degree (χ2 = 13.06, df = 1, p = .0003), with the nieces and nephews (M = 3.7) of our homosexual male probands being just as likely to be offspring of their brothers (M = 1.9) as of their sisters (M = 1.8), and similarly the nieces and nephews (M = 2.1) of our heterosexual male probands being just as likely to be offspring of their brothers (M = 1.0) as of their sisters (M = 1.1). As expected, homosexual men had fewer offspring (M = 0.17) than heterosexual men (M = 0.60) (χ2 = 129.20, df = 1, p < .0001). We note that the overall results changed little when we controlled for race/ethnicity (white/non-Hispanic versus the remaining groups together). When controlling for race/ethnicity, all previously significant family size differences remained significant, but the trend level results for paternal complete generation and paternal total disappeared. We found no significant interactions between race/ethnicity and number of relatives in the various classes for homosexual versus heterosexual male probands, suggesting that the association between sexual orientation and family size was similar for different races/ethnicities. In general, the results were very similar to those without controlling for race/ethnicity (Table 3).
Birth Order
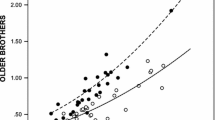
Homosexual male probands had more older brothers than did their heterosexual counterparts (p < .00064), after controlling for proband age (Table 3 and Fig. 2). The relationship between number of older brothers and proband sexual orientation can be seen in Fig. 3: the likelihood of a homosexual proband rises as the number of older brothers increases. In addition, as can be derived from the OR in Table 3 (and by employing a previously used methodology; Blanchard & Bogaert, 1996; Blanchard, Zucker, Siegelman, Dickey, & Klassen, 1998), each additional older brother increased the odds of male homosexuality by 20%.
Mean sibling number by sibling and proband type: Mean sibling number with 95% confidence interval, by sibling type, for homosexual (N = 677) versus heterosexual (N = 873) probands with family tree data. Homosexual probands had more older brothers, older sisters, and younger sisters than heterosexual probands, controlling for proband age (confer Table 3). Abbreviations: OB = older brothers; OS = older sisters; YB = younger brothers; YS = younger sisters; Ho = homosexual male probands; and He = heterosexual male probands
Number of older brothers by proband sexual orientation: Relationship between number of older brothers for homosexual (N = 677) versus heterosexual (N = 873) male probands, and percentage of probands with family tree data who are homosexual. The parenthetical numbers are the total of homosexual plus heterosexual probands with that number (0, 1, 2, 3, or ≥4) of older brothers. If each category had an equal likelihood of the proband being homosexual, one would expect a flat curve with each category consisting of approximately 43.7% (677/1,550) homosexual probands
In addition, our sample had a sibling sex ratio, for both homosexual and heterosexual probands, very similar to the proportion of male live births in the general white population of 0.515 (106 males per 100 females) (James, 1987). Our homosexual probands had a total of 980 brothers and 911 sisters for a sibling sex ratio of 0.518, and our heterosexual probands had a total of 994 brothers and 898 sisters for a sibling sex ratio of 0.525; neither ratio was significantly different from the expected 0.515 or each other (two-tailed binomial test). This suggests that our sample was comparable to the general population regarding proportions of male and female siblings. When separated into older versus younger siblings (Table 4), we find excess older brothers relative to older sisters (p = .040) and a trend for a deficit of younger brothers compared to younger sisters (p = .083) for the homosexual probands, but no differences for the heterosexual probands.
Table 3 and Fig. 2 also illustrate that the homosexual male probands had more older sisters and more younger sisters than the heterosexual male probands. This is noteworthy because the previous literature suggests, in general, an “older brother effect,” but not an “older sister effect” (e.g., Blanchard, 2008). In our sample, the number of older sisters was correlated with number of older brothers (p < .0001, r = .33). We therefore investigated whether this “older sister effect” was its own, independent effect and not merely driven by its correlation with older brothers. We conducted another logistic regression analysis to test whether the association of proband sexual orientation with the number of older sisters held true when controlling for older brothers (in addition to age). That is, we predicted the probands’ sexual orientation simultaneously by older brothers, older sisters, and age. The number of older sisters was no longer significantly higher for homosexual than heterosexual probands, though there was a trend (χ2 = 3.09, p = .08, OR = 1.11, 95% CI = 0.98–1.25). Thus, the association of number of older sisters with proband sexual orientation seemed partially explained by the finding that men with older brothers were also likely to have older sisters. The effect of older brothers remained significant in this model (χ2 = 7.51, p = .006, OR = 1.16, 95% CI = 1.04–1.30). Similar analyses suggested that the effects of younger sisters remained significant whether controlling for the effect of older brothers (χ2 = 10.55, p = .001, OR = 1.23, 95% CI = 1.08–1.39) or of younger brothers (χ2 = 7.77, p = .005, OR = 1.19, 95% CI = 1.05–1.35).
Homosexual probands tend to come from larger sibships than heterosexual probands due to both more older brothers (since some proportion of homosexual probands with multiple older brothers are homosexual due to the fraternal birth order effect) and a secondary excess of older sisters (Blanchard & Bogaert, 1996). Larger sibships would predict larger numbers of their offspring (i.e., nieces and nephews) as well. It has also been noted that family size ideals depend in large part on the size of the family of origin (Clay & Zuiches, 1980). Hence, we were concerned that a combination of a statistical artifact of the fraternal birth order effect leading to larger sibship sizes (and concomitant larger numbers of nieces and nephews) for homosexual versus heterosexual probands with some cultural transmission of ideal family size might be the sole contributors to the increased family sizes we found for homosexual probands for some relative classes. To examine such a possibility, we re-ran the family size analyses controlling for older brothers, and found that the larger family sizes previously found for homosexual versus heterosexual probands (namely, paternal aunts and uncles, siblings, and nieces and nephews) remained significant with the same pattern of results. However, there was some correlation between the number of older brothers and the total number of maternal and paternal relatives (after removing the older brothers from the latter group) (r = .11, p < .0001). Therefore, the fraternal birth order effect explains part, but not all, of the increased family size findings.
Since having more older brothers increases the chance of being homosexual in men due to the fraternal birth order effect, a smaller proportion of the variance in such instances remains to be explained by hereditary and/or other contributors to variation in sexual orientation. Thus, we speculated that homosexual probands with no older brothers might have a larger number of other gay male relatives (part of the familiality representing heritable contributions to sexual orientation). We generated a homosexual familial factor for each proband with the numerator being the number of gay male relatives adjusted for the degree of relationship, i.e., numerator = (total number of first degree gay male relatives × 0.5) + (total number of second degree gay male relatives × 0.25) + (assayed number of third degree gay male relatives × 0.125). The denominator was the same as the numerator, except for including all male relatives, regardless of sexual orientation. However, we found (controlling for age) that both the number of older brothers and the homosexual familial factor predicted homosexuality (χ2 = 10.49, df = 1, p = .0012, and χ2 = 48.27, df = 1, p < .0001, respectively) with the latter being more important (predictive), but there was no interaction, both when looking at the entire sample (χ2 = 0.41, df = 1, p = .52) and when restricting the analysis to the probands with homosexual brothers (χ2 = 0.13, df = 1, p = .72).
Handedness
Overall, the prevalence of NRH men among homosexual (96/690 = 13.9%) and heterosexual (140/887 = 15.8%) probands did not differ significantly (χ2 = 1.07, df = 1, p = .30). This was also the case when just considering the writing hand where the prevalence of NRH (left or both) writing hand for our male homosexual (88/690 = 12.8%) versus heterosexual (125/885 = 14.1%) probands was no different (χ2 = 0.62, df = 1, p = .43). We note that there was a high correlation (r = .81) between the handedness classification we used (cutoff at 70% of a list of 10 common tasks) with that achieved solely considering the writing hand. There was no correlation of handedness (either classification) with age, overall or within the homosexual or heterosexual groups, and controlling for age did not result in a significant association of handedness (70% cutoff, or writing hand) and sexual orientation (χ2 = 0.78, df = 1, p = .38 and χ2 = 0.72, df = 1, p = .40, respectively).
In the present study, an association of homosexuality with an increased number of older brothers was found both for RH (χ2 = 6.84, p = .009, OR = 1.16, 95% CI = 1.04–1.30) and NRH individuals (χ2 = 5.21, p = .02, OR = 1.40, 95% CI = 1.06–1.91). We conducted another logistic regression that tested for the difference in this effect by computing the interaction between number of older brothers and handedness. There was no difference in this effect between RH and NRH individuals (χ2 = 1.34, p = .25, OR = 1.09, 95% CI = 0.94–1.28). Therefore, the older brother effect was generally significant, regardless of handedness in our sample.
Hair Whorl
The prevalence of a CCW hair whorl in homosexual men (106/537 = 19.7%) and heterosexual men (132/769 = 17.2%) did not differ significantly (χ2 = 1.41, df = 1, p = .24). Also, the prevalence of a CCW hair whorl among RH men (218/1,168 = 18.7%) and NRH men (33/206 = 16.0%) did not differ (χ2 = 0.82, df = 1, p = .37) for the aggregated sample (all sexual orientations combined). The analyses done separately by sexual orientation were also negative. For the homosexual men, the prevalence of a CCW hair whorl among RH men (91/461 = 19.7%) and NRH men (15/73 = 20.5%) did not differ (χ2 = 0.03, df = 1, p = .86); and for the heterosexual men, the prevalence of a CCW hair whorl among RH men (115/650 = 17.7%) and NRH men (17/116 = 14.7%) did not differ (χ2 = 0.64, df = 1, p = .42).
Discussion
Familiality
Consistent with previous family studies (Bailey & Bell, 1993; Bailey & Pillard, 1991; Bailey et al., 1999; Hamer et al., 1993; Pillard & Weinrich, 1986), we found that male sexual orientation was familial. Further, we found that the familiality decreased as the degree of relatedness decreased, i.e., first- versus second-degree relatives. It is more difficult to know why the third-degree relatives appeared intermediate, though we only have information on one type of third-degree relative (first cousins). The rate of homosexual brothers of homosexual probands in our sample (90/999 = 9.0%) was very similar to that previously found (25/259 = 9.7%) for 167 homosexual festival probands (Bailey et al., 1999).
Generational (age-cohort) effects in terms of knowledge of sexual orientation in other family members have been noted previously with younger cohorts coming out at earlier ages (Floyd & Bakeman, 2006; Grov, Bimbi, Nanin, & Parsons, 2006)––consistent with our finding that the most distant and eldest generation of relatives of the proband we assayed (grandfathers) had the lowest rate of reported homosexuality, and this was lower than prevalence estimates of male homosexuality in the general population (e.g., 2.4% of men with only homosexual attraction as shown in Table 8.3B in Laumann et al., 1994). We note that for the relative class for which one would expect the proband to have the most complete knowledge in terms of closeness of relationship and being in the same age cohort (i.e., brothers), the heterosexual probands reported a rate of homosexuality of 2.3% (23/1,005), which is indistinguishable from the aforementioned general population prevalence of 2.4% (Laumann et al., 1994). This arguably lends support to having the most confidence for these closer relationships, in terms of proband’s knowledge of sexual orientation in other family members. Furthermore, the only relatives of male homosexual probands in the same generation as the first cousins whom we assayed were the brothers, who had higher rates of male homosexuality than the first cousins (χ2 = 29.53, df = 1, p < .0001). This result also supports the hypothesis that closeness to relatives may affect reported rates of homosexuality (since probands would be closer to their brothers than to their cousins). It also, however, supports the hypothesis that homosexuality is heritable and that genetically closer individuals are more alike in their sexual orientation. Our calculated λsibs of 3.9, was consistent with previous estimates of λs of 3.0 and 4.0 (from two different samples) (Bailey et al., 1999). To put this into perspective within the field of complex genetics, an estimate of λsibs of 4 or less is often seen in about a third of common and complex genetic conditions (Altmuller et al., 2001) though, of course, we are discussing a trait, male sexual orientation, here.
Our finding that sisters of homosexual male probands had a higher rate of homosexuality than sisters of heterosexual male probands (7.1% vs. 0.7%) shows at least partial familial clustering of male and female homosexuality. This has been found in some studies (Bailey & Bell, 1993; Bailey et al., 1999; Pattatucci & Hamer, 1995) though not all (Bailey & Benishay, 1993; Bailey, Pillard, Neale, & Agyei, 1993). Thus, there remains some uncertainty as to the degree to which male sexual orientation and female sexual orientation are co-familial, and corresponding uncertainty for the utility of studying the traits separately or concurrently.
None of our findings supported the hypothesis that maternal inheritance of male sexual orientation was a more significant factor than paternal inheritance: maternal and paternal inheritance appeared equally important in the present study. This result was consistent with some (Bailey et al., 1999; Rice et al., 1999), but not all (Hamer et al., 1993) previous research. The lack of elevated “transmission” of male sexual orientation in the maternal line in the current study supports the notion that hereditary contributions to male sexual orientation do not predominantly reside on the X chromosome, but instead primarily reside elsewhere, that is, on the autosomes (i.e., chromosomes 1 through 22).
Family Size
Our overall pattern of increased family sizes for homosexual probands was more similar to that found in larger English samples (King et al., 2005; Rahman et al., 2008), where the effect was more pronounced on the paternal side overall, than in the smaller Italian samples that found more of an effect on the maternal side (Camperio-Ciani et al., 2004; Iemmola & Camperio Ciani, 2008). Since we found no evidence of maternal inheritance being more prominent than paternal inheritance in the family history portion of the study, we analyzed our data with less of a focus on likelihood of sharing of the X chromosome than some previous studies (Camperio-Ciani et al., 2004; Iemmola & Camperio Ciani, 2008; Rahman et al., 2008). We also examined one of the most consistent findings from the Italian studies (Camperio-Ciani et al., 2004; Iemmola & Camperio Ciani, 2008), namely whether maternal aunts (who on average share the X chromosome with the male probands 75% of the time) have higher mean fecundity for homosexual probands (versus heterosexual ones) than paternal aunts who do not share their X chromosome with the male probands; however, we did not find such a difference. In sum, this is another way in which we did not find evidence to consider chromosome X to be more important than the autosomes.
We did notice that, for all relative classes (except paternal cousins), our U.S. sample had somewhat larger mean family sizes irrespective of sexual orientation compared to an English sample (i.e., our Table 3 versus Table 2 in King et al., 2005). The most notable difference was the average number of offspring of the homosexual probands––0.17 in our U.S. sample and 0.002 in the English sample. This pattern may be due, in part, to our U.S. sample being older (M = 40.4 years for homosexual probands, and M = 34.2 years for heterosexual probands) than the English sample (36.9 years and 32.1 years, respectively) (King et al., 2005). Part of this may reflect more general national differences: while indices such as period total fertility rate fluctuate with age cohort, values for relevant periods over the past several decades, though similar, are somewhat lower in England (Armitage & Babb, 1996) (or Europe in general: Lutz, O’Neill, & Scherbov, 2003) than in the U.S. (Schoen, 2004). Another possible explanation is the different ascertainment; the initial English sample was recruited from urban London sexually transmitted disease clinics (King et al., 2005), and our sample was recruited from festivals. In this latter sense, our festival sample might be more comparable to other studies recruiting from such non-clinical sources as clubs, bars, organizations, beaches, hotels, and universities (Camperio-Ciani et al., 2004; Iemmola & Camperio Ciani, 2008; Rahman et al., 2008).
Notably, homosexual male probands had more nieces and nephews than heterosexual male probands in our sample, and the next largest study (King et al., 2005) also found substantially higher numbers of such relatives (p < .005, OR = 1.11) for homosexual men (M = 2.7) compared to heterosexual men (M = 1.4). Our larger sample size enabled a strong corroboration of this interesting finding. Perhaps this effect reflects an androphilic genetic tendency due to which the sisters of male homosexual probands had more children than the sisters of the heterosexual probands. However, we did not find this to be the case in our data––the nieces and nephews of our homosexual male probands were just as likely to be offspring of their brothers as of their sisters, and similarly the nieces and nephews of our heterosexual male probands were just as likely to be offspring of their brothers as of their sisters.
It also seems possible that genetic variants contributing to male homosexuality might enhance the number of offspring in siblings through some other channel than an androphilic tendency, such as aspects of personality (e.g., sensitivity, kindness, empathy) that might facilitate reproductive fitness in heterosexual carriers as previously proposed (Miller, 2000). Our data were indeed more suggestive of a general familial effect. Besides the balancing selection explanation to the Darwinian conundrum of male sexual orientation, a competing explanation is the kin-selection hypothesis (Wilson, 1975). For homosexual probands who do not have children of their own, their nieces and nephews are their closest relatives in the next generation. According to the kin-selection hypothesis, by contributing to the rearing of their nephews and nieces––such as via providing some material or personal assistance––the homosexual men would be indirectly helping to propagate their genes via increasing their inclusive fitness, the evolutionary fitness measured in terms of the propagation of one’s genes by the survival and reproduction of both oneself and one’s kin. However, we obtained no information on the possibility of kin selection operating in our sample, and previous studies with Western samples have not supported this notion (Bobrow & Bailey, 2001; Rahman & Hull, 2005), though studies in Samoa have been consistent with the kin-selection hypothesis (Vasey, Pocock, & Vanderlaan, 2007; Vasey & Vanderlaan, 2008). Certainly, it is possible that more than one phenomenon (e.g., kin-selection and balancing selection) might be operating, or that kin-selection might be more apparent in some cultures than others––there are important differences between Western and Samoan cultures that might bear on this (Bobrow & Bailey, 2001; Vasey et al., 2007; Vasey & Vanderlaan, 2008).
Birth Order and Handedness
We replicated the fraternal birth order effect, as have many others (for review, see Blanchard, 2008). We saw this effect in both analyses performed, suggesting its robustness. Furthermore, our older sibling sex ratio for homosexual probands of 0.548 (expressed another way as 539/445 × 100 = 121) is virtually the same as seen in a previous meta-analysis (i.e., Fig. 2 in Blanchard, 2004) and as seen for right-handers in the largest such study (i.e., Table 6 in Blanchard & Lippa, 2007). In our data (Fig. 3), the increasing likelihood of homosexuality in a proband seen with increasing numbers of older brothers was more prominent with the larger number of older brothers (3 and ≥4), but otherwise the curve resembled ones previously reported (Blanchard & Bogaert, 1996; Blanchard et al., 1998). Interestingly, besides finding that our male homosexual probands had more older brothers than their heterosexual counterparts, we also found them to have more younger sisters in the logistic regression analysis.
An extension of the immunological hypothesis (Blanchard & Klassen, 1997) could possibly explain this effect. That is, if mothers form antibodies against male antigens of earlier sons, it is possible that subsequent male fetuses are more likely to be spontaneously aborted or otherwise not survive. Female fetuses, however, might escape unscathed or be less impacted, increasing the number of later born sisters. Previous studies have found that male offspring with older brothers do have lower average birth weight compared to those with older sisters (Blanchard & Ellis, 2001; Cote, Blanchard, & Lalumiere, 2003; Nielsen et al., 2008), which could be consistent, as would the trend (p = .083, two-tailed binomial test) in our data for homosexual probands having a relative deficit of younger brothers to younger sisters. However, some of the present results did not support the hypothesis (e.g., the male homosexual probands did not have a decreased number of younger brothers relative to the male heterosexual probands in the logistic regression analysis). Thus, the findings for the younger siblings were less clear than our replication of the fraternal birth order effect.
Collecting the various measures on the same large sample enabled us to check their impact on each other, such as finding that the fraternal birth order effect explains part, but not all, of the increased family size findings. We also found that while the fraternal birth order effect and familiality both predicted homosexuality, they did so independently of each other, as has been found by others using history of legal marriage or cohabitation as a proxy variable for sexual orientation in relatives of probands, i.e., bachelorhood after age 40 indicating a high likelihood of male homosexuality (Blanchard & Bogaert, 1997). We speculate that this might not empirically be the case if the homosexual proband sample derived from pairs of homosexual brothers (though we found no evidence of their interaction in our subset of probands with a gay brother), and in such instances linkage analyses should be adjusted taking into account the birth order as suggested by others (Cantor et al., 2002).
While there has been some heterogeneity of findings, most studies have found homosexual men more likely than heterosexual men to be NRH (for reviews, see Blanchard, 2008; Lalumiere et al., 2000), but we did not find this in our sample. This was the case whether we classified subjects as NRH if they were RH for less than 70% of a list of 10 common tasks or solely based on which hand they used for writing (e.g., Blanchard & Lippa, 2007). We noted that our NRH writing hand for male homosexual (12.8%) versus heterosexual (14.1%) probands closely bracketed the overall NRH (summing LH, mostly LH, and both hands from Table 2 of Peters, Reimers, & Manning, 2006) writing hand rate of 13.5% found for white males (including all sexual orientations) found in the BBC internet sample (90% of our sample had a predominant race of white). Studies on older historical cohorts (Davis & Annett, 1994; Gilbert & Wysocki, 1992) have found a decreasing proportion of NRH with increasing age, but we did not find this pattern in our sample and controlling for age did not alter our null handedness findings. Since we found no relationship between sexual orientation and handedness in our sample, it is perhaps not surprising that we did not find a relationship between handedness and the older brother effect that other studies have found (Blanchard, 2008; Blanchard et al., 2006; Blanchard & Lippa, 2007). One possible explanation is that the fraternal birth order effect is a more robust finding than the association of handedness with male sexual orientation (Blanchard, 2008; Lalumiere et al., 2000).
Hair Whorl
We found no significant relationship between hair whorl rotation pattern and handedness, contrasting with two studies (Beaton & Mellor, 2007; Klar, 2003), but consistent with the others (Annett, 1985; Collins, 1977; Jansen et al., 2007; Newman, 1934; Rahman et al., 2009). Also, we found no significant relationship between hair whorl rotation pattern and sexual orientation, contrasting with previous results in a much smaller sample (Klar, 2004), but consistent with another smaller sample (Rahman et al., 2009). Based on our experience, we agree with others (Jansen et al., 2007; Rahman et al., 2009) about the importance of close and direct examination of the hair whorl, as opposed to unobtrusive observation from a distance without the subject’s awareness (Klar, 2004). We noted that our overall rate of CCW hair whorl (18%) matches very closely with the other large study utilizing direct examination where, 19% of Germans with single whorls had CCW ones (Jansen et al., 2007). Similarly, we achieved a more accurate classification of sexual orientation by directly asking individuals as did the other negative study (Rahman et al., 2009), compared to assuming all the men in particular areas (e.g., predominantly gay beach area, generic beach, shopping mall) were of a particular sexual orientation as in the previous positive study (Klar, 2004). Thus, in sum, the majority of relevant research can support neither an association between hair whorl rotation patterns and handedness nor an association between hair whorl rotation patterns and sexual orientation.
Limitations
The homosexual and heterosexual samples were demographically similar to each other except for age, which obviously affected the numbers of some types of relatives. We attempted to control statistically for the age difference, but future research should aim to minimize such a difference. Both the homosexual and heterosexual samples included many participants of relatively high employment and educational status, and were predominantly white and non-Hispanic, thus limiting any generalizations of findings to other populations. Furthermore, the samples may not be fully representative of homosexual and heterosexual men in general, given the festival ascertainment. However, it seems unlikely that these concerns would have much impact on findings on some measures, especially number of older brothers, handedness, and hair whorl rotation pattern. Finally, the study relied on self-report about both the participant and their family members and is vulnerable to differential recall or knowledge of sexual orientation in other family members of the homosexual probands versus the heterosexual probands (discussed in Bailey et al., 1999), but this would likely not extend to recollections of the number of various relative classes.
Conclusions
Overall, this study replicated and extended several previous findings––especially with regard to family size and composition (homosexual men having more relatives, more gay relatives, and more older brothers)––but contrasted with some of the other previous research findings, especially with regards to the significance of maternal lines, handedness, and hair whorl rotation pattern. Due to significant methodological advantages of this study over the previous research on hair whorl rotation pattern and sexual orientation, our study casts significant doubt on the previously suggested relationship between hair whorl rotation pattern and sexual orientation in men. The family size findings from this study contribute to the understanding of sexual orientation in men and of the evolutionary conundrum of male homosexuality.
References
Alanko, K., Santtila, P., Harlaar, N., Witting, K., Varjonen, M., Jern, P., et al. (2009). Common genetic effects of gender atypical behavior in childhood and sexual orientation in adulthood: A study of Finnish twins. Archives of Sexual Behavior. doi:10.1007/s10508-008-9457-3.
Altmuller, J., Palmer, L. J., Fischer, G., Scherb, H., & Wjst, M. (2001). Genomewide scans of complex human diseases: True linkage is hard to find. American Journal of Human Genetics, 69, 936–950.
Annett, M. (1970). A classification of hand preference by association analysis. British Journal of Psychology, 61, 303–321.
Annett, M. (1985). Left, right, hand and brain: The right shift theory. London, UK: Erlbaum.
Armitage, B., & Babb, P. (1996). Population review: (4). Trends in fertility. Population Trends, 84, 7–13.
Bailey, J. M., & Bell, A. P. (1993). Familiality of female and male homosexuality. Behavior Genetics, 23, 313–322.
Bailey, J. M., & Benishay, D. S. (1993). Familial aggregation of female sexual orientation. American Journal of Psychiatry, 150, 272–277.
Bailey, J. M., Dunne, M. P., & Martin, N. G. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal of Personality and Social Psychology, 78, 524–536.
Bailey, J. M., Gaulin, S., Agyei, Y., & Gladue, B. A. (1994). Effects of gender and sexual orientation on evolutionarily relevant aspects of human mating psychology. Journal of Personality and Social Psychology, 66, 1081–1093.
Bailey, J. M., & Pillard, R. C. (1991). A genetic study of male sexual orientation. Archives of General Psychiatry, 48, 1089–1096.
Bailey, J. M., Pillard, R. C., Dawood, K., Miller, M. B., Trivedi, S., Farrer, L. A., et al. (1999). A family history study of male sexual orientation using three independent samples. Behavior Genetics, 29, 79–86.
Bailey, J. M., Pillard, R. C., Neale, M. C., & Agyei, Y. (1993). Heritable factors influence sexual orientation in women. Archives of General Psychiatry, 50, 217–223.
Beaton, A. A., & Mellor, G. (2007). Direction of hair whorl and handedness. Laterality, 12, 295–301.
Bell, A. P., & Weinberg, M. S. (1978). Homosexualities: A study of diversity among men and women. New York: Simon and Schuster.
Blanchard, R. (1997). Birth order and sibling sex ratio in homosexual versus heterosexual males and females. Annual Review of Sex Research, 8, 27–67.
Blanchard, R. (2004). Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. Journal of Theoretical Biology, 230, 173–187.
Blanchard, R. (2008). Review and theory of handedness, birth order, and homosexuality in men. Laterality, 13, 51–70.
Blanchard, R., & Bogaert, A. F. (1996). Homosexuality in men and number of older brothers. American Journal of Psychiatry, 153, 27–31.
Blanchard, R., & Bogaert, A. F. (1997). Additive effects of older brothers and homosexual brothers in the prediction of marriage and cohabitation. Behavior Genetics, 27, 45–54.
Blanchard, R., & Bogaert, A. F. (2004). Proportion of homosexual men who owe their sexual orientation to fraternal birth order: An estimate based on two national probability samples. American Journal of Human Biology, 16, 151–157.
Blanchard, R., Cantor, J. M., Bogaert, A. F., Breedlove, S. M., & Ellis, L. (2006). Interaction of fraternal birth order and handedness in the development of male homosexuality. Hormones and Behavior, 49, 405–414.
Blanchard, R., & Ellis, L. (2001). Birth weight, sexual orientation and the sex of preceding siblings. Journal of Biosocial Science, 33, 451–467.
Blanchard, R., & Klassen, P. (1997). H-Y antigen and homosexuality in men. Journal of Theoretical Biology, 185, 373–378.
Blanchard, R., & Lippa, R. A. (2007). Birth order, sibling sex ratio, handedness, and sexual orientation of male and female participants in a BBC internet research project. Archives of Sexual Behavior, 36, 163–176.
Blanchard, R., Zucker, K. J., Siegelman, M., Dickey, R., & Klassen, P. (1998). The relation of birth order to sexual orientation in men and women. Journal of Biosocial Science, 30, 511–519.
Bobrow, D., & Bailey, J. M. (2001). Is male homosexuality maintained via kin selection? Evolution and Human Behavior, 22, 361–368.
Bogaert, A. F. (2006). Biological versus nonbiological older brothers and men’s sexual orientation. Proceedings of the National Academy of Sciences of the United States of America, 103, 10771–10774.
Buhrich, N., Bailey, J. M., & Martin, N. G. (1991). Sexual orientation, sexual identity, and sex-dimorphic behaviors in male twins. Behavior Genetics, 21, 75–96.
Camperio-Ciani, A., Corna, F., & Capiluppi, C. (2004). Evidence for maternally inherited factors favouring male homosexuality and promoting female fecundity. Proceedings of the Royal Scoiety of London, Series B: Biological Sciences, 271, 2217–2221.
Cantor, J. M., Blanchard, R., Paterson, A. D., & Bogaert, A. F. (2002). How many gay men owe their sexual orientation to fraternal birth order? Archives of Sexual Behavior, 31, 63–71.
Chivers, M. L., Rieger, G., Latty, E., & Bailey, J. M. (2004). A sex difference in the specificity of sexual arousal. Psychological Science, 15, 736–744.
Clay, D. C., & Zuiches, J. J. (1980). Reference groups and family size norms. Population and Environment, 3, 262–279.
Collins, R. L. (1977). Origins of the sense of asymmetry: Mendelian and non-Mendelian models of inheritance. Annals of the New York Academy of Sciences, 299, 283–305.
Cote, K., Blanchard, R., & Lalumiere, M. L. (2003). The influence of birth order on birth weight: Does the sex of preceding siblings matter? Journal of Biosocial Science, 35, 455–462.
Davis, A., & Annett, M. (1994). Handedness as a function of twinning, age and sex. Cortex, 30, 105–111.
Floyd, F. J., & Bakeman, R. (2006). Coming-out across the life course: Implications of age and historical context. Archives of Sexual Behavior, 35, 287–296.
Gates, G., & Ost, J. (2004). The gay and lesbian atlas. Washington, DC: Urban Institute Press.
Geschwind, D. H., Miller, B. L., DeCarli, C., & Carmelli, D. (2002). Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proceedings of the National Academy of Sciences of the United States of America, 99, 3176–3181.
Gilbert, A. N., & Wysocki, C. J. (1992). Hand preference and age in the united states. Neuropsychologia, 30, 601–608.
Grov, C., Bimbi, D. S., Nanin, J. E., & Parsons, J. T. (2006). Race, ethnicity, gender, and generational factors associated with the coming-out process among lesbian, and bisexual individuals. Journal of Sex Research, 43, 115–121.
Hamer, D. H., Hu, S., Magnuson, V. L., Hu, N., & Pattatucci, A. M. (1993). A linkage between DNA markers on the X chromosome and male sexual orientation. Science, 261, 321–327.
Hershberger, S. L. (1997). A twin registry study of male and female sexual orientation. Journal of Sex Research, 34, 212–222.
Heston, L. L., & Shields, J. (1968). Homosexuality in twins: A family study and a registry study. Archives of General Psychiatry, 18, 149–160.
Hu, S., Pattatucci, A. M., Patterson, C., Li, L., Fulker, D. W., Cherny, S. S., et al. (1995). Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nature Genetics, 11, 248–256.
Iemmola, F., & Camperio Ciani, A. (2008). New evidence of genetic factors influencing sexual orientation in men: Female fecundity increase in the maternal line. Archives of Sexual Behavior. doi:10.1007/s10508-10008-19381-10506.
James, W. H. (1987). The human sex ratio: Part I: A review of the literature. Human Biology, 59, 721–752.
Jansen, A., Lohmann, H., Scharfe, S., Sehlmeyer, C., Deppe, M., & Knecht, S. (2007). The association between scalp hair-whorl direction, handedness and hemispheric language dominance: Is there a common genetic basis of lateralization? Neuroimage, 35, 853–861.
Jones, M. B., & Blanchard, R. (1998). Birth order and male homosexuality: Extension of Slater’s index. Human Biology, 70, 775–787.
Kallmann, F. J. (1952). Twin and sibship study of overt male homosexuality. American Journal of Human Genetics, 4, 136–146.
Kendler, K. S., Thornton, L. M., Gilman, S. E., & Kessler, R. C. (2000). Sexual orientation in a U.S. national sample of twin and nontwin sibling pairs. American Journal of Psychiatry, 157, 1843–1846.
King, M., Green, J., Osborn, D. P., Arkell, J., Hetherton, J., & Pereira, E. (2005). Family size in white gay and heterosexual men. Archives of Sexual Behavior, 34, 117–122.
King, M., & McDonald, E. (1992). Homosexuals who are twins: A study of 46 probands. British Journal of Psychiatry, 160, 407–409.
Kinsey, A. C., Pomeroy, W. B., & Martin, C. E. (1948). Sexual behavior in the human male. Philadelphia: W.B. Saunders Company.
Kirk, K. M., Bailey, J. M., Dunne, M. P., & Martin, N. G. (2000). Measurement models for sexual orientation in a community twin sample. Behavior Genetics, 30, 345–356.
Kirk, K. M., Bailey, J. M., & Martin, N. G. (1999). How accurate is the family history method for assessing siblings’ sexual orientation? Archives of Sexual Behavior, 28, 129–137.
Klar, A. J. (2003). Human handedness and scalp hair-whorl direction develop from a common genetic mechanism. Genetics, 165, 269–276.
Klar, A. J. (2004). Excess of counterclockwise scalp hair-whorl rotation in homosexual men. Journal of Genetics, 83, 251–255.
Lalumiere, M. L., Blanchard, R., & Zucker, K. J. (2000). Sexual orientation and handedness in men and women: A meta-analysis. Psychological Bulletin, 126, 575–592.
Lander, E., & Kruglyak, L. (1995). Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nature Genetics, 11, 241–247.
Langstrom, N., Rahman, Q., Carlstrom, E., & Lichtenstein, P. (2008). Genetic and environmental effects on same-sex sexual behavior: A population study of twins in Sweden. Archives of Sexual Behavior. doi:10.1007/s10508-008-9386-1.
Laumann, E. O., Gagnon, J. H., Michael, R. T., & Michaels, S. (1994). The social organization of sexuality: Sexual practices in the United States. Chicago: The University of Chicago Press.
Longstreth, L. E. (1980). Human handedness: More evidence of genetic involvement. Journal of Genetic Psychology, 137, 275–283.
Lutz, W., O’Neill, B. C., & Scherbov, S. (2003). Demographics. Europe’s population at a turning point. Science, 299, 1991–1992.
Miller, E. M. (2000). Homosexuality, birth order, and evolution: Toward an equilibrium reproductive economics of homosexuality. Archives of Sexual Behavior, 29, 1–34.
Mustanski, B. S., Chivers, M. L., & Bailey, J. M. (2002). A critical review of recent biological research on human sexual orientation. Annual Review of Sex Research, 13, 89–140.
Mustanski, B. S., Dupree, M. G., Nievergelt, C. M., Bocklandt, S., Schork, N. J., & Hamer, D. H. (2005). A genomewide scan of male sexual orientation. Human Genetics, 116, 272–278.
Newman, H. H. (1934). Dermatoglyphics and the problem of handedness. American Journal of Anatomy, 55, 277–322.
Nielsen, H. S., Mortensen, L., Nygaard, U., Schnor, O., Christiansen, O. B., & Andersen, A. M. (2008). Brothers and reduction of the birth weight of later-born siblings. American Journal of Epidemiology, 167, 480–484.
Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113.
Pattatucci, A. M. L., & Hamer, D. H. (1995). Development and familiality of sexual orientation in females. Behavior Genetics, 25, 407–420.
Peters, M., Reimers, S., & Manning, J. T. (2006). Hand preference for writing and associations with selected demographic and behavioral variables in 255, 100 subjects: The BBC internet study. Brain and Cognition, 62, 177–189.
Pillard, R. C., & Weinrich, J. D. (1986). Evidence of familial nature of male homosexuality. Archives of General Psychiatry, 43, 808–812.
Rahman, Q., Clarke, K., & Morera, T. (2009). Hair whorl direction and sexual orientation in human males. Behavioral Neuroscience, 123, 252–256.
Rahman, Q., Collins, A., Morrison, M., Orrells, J. C., Cadinouche, K., Greenfield, S., et al. (2008). Maternal inheritance and familial fecundity factors in male homosexuality. Archives of Sexual Behavior, 37, 962–969.
Rahman, Q., & Hull, M. S. (2005). An empirical test of the kin selection hypothesis for male homosexuality. Archives of Sexual Behavior, 34, 461–467.
Rice, G., Anderson, C., Risch, N., & Ebers, G. (1999). Male homosexuality: Absence of linkage to microsatellite markers at Xq28. Science, 284, 665–667.
Sanders, A. R., Cao, Q., Zhang, J., Badner, J. A., Goldin, L. R., Guroff, J. J., et al. (1998). Genetic linkage study of male homosexual orientation (New Research Abstracts NR149). Toronto, Ontario, Canada: American Psychiatric Association.
Sanders, A. R., & Dawood, K. (2003). Sexual orientation. In Encyclopedia of life sciences. Chichester: John Wiley & Sons. http://www.els.net/ [doi:10.1038/npg.els.0001480].
Schoen, R. (2004). Timing effects and the interpretation of period fertility. Demography, 41, 801–819.
Vasey, P. L., Pocock, D. S., & Vanderlaan, D. P. (2007). Kin selection and male androphilia in Samoan fa’afafine. Evolution and Human Behavior, 28, 159–167.
Vasey, P. L., & VanderLaan, D. P. (2007). Birth order and male androphilia in Samoan fa’afafine. Proceedings of the Royal Scoiety of London, Series B: Biological Sciences, 274, 1437–1442.
Vasey, P. L., & Vanderlaan, D. P. (2008). Avuncular tendencies and the evolution of male androphilia in Samoan fa’afafine. Archives of Sexual Behavior. doi:10.1007/s10508-10008-19404-10503.
Whitam, F. L. (1983). Culturally invariable properties of male homosexuality: Tentative conclusions from cross-cultural research. Archives of Sexual Behavior, 12, 207–226.
Whitam, F. L., Diamond, M., & Martin, J. (1993). Homosexual orientation in twins: A report on 61 pairs and three triplet sets. Archives of Sexual Behavior, 22, 187–206.
Wilson, E. O. (1975). Sociobiology: The new synthesis. Cambridge: Harvard University Press.
Wilson, E. O. (1978). On human nature. Cambridge: Harvard University Press.
Acknowledgments
We thank the anonymous participants for their time and cooperation. We thank Drs. J. Michael Bailey and Joan A. Linsenmeier for insightful comments on an earlier draft of the article, and the thorough reviews and helpful suggestions from the Editor and the three peer reviewers. This research was supported by a grant to RK from the Chicago Area Undergraduate Research Symposium, a Summer Undergraduate Research Grant to GS from Northwestern University, and funds from the Behavior Genetics Unit of NorthShore University HealthSystem.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwartz, G., Kim, R.M., Kolundzija, A.B. et al. Biodemographic and Physical Correlates of Sexual Orientation in Men. Arch Sex Behav 39, 93–109 (2010). https://doi.org/10.1007/s10508-009-9499-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-009-9499-1