Abstract
In the present study, three physical development characteristics—weight, height, and age of menarche—were examined for their relation to sexual orientation. Participants were men and women comprising the National Survey of Sexual Attitudes and Lifestyles-2000 (N > 11,000). Participants completed self-report measures of sexual orientation, height, weight, and, for women, age of menarche. Results indicated that gay/bisexual men were significantly shorter and lighter than heterosexual men. There were no significant differences between lesbians and heterosexual women in height, weight, and age of puberty. The results add to literature suggesting that, relative to heterosexual men, gay/bisexual men may have different patterns of growth and development because of early biological influences (e.g., exposure to atypical levels of androgens prenatally). However, the present results do not support a number of studies suggesting that lesbian/bisexual women are taller and heavier than heterosexual women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some recent research has examined aspects of physical development (e.g., height, weight, and age of puberty) and their relation to sexual orientation. This research has shown that gay/bisexual men are shorter and lighter than heterosexual men and that lesbian/bisexual women are taller and heavier than heterosexual women (e.g., Blanchard & Bogaert, 1996a; Bogaert, 1998; Bogaert & Blanchard, 1996). These body size differences have been observed in samples from different eras. For example, Blanchard and Bogaert (1996a) and Bogaert (1998) found evidence for a body size/sexual orientation relation in the original Kinsey data (compiled from the 1930s–1960s), while Bogaert and Friesen (2002) and Bogaert and Blanchard (1996) found evidence for such a relation in contemporary samples. However, there are notable failures to show such a relation (e.g., Perkins, 1981) and all of the supportive findings on body size and men’s sexual orientation have been in convenience samples. Some research on the relation between age of puberty and sexual orientation has suggested that gay/bisexual men enter puberty earlier than heterosexual men (e.g., Bogaert, Friesen, & Klentrou, 2002), but the findings are inconsistent (e.g., Savin-Williams & Ream, 2006), and there is no evidence of a reliable relation between pubertal onset and sexual orientation in women (Bogaert, 1998; Bogaert & Friesen, 2002; Tenhula & Bailey, 1998).
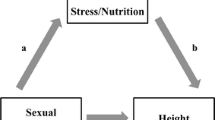
One explanation for these putative physical differences between gays/lesbians and heterosexual people is variation in prenatal hormones. Prenatal sex hormones play a large role in gender differentiation (e.g., Arnold, 2002), and these hormones have been argued to underlie sexual orientation (Ellis & Ames, 1987; Meyer-Bahlburg et al., 1995). If so, one would expect relatively broad effects of prenatal hormones to occur in brain/body organization, such that sex-dimorphic “correlates” of sexual orientation should be detectable. These “correlates” should be most evident for characteristics exhibiting large sex dimorphisms, including height, weight, and age of puberty, as men are significantly taller, heavier (even for their height), and enter puberty later than women (e.g., Grumbach & Styne, 1992; Underwood & Van Wyk, 1992). Thus, evidence that gay men and lesbians evince a pattern of scores on these sex dimorphic characteristics similar to the opposite sex—gay men smaller and enter puberty earlier, and lesbians larger and enter puberty later—provides additional support for the theory that prenatal hormones contribute to sexual orientation development. Indeed, it would suggest that sex-dimorphic brain processes (e.g., sites in the hypothalamus; cf. LeVay, 1991) governing growth and development are differentiated via prenatal hormones into a pattern typical of the opposite-sex in gay men and lesbians. Interestingly, aside from these basic physical development features, other sex-dimorphic characteristics (e.g., somatic and behavioral features) have been linked to sexual orientation (for reviews, see Mustanski, Chivers, & Bailey, 2002; Rahman & Wilson, 2003; Wilson & Rahman, 2005), and this pattern of results also provide evidence that prenatal hormones may underlie sexual orientation development.
Although variation in prenatal hormones is, arguably, the leading biological explanation of sexual orientation development, other biological theories may account for these putative physical development differences between gays/lesbians and heterosexual people. First, genetic factors may play a role. There is evidence that genetic factors are relevant to both sexual orientation (e.g., Bailey, Dunne, & Martin, 2000; Hamer, Hu, Magnussion, Hu, & Pattatucci, 1993; Mustanski et al., 2005) and body size (e.g., Mueller, 1976). Thus, common genes may affect developmental processes involved with both sexual orientation and physical growth. Another biological explanation is developmental instability, or phenotypic anomalies, which develop as result of environmental and/or genetic stressors. For example, one potential source of environmental vulnerability during prenatal development is maternal stress. There is evidence that stress is elevated in pregnant mothers who give birth to gay/bisexual sons (e.g., Ellis & Cole-Harding, 2001) and that maternal stress during pregnancy can affect growth and development of the fetus (e.g., Lobel, Dunkel-Schetter, & Scrimshwa, 1992; cf. Lobel, 1994). Thus, maternal stress, or other environmental factors contributing to possible developmental instability during pregnancy, may affect developmental processes involved with both sexual orientation and physical characteristics of the fetus. Another biological explanation is immunological in origin. Originally formulated to account for the finding that gay men have a greater number of older brothers than heterosexual men (e.g., Blanchard & Bogaert, 1996b), the immunological explanation of sexual orientation suggests that factors specifically associated with male (as opposed to female) fetal development can provoke an immune response in pregnant mothers. An immune response of this kind may alter brain organization related to sexual orientation but also physical development of some male fetuses. Of course, such an explanation is gender-specific in nature, and thus is most applicable to putative physical development differences in male (versus female) sexual orientation.
The present study examined whether three physical development characteristics—weight, height, and age of menarche—were related to sexual orientation in men and women. As this study employed a large national probability sample—National Survey of Sexual Attitudes and Lifestyles-2000 (NATSAL 2000; Erens et al., 2001)—it contains one of the largest samples ever to investigate these issues, along with a broadly representative one of the population of Britain. Most previous investigations examining these issues have used convenience or clinical samples (cf. Bogaert & Friesen, 2002; Bogaert et al., 2002).
Method
Sample
NATSAL-2000 used a probability sample of Britain (England, Wales, and Scotland; Erens et al., 2001). The survey assessed young adults, with ages ranging from 16 to 44. Some of the measures (e.g., demographics, sexual orientation) were posed to all participants via an initial face-to-face interview; the remainder of the measures (e.g., age of menarche; sexuality) were administered later via a computerized self-assessment. However, this computer self-assessment only occurred for those who were sexually experienced, and thus some of the measures, including age of menarche, had somewhat elevated missing data.
Two samples (“core” and “ethnic-boost” sample) were gathered in NATSAL-2000. As the core or general population sample was the main sample (N = 11,161) and broadly represents the population of Britain, this was one was used in the present study. NATSAL-2000 data are typically weighted (Erens et al., 2001; FINAL_WT) to adjust for inequities in sampling (e.g., residence differences in inner versus outer London, along with gender and age disparities). This weight was used in the present study. In addition, 71 participants who the interviewers assessed as having “severe” language, literacy, or other problems during the interview/questionnaire process were eliminated. The remaining participants in the sample comprised 5,637 men and 5,453 women.
Measures
Menarche, Height, and Weight
Age at menarche was recorded in full years. A small (but elevated) percentage of the women (4.0%) did not have a valid response for this milestone in part because, as previously mentioned, they were not posed the menarche question if they did not have sexual experience with a partner. Note that men were not asked their age of puberty in NATSAL-2000. Participants reported on their height and weight and the responses were converted to kilograms and centimetres. Height and weight was also used to calculate the participants’ body mass index (BMI), an indicator of excess weight or body fat.
Sexual Attraction and Experience
Participants were handed a card on which was written “I have felt sexually attracted to…”. They were given six options to which they could respond: (1) “only the opposite sex” (male n = 5176, female n = 4804); (2) “more often to opposite sex, and at least once to a same-sex” (male n = 301, female n = 530); (3) “about equally often to males and females” (male n = 26, female n = 30); (4) “more often to same-sex, and at least once to the opposite sex” (male n = 57, female n = 22); (5) “only same-sex, never to opposite sex” (male n = 51, female n = 13); and (6) “I have never felt sexually attracted to anyone at all” (male n = 18, female n = 31). The 27 men and 41 women who reported they did not have any attraction to men or women or did answer the question on sexual attraction were eliminated from further analyses.
Participants were handed a card on which was written “Sexual experience is any kind of contact with another person that you felt was sexual (it could just be kissing or touching, or intercourse or any other form of sex). I have had some experience…”. They were given six options to which they could respond: (1) “only the opposite sex” (male n = 5,173, female n = 4,991); (2) “more often to opposite sex, and at least once to a same-sex” (male n = 266, female n = 336); (3) “about equally often to males and females” (male n = 13, female n = 20); (4) “more often same-sex, and at least once with the opposite sex” (male n = 59, female n = 21); (5) “only same-sex, never to opposite sex” (male n = 43, female n = 6); and (6) “I have never had sexual experience with anyone at all” (male n = 77, female n = 70). Seven men and 8 women did not respond to this question and were eliminated from further analysis.
The attraction and sexual experience measures were averaged. Those who reported, on average, a predominant or exclusive opposite-sex attraction/experience (i.e., scoring an average of attraction and experience of 2 or less) were categorized as straight/heterosexual; the remainder (scoring an average of attraction and experience of more than 2) were categorized as gay/bisexual. For those reporting no sexual experience, their categorization of sexual orientation was based on their sexual attraction score (i.e., scoring 2 or less straight/heterosexual; the remainder gay/bisexual). Note that the lack of sexual experience is not necessarily a limitation in this categorization, as sexual attraction is often used by itself to measure sexual orientation (e.g., Bogaert, 2003). There were 132 men and 75 women categorized as gay/bisexual and 5,472 men and 5,335 women categorized as heterosexual using these criteria.
Additional Demographics
Included were age (in years) and education (1 = “degree,” 2 = “higher education, but below degree level,” 3 = “0 level or equivalent,” 4 = “other/foreign,” or 5 = “none/no exams passed”). Education was reverse coded so that those with higher education levels had higher scores (i.e., 1 = “none/no exams passed” to 5 = “degree”). Race-ethnicity (1 = “White,” 2 = “Black,” 3 = “Asian,” or 4 = “other”) was also included. Race-ethnicity was recoded so that 0 = “White” and 1 = “non-White.”
Included too was number of siblings, as there is evidence that birth order (i.e., older brothers) may be related to both body size (e.g., birth weight; Côté, Blanchard, & Lalumière, 2003) and men’s sexual orientation (for a review, see Blanchard, 2004). Participants were asked whether they had only sisters, only brothers, or both brothers and sisters (or none). They were also asked for birth order using three categories—first born, last born, and in-between—along with their total number of siblings. From these variables, number of older brothers, older sisters, younger brothers, and younger sisters were constructed using a series of decision rules (see also Bogaert, 2003, 2005). However, because of the truncated birth order information, these data are less than optimal for the reconstruction of sibling characteristics, giving both exact and estimated quantities for these characteristics (including fractions; see Bogaert, 2003).
Results
Tables 1 and 2 show descriptive statistics for the demographic and physical development variables as a function of sexual orientation and gender. No significant sexual orientation differences occurred in age, race/ethnicity, or education. Also, note that, although in the predicted direction, there was no significant older brother (or fraternal birth order) effect in these data, contrary to much prior research (e.g., Blanchard, 2004). However, as mentioned, these data were less than optimal to examine sibling characteristics (but see Bogaert, 2003). Gay/bisexual and heterosexual men differed significantly in body size, with gay men reporting being shorter and lighter (even for their height; see body mass results) than heterosexual men. The lesbian/bisexual women did not differ from the heterosexual women on any of the three physical variables. However, the Levene’s tests for equality of variances indicated that weight and body mass (p = .02; p = .04, respectively) and height (marginal; p = .06) were more variable in lesbians/bisexual women than in heterosexual women.
Discussion
In a national probability sample, physical development characteristics were assessed for their relation to sexual orientation in both men and women. Gay/bisexual men reported being shorter and lighter than heterosexual men. No significant differences in three dimensions of physical development—height, weight, and onset of puberty—were found between lesbian/bisexual and heterosexual women. Null findings for age of menarche were consistent with previous research (Bogaert, 1998; Bogaert & Friesen, 2002; Tenhula & Bailey, 1998), but the lack of a height and weight difference between lesbian/bisexual and heterosexual women was somewhat surprising because these differences have been found a number of times before, including in a similar large representative sample from Britain (Bogaert & Friesen, 2002).
The height and weight differences between gay/bisexual and heterosexual men have been found before, although the previous supportive studies used clinical or convenience samples. The only other study using a national probability sample to examine physical development differences between heterosexual and gay/bisexual men found no evidence that gay/bisexual men were shorter or lighter than heterosexual comparisons (Bogaert & Friesen, 2002). Thus, the present results, based on this recent large national probability sample from Britain, are important because they verify physical development differences between heterosexual and gay/bisexual men found to occur in nonrepresentative samples. These results, then, add to a body of research studies indicating that gay/bisexual men evince a pattern of scores on some sex-dimorphic somatic and cognitive characteristics similar to women (e.g., for a review, see Mustanski et al., 2002; Wilson & Rahman, 2005). These results also raise the possibility that homosexual men have a degree of somatic feminization, or de-masculinization, via prenatal hormonal influences on growth and development mechanisms (but also see Alias, 2004; Bogaert & Hershberger, 1999; McFadden & Champlin, 2000).
A height difference between gay/bisexual and heterosexual men may be especially relevant to biological theories of sexual orientation (e.g., prenatal hormones) because final adult height is relatively immutable, i.e., not open to change significantly due to most medical, psychological, or environmental influences after puberty (e.g., Underwood & Van Wyk, 1992). Weight, in contrast, can vary significantly during adulthood because of lifestyle and health issues. For example, gay/bisexual men may be more motivated than heterosexual men to maintain a slender body (e.g., Siever, 1994) because physical appearance issues (e.g., trim body, youthful appearance) are particularly relevant to male/male romantic/sexual relationships (e.g., Sergios & Cody, 1986). There is also evidence that gay men are more prone than heterosexual men to eating disorders (e.g., Herzog, Norman, Gordon, & Pepose, 1984; Robinson & Holden, 1986). On the other hand, a biological explanation of the weight difference should still be considered, as gay/bisexual men may have, on average, a smaller frame and bone structure relative to heterosexual men, and this may partially account for why gay/bisexual men are lighter than heterosexual men.
As indicated, other biological explanations, potentially unrelated to variations in prenatal exposure to androgens, may partially account for the physical differences between bisexual/gay and heterosexual men. One is developmental instability that occurs as result of environmental and/or genetic stressors. One source of environmental vulnerability is maternal stress during pregnancy, which may affect processes involved with both sexual orientation (e.g., Ellis & Cole-Harding, 2001) and physical characteristics of the developing fetus (e.g., Lobel et al., 1992; cf. Lobel, 1994). Such stress effects—via, for example, the production of stress hormones—may partially operate through alterations of prenatal androgens to affect brain structures underlying sexual orientation (e.g., Ward & Weisz, 1984), but it should not be discounted that stress hormones may have important effects independent of prenatal androgens, particularly on fetal growth and development.
Second, genetic factors may be relevant, as they have been linked to both sexual orientation (e.g., Bailey et al., 2000; Hamer et al., 1993; Mustanski et al., 2005) and body size (e.g., Mueller, 1976). Thus, common genes may affect processes involved with both sexual orientation and physical development, again potentially independent of prenatal androgens.
Third, an immunological explanation of sexual orientation suggests that factors specifically associated with male (as opposed to female) fetal development can provoke an immune response in pregnant mothers. An immune response would presumably alter brain organization related to sexual orientation in male fetuses; it would also presumably affect physical development of male fetuses. Of course, such an explanation is gender-specific in nature, and thus, is most applicable to putative physical development differences in male (versus female) sexual orientation. Thus, it may be able to explain the body size differences between gay and heterosexual men observed in the present study.
Although there was no relation between physical development and sexual orientation in women, body size (particularly weight) was more variable in lesbians/bisexual women than in heterosexual women. This raises the possibility that certain biological factors (e.g., high prenatal androgens) increase variability in physical traits in bisexual/lesbian women. Or, alternatively, there may be multiple biological factors, each with its own pathway increasing the likelihood of a same-sex orientation in women, with, for example, one (e.g., prenatal androgens) increasing body size, and while another (e.g., developmental instability) decreasing body size. Another alternative is that there may be multiple factors, again each with its own pathway increasing the likelihood of same-sex orientation, but one of these pathways may be psychosocial in nature. For example, there is less evidence of a prenatal masculinization effect in “femme” lesbians than in “butch” lesbians (e.g., Brown, Finn, Cooke, & Breedlove, 2002), raising the possibility that psychosocial factors may play a significant role in the “femme” subgroup of lesbians. Thus, these putative multiple pathways, stemming from very different etiological sources, may also increase variability in lesbian/bisexual women, including perhaps in body size. More research needs to examine these possibilities.
An important question to answer is why the present findings differ from Bogaert and Friesen (2002), who used a similar national probability sample of Britain (NATSAL-1990), and yet did not, for example, find body size differences between gay and heterosexual men. The answer may lie in the fact that, although the present study and Bogaert and Friesen (2002) used similar British national samples, there were also differences between these samples. First, there were differences in recruitment, including more extensive surveying of greater London in NATSAL-2000 relative to NATSAL-1990 (see Erens et al., 2001). There was also a ten year difference between NATSAL-2000 and NATSAL-1990, and there may have been some relevant changes in aspects of the social/psychological environment affecting sexual development or at least in the willingness to report aspects of sexuality (e.g., same-sex attractions; for a discussion, see Johnson et al., 2001). The age difference between the two samples may also be important. The age range in NATSAL-2000 was restricted to 16–44, whereas the first survey had an age range of 16–59. How these sample differences could have affected the findings is unknown, but given that the effects are small (e.g., a height difference of less than two centimetres), it is not surprising that even similar samples show somewhat different findings.
Finally, it should be noted that alternative, nonbiological explanations may still be plausible to account for the height difference between gay/bisexual and heterosexual men. One possibility is that young heterosexual men exaggerate their physical size to conform to a stereotypically “masculine” or “hypermasculine” ideal. Young homosexual men may be less inclined to conform to a stereotypically “masculine” ideal, as a masculine or hypermasculine gender role may not represent the norm in gay/bisexual men (e.g., Bailey & Zucker, 1995). Research suggests that actual height correlates highly with self-report height (Himes & Roche, 1982), but there is also evidence that men may over-report their heights, and they do so more than women do (Giles & Hutchinson, 1991). Whether this gender difference in the over reporting of height varies across sexual orientation, however, is unknown.
References
Alias, A. B. (2004). A role for 5-alpha-reductase activity in the development of male homosexuality? Annals of the New York Academy of Sciences, 1032, 237–244.
Arnold, A. P. (2002). Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In D. W. Pfaff, A. P. Arnold, A. Etgen, S. Fahrbach, & R. Rubin (Eds.), Hormones, brain, and behavior (pp. 105–135). San Diego, CA: Academic Press.
Bailey, J. M., Dunne, M. P., & Martin, N. G. (2000). Genetic and environmental influences on sexual orientation and its correlates in an Australian twin sample. Journal of Personality and Social Psychology, 78, 524–536.
Bailey, J. M., & Zucker, K. J. (1995). Childhood sex-typed behavior and sexual orientation: A conceptual analysis and quantitative review. Developmental Psychology, 31, 43–55.
Blanchard, R. (2004). Quantitative and theoretical analyses of the relation between older brothers and homosexuality in men. Journal of Theoretical Biology, 230, 173–187.
Blanchard, R., & Bogaert, A. F. (1996a). Biodemographic comparisons of homosexual and heterosexual men in the Kinsey interview data. Archives of Sexual Behavior, 25, 551–579.
Blanchard, R., & Bogaert, A. F. (1996b). Homosexuality in men and number of older brothers. American Journal of Psychiatry, 153, 27–31.
Bogaert, A. F. (1998). Physical development and sexual orientation in women: Height, weight, and age at puberty comparisons. Personality and Individual Differences, 24, 115–121.
Bogaert, A. F. (2003). Number of older brothers and sexual orientation: New tests and attraction/behavior distinction in two national probability samples. Journal of Personality and Social Psychology, 84, 644–652.
Bogaert, A. F. (2005). Sibling sex ratio and sexual orientation in men and women: New tests in two national probability samples. Archives of Sexual Behavior, 34, 111–116.
Bogaert, A. F., & Blanchard, R. (1996). Physical development and sexual orientation in men: Height, weight, and age of puberty differences. Personality and Individual Differences, 21, 77–84.
Bogaert, A. F., & Friesen, C. (2002). Sexual orientation and height, weight, and age of puberty: New tests from a British national probability sample. Biological Psychology, 59, 135–145.
Bogaert, A. F., Friesen, C., & Klentrou, P. (2002). Age of puberty and sexual orientation in a national probability sample. Archives of Sexual Behavior, 31, 73–81.
Bogaert, A. F., & Hershberger, S. (1999). The relation between sexual orientation and penile size. Archives of Sexual Behavior, 28, 213–221.
Brown, W. M., Finn, C. J., Cooke, B. M., & Breedlove, S. M. (2002). Differences in finger length ratios between self-identified “butch” and “femme” lesbians. Archives of Sexual Behavior, 31, 123–127.
Côté, K., Blanchard, R., & Lalumière, M. L. (2003). The influence of birth order on birth weight: Does sex of preceding siblings matter? Journal of Biosocial Science, 35, 455–462.
Ellis, L., & Ames, M. A. (1987). Neurohormonal functioning and sexual orientation: A theory of homosexuality-heterosexuality. Psychological Bulletin, 101, 233–258.
Ellis, L., & Cole-Harding, S. (2001). Effects of prenatal stress, and of prenatal alcohol and nicotine exposure, on human sexual orientation. Physiology & Behavior, 74, 213–226.
Erens, B., McManus, S., Field, J., Korovessis, C., Johnson, A., Fenton, K., & Wellings, K. (2001). National Survey of Sexual Attitudes and Lifestyles II: Technical Report. London: National Centre for Social Research.
Giles, E., & Hutchinson, D. L. (1991). Stature and age related bias in self-reported stature. Journal of Forensic Sciences, 36, 760–780.
Grumbach, M. M., & Styne, D. M. (1992). Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In J. D. Wilson & D. W. Foster (Eds.), Williams textbook of endocrinology (8th ed., pp. 1139–1221). Philadelphia: W. B. Saunders.
Hamer, D. H., Hu, S., Magnussion, V. L., Hu, N., & Pattatucci, A. M. L. (1993). A linkage between DNA markers on the X chromosome and male sexual orientation. Science, 261, 321–327.
Herzog, D. B., Norman, K. D., Gordon, C., & Pepose, M. (1984). Sexual conflict and eating disorders in 27 males. American Journal of Psychiatry, 141, 989–990.
Himes, J. H., & Roche, A. F. (1982). Reported versus measured adult stature. American Journal of Physical Anthropology, 58, 335–341.
Johnson, A., Mercer, C. H., Erens, B., Copas, A. J., McManus, S., Wellings, K., et al. (2001). Sexual behaviour in Britain: Partnerships, practices, and HIV risk behaviours. Lancet, 358, 1935–1842.
LeVay, S. (1991). A difference in hypothalamic structure between heterosexual and homosexual men. Science, 253, 1034–1037.
Lobel, M. (1994). Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. Journal of Behavioral Medicine, 17, 225–272.
Lobel, M., Dunkel-Schetter, C., & Scrimshwa, S. C. M. (1992). Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychology, 11, 32–40.
McFadden, D., & Champlin, C. A. (2000). Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. Journal of the Association for Research in Otolaryngology, 1, 89–99.
Meyer-Bahlburg, H. F. L., Ehrhardt, A. A., Rosen, L. R., Gruen, R. S., Veridiano, N. P., Vann, F. H., et al. (1995). Prenatal estrogens and the development of homosexual orientation. Developmental Psychology, 31, 12–21.
Mueller, W. H. (1976). Parent-child correlations for stature and weight among school aged children: A review of 24 studies. Human Biology, 48, 379–397.
Mustanski, B. S., Chivers, M. L., & Bailey, J. M. (2002). A critical review of recent biological research on human sexual orientation. Annual Review of Sex Research, 13, 89–140.
Mustanski, B. S., Dupree, M. G., Nievergelt, C. M., Bocklandt, S., Schork, N. J., & Hamer, D. H. (2005). A genomewide scan of male sexual orientation. Human Genetics, 116, 272–278.
Perkins, M. W. (1981). Female homosexuality and body build. Archives of Sexual Behavior, 10, 337–345.
Rahman, Q., & Wilson, G. D. (2003). Born gay? The psychobiology of human sexual orientation. Personality and Individual Differences, 34, 1337–1382.
Robinson, P. H., & Holden, N. L. (1986). Bulimia nervosa in the male: A report of nine cases. Psychological Medicine, 16, 795–803.
Savin-Williams, R. C., & Ream, G. L. (2006). Pubertal onset and sexual orientation in a national probability sample. Archives of Sexual Behavior, 35, 279–286.
Sergios, P., & Cody, J. (1986). Importance of physical attractiveness and social assertiveness skills in male homosexual dating behaviour and partner selection. Journal of Homosexuality, 12, 71–84.
Siever, M. D. (1994). Sexual orientation and gender as factors in socioculturally acquired vulnerability to body dissatisfaction and eating disorders. Journal of Consulting and Clinical Psychology, 62, 252–260.
Tenhula, W. N., & Bailey, J. M. (1998). Female sexual orientation and pubertal onset. Developmental Neuropsychology, 14, 369–383.
Underwood, L. E., & Van Wyk, J. J. (1992). Normal and aberrant growth. In J. D. Wilson & D. W. Foster (Eds.), Williams textbook of endocrinology (8th ed., pp. 1079–1138). Philadelphia: W. B. Saunders.
Ward, I. L., & Weisz, J. (1984). Differential effects of maternal stress on circulating levels of corticosterone, progesterone, testosterone in male and female rat fetuses and their mothers. Endocrinology, 114, 1635–1644.
Wilson, G., & Rahman, Q. (2005). Born gay: The biology of sex orientation. London: Peter Owen Publishers.
Acknowledgements
I thank Luanne K. Jamieson for comments on a previous draft of this article. I also thank the investigators at the National Centre for Social Research for making these data accessible to researchers. Finally, I thank three anonymous reviewers and the Editor for helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bogaert, A.F. Physical Development and Sexual Orientation in Men and Women: An Analysis of NATSAL-2000. Arch Sex Behav 39, 110–116 (2010). https://doi.org/10.1007/s10508-008-9398-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-008-9398-x