Abstract
This study aimed to investigate the chemical composition and effects of dietary supplementation with Citrus limon extract (CLE) (Nor-Spice AB®) on growth, hematological, and innate immunity parameters of striped catfish juveniles (Pangasius hypophthalmus). Chemical composition was detected using nuclear magnetic resonance and mass spectrometry. Six diets with different levels of CLE —0.0 (control), 0.2, 0.4, 0.8, 1.6, and 3.2 g kg diet–1 — were evaluated for 90 days, followed by 8 days of bacterial infection by Aeromonas hydrophila. CLE presented phenolic compounds (mainly flavonoids) and polysaccharides as major constituents. Fish supplemented with 0.4 g CLE kg diet–1 showed final weight, weight gain, final biomass, specific growth rate, and feed conversion ratio higher than fish of the control group, or those supplemented with diets between 0.8 and 3.2 g CLE kg–1. Fish supplemented with 3.2 g CLE kg diet–1 showed plasma albumin levels, respiratory burst total thrombocytes, total leukocytes, lymphocytes, and monocytes significantly higher than fish supplemented with other diets. After bacterial infection, fish fed CLE maintained biochemical, hematological, and immunological parameters similar to the control group, except for plasma total proteins and neutrophils levels that decreased as the concentration of CLE was increased in the diet. In conclusion, the addition of 0.4 g CLE kg diet–1 is recommended for improving the growth and immune resistance of striped catfish in intensive culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Striped catfish (Pangasius hypophthalmus, Sauvage, 1878) are one of the most extensively farmed fish globally (Naylor et al. 2021). It is a profitable farmed fish because of its high demand in local and international markets and its high nutritional value at a low consumer cost (Hoque et al. 2021). This species presents favorable characteristics for cultivation, such as tolerance of low dissolved oxygen (Lefevre et al. 2011), omnivorous feeding habits, and fast growth (Patel et al. 2022). However, when this species is cultured under high stocking density in aquaculture, it is highly vulnerable to infectious diseases caused by pathogenic bacteria such as Aeromonas hydrophila (Hayatgheib et al. 2020), resulting in great economic losses for fish farmers. Aeromonas sp. infection can decrease the functions of the immune system (Souza et al. 2020), and motile Aeromonas septicemia, also known as red spot disease or hemorrhage disease, leads to a mortality of up to 80% in striped catfish (Dung et al. 2008). Although antibiotics can be used for growth promotion and to treat A. hydrophila infections (Dawood et al. 2018), they increase the risk of selection of resistant pathogens (Souza et al. 2018), contamination of the environment (e.g., recirculating aquaculture system), and the death of bacteria beneficial to aquaculture (Felix e Silva et al. 2022). This reduces consumer acceptance of striped catfish aquaculture and discredits their nutritional benefits (Hasan et al. 2021). Therefore, it is necessary to use dietary supplements that promote greater performance and strengthen the health of fish, with a consequent contribution to the food safety of consumers.
Plant extracts have been highlighted among the supplements to be used as additives in fish diets because they are a safe food derived from eco-friendly farming practices (Morante et al. 2021; Souza et al. 2021) and a natural alternative to synthetic drugs (Yilmaz 2019). In addition, some plants have potential bioactive compounds such as polysaccharides (carbohydrates), tannins, flavonoids, and organic acids (Pan et al. 2011) that are closely linked to factors related to growth and metabolism in fish (Zhu 2020). The beneficial effects of plant-derived products on the development, survival, and health of striped catfish have been reported from previous studies (Güroy et al. 2014; Labh et al. 2017; Nhu et al. 2019; 2020; Hasan et al. 2021; Maiti et al. 2021; Patel et al. 2022). The mode of action of these herbs is usually the enhancement of the immune response through the elevation of immune parameters and control of infectious diseases by mitigating many side effects involving the synthesis of antimicrobials (Punitha et al. 2008).
Citrus genus plants are grown in over 140 tropical and subtropical countries, producing more than 140 million tons per hectare (FAO 2021). Lemon (Citrus limon) extract (hereinafter CLE) is rich in polysaccharides (pectin), flavonoids, essential oils, vitamins, and minerals (González-Molina et al. 2010). Citrus pectin has already demonstrated prebiotic potential (Ho et al. 2017) and growth promotion in fish (Hosseini et al. 2020), as well as antibacterial activity against Lactobacillus paracasei and Bifidobacterium bifidum (Zhang et al. 2017). Flavonoids play a role in growth promotion (Samavat et al. 2019; Morante et al. 2021; Souza et al. 2021) and antioxidant action in fish (Nazeri et al. 2017). The antibacterial activities of Citrus are also associated with flavonoid contents (Negi and Jayaprakasha 2001; Samavat et al. 2019). Polysaccharides have prebiotic properties, increasing nutrient digestibility, absorption, and assimilation capacity in fish through improved gastrointestinal morphology or digestive systems (Heidarieh et al. 2013; Gabriel and González-Redondo 2021). In general, Citrus are biologically active in improving fish health through antimicrobial, antioxidant, physiological, metabolic, immunological, and intestinal benefits (Baba et al. 2016; Beltrán et al. 2017; Ngugi et al. 2017; Rahman et al. 2019; Samavat et al. 2019; Kesbiç et al. 2020; Harikrishnan et al. 2020; Chekani et al. 2021).
Although lemons have several bioactive compounds, there is no study on the effect of dietary CLE on striped catfish. Hence, in this study, different concentrations of CLE were incorporated into the diet fed to striped catfish juveniles to investigate its effects on growth performance, metabolism, hematology, immunology, and resistance to A. hydrophila infection.
Materials and methods
Location, fish, and experimental conditions
Striped catfish juveniles were supplied by a commercial fish farm in Juazeiro do Norte, CE, Brazil. The fish were transported to the aquaculture laboratory at Universidade Federal do Vale do São Francisco (UNIVASF). During acclimatization, the fish were fed extruded commercial feed containing 40% crude protein and 3000 kcal kg−1 of digestible energy (GUABI, P-40, 2 mm) six times a day (07:00, 09:00, 11:00, 13:00, 15:00, and 17:00 h) at a rate of 20% of the initial biomass. The experiment lasted 90 days, followed by 8 days of bacterial infection by A. hydrophila.
After two weeks of acclimatization, 234 fish (1.74 ± 0.05 g) were distributed in a completely randomized experimental design with six treatments and three replicates in 1000-L fiberglass tanks (n = 13 fish per tank) in a recirculating aquaculture system, with constant aeration, physical and biological filters, water heated to 28.0 °C (Full Gauge, model Tic-17rgti, Canoas, Brazil), and a natural photoperiod (about 12 L:12 D).
The water quality parameters remained stable during the experiment. The water quality parameters for dissolved oxygen (5.90 ± 0.40 mg O2 L−1) and water temperature (28.0 ± 0.30 °C) were monitored with the aid of an oximeter (Pol-60, Politerm®, São Paulo, Brazil), and pH (6.80 ± 0.30) was monitored using a pH meter (HI 98,130, Hanna®, Barueri, Brazil) every day. Alkalinity (50.00 ± 0.01 mg CaCO3 L−1), non-ionized ammonia (0.22 ± 0.08 mg NH3 L−1), and nitrite (0.05 ± 0.01 mg N-NO2 L−1) were monitored by kit (Alfatecnoquímica, Florianópolis, Brazil) twice a week. The tanks were cleaned daily with a siphon to remove excess feces and feed residues.
Composition of Citrus limon extract
The chemical characterization of CLE was performed by nuclear magnetic resonance (NMR) analysis. For this, 1D and 2D NMR data were acquired at 298 K in DMSO-d6 on a Bruker AVANCE III 400 NMR spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) operating at 9.4 T, observing 1H and 13C at 400 and 100 MHz, respectively. The NMR spectrometer was equipped with a 5-mm direct detection probe with a z-gradient. One-bond (1H-13C HSQC) and long-range (1H-1H COSY, 1H-1H TOCSY, 1H-13C HMBC) NMR correlation experiments were optimized for average coupling constants 1 J(C,H) and LRJ(C,H) of 140 and 8 Hz, respectively. All 1H and 13C NMR chemical shifts (δ) were given in ppm related to the TMS signal at 0.00 as an internal reference and the coupling constants (J) in Hz.
A stock solution (1 mg mL–1) of CLE was prepared in methanol. A 5-µL aliquot of the stock solution was further diluted to 5 µg mL–1, and the resulting solution was analyzed by direct infusion in the mass spectrometer (Bruker Daltonics, Germany). All mass spectra were recorded in continuous monitoring mode using TSQ Quantum Access equipment with an Atmospheric pressure chemical ionization (APCI) source and operating in positive and negative acquisition mode for mass spectrometry (MS) and MS/MS analyses. The spectra were obtained from the average of at least ten acquired spectra. The samples were infused into the APCI source through the equipment's syringe pump (10 μL min–1). The MS/MS spectra were obtained from tap-plying energy from 25 to 35 eV.
Performance assay and diet preparation
The experimental diets were supplemented with CLE (Nor-Spice AB®, Nor-Feed, Beaucozé, France; organic production certificate CE n°834/2007 & CE n°889/2008 by FR-BIO 10). Six isoproteic and isocaloric diets (0.0 (control), 0.2, 0.4, 0.8, 1.6, and 3.2 g CLE kg diet–1 g CLE kg diet–1) were formulated (Table 1).
CLE was added along with the other ingredients. The ingredients were ground with a hammer grinder in 1 mm sieves, mixed, and processed to produce the experimental diets. Diet extrusion was performed by wetting the mixture with water (30%) in a commercial extruder (Inbramaq, São Paulo, Brazil) using a 5-mm die plate. Subsequently, the pellets were extruded at 90 °C by 2 s and dehydrated in a forced-air recycling system oven for 24 h at 55 °C. The pellets were refrigerated (− 20 °C) in glass containers with hermetically sealed caps until use.
Growth performance variables
The fish fasted for 24 h before growth performance analysis and sample collection. The weight (g) of striped catfish from all experimental units was measured to calculate growth performance (day 90), except for hepatosomatic index and viscerosomatic index, where three fish per treatment were randomly sampled, removed from the tanks, and anesthetized with benzocaine (30 mg L−1) for tissue collection. The production variables were:
-
Weight gain (WG, g) = final body weight (g) − initial body weight (g);
-
Specific growth rate (SGR, % per day–1) = 100 × (Ln final weight (g) − Ln initial weight (g))/time (days);
-
Feed conversion ratio (FCR) = feed intake (g) / weight gain (g);
-
Hepatosomatic index (HSI, %) = 100 x (liver weight (g)/body weight (g);
-
Viscerosomatic index (VSI, %) = 100 x (viscera weight (g)/fish weight (g));
-
Survival (%) = (final fish number / initial fish number) × 100.
Sample collection
At the end of the experimental period (90 days), the same three fish per treatment used for HSI and VSI analyses were used for blood collection, and 2 mL of blood was collected from each fish via a venocaudal puncture using a sterile syringe containing ethylenediaminetetraacetic acid (EDTA). Two blood aliquots were collected. The first aliquot (1 mL) was used for hematological analyses. The second aliquot (1 mL) was centrifuged at 3000 × g at 4 °C for 10 min (UniCen M, Helolab) to separate the plasma, and the samples were stored at − 20 °C for biochemical determinations.
Hematological and plasma analyses
The blood smears were made immediately after blood collection for the erythrocyte analysis. The erythrocytes were counted in a Neubauer counting chamber (1 × 106 μL–1) using a microscope at 400 × magnification. The hemoglobin concentration was determined by the cyanmethemoglobin method. Hematocrit was determined in duplicate using heparinized capillary tubes, filled to two-thirds of its total volume, and centrifuged at 12,000 × g for 5 min. The mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were calculated according to the following formulae:
Blood smears were prepared and panchromatically stained with May Grünwald-Giemsa-Wright for differential leukocyte counts. Two hundred cells of bloodstaining were counted to perform the differential count of leucocytes, defining the percentage of each component of interest (Tavares-Dias et al. 2007). These blood extensions were also used to determine the number of total leukocytes and thrombocytes. Total leucocytes and thrombocyte count were performed considering the total erythrocyte amount as obtained by the Neubauer chamber according to the following formulae:
The total thrombocyte and leukocyte count and the differential leukocytes, lymphocytes, neutrophils, monocytes, basophils, and eosinophils were performed by staining air-dried blood smears with May–Grünwald–Giemsa–Wright (Rosenfeld 1947). An optical microscope performed the observations at 100 × magnification.
To determine respiratory burst activity, 100 μL of blood was added to 100 μL of 0.2% nitro blue tetrazolium solution (Sigma, St. Louis, MO, USA). The final solution was homogenized and then incubated for 30 min at 25 °C. After incubation, we performed a second homogenization. Then, 50 μL from the solution was added to 1 ml of N, N-dimethyl formamide (Sigma). This solution was then homogenized and centrifuged at 3000 × g for 5 min. The optical density of supernatant was determined on a spectrophotometer (Biospectro SP-220, Curitiba, Brazil) at 540 nm (Biller-Takahashi et al. 2013).
A semi-automated biochemical analyzer (Doles®, model D250, Goiânia, GO, Brazil) was used for the plasma biochemical analyses. The plasma obtained was used to determine glucose (mg dL−1), cholesterol (mg dL−1), triglycerides (mg dL−1), aspartate aminotransferase (AST) (U L−1), total proteins (g dL−1), and albumin (g dL−1) (Labtest®; Lagoa Santa, MG, Brazil) by the colorimetric enzymatic method using commercial kits; measurements were made with a spectrophotometer (Biospectro SP-220).
Aeromonas hydrophila challenge
Aeromonas hydrophila isolated from the bacterial collection of the Laboratory of Microbiology and Animal Immunology of UNIVASF were obtained as described by Felix e Silva et al. (2022). The A. hydrophila strain (IF2) used in the current study was determined by 16S ribosomal DNA (rDNA) polymerase chain reaction (PCR) and sequencing previously performed by Freire et al. (2019).
The lethal dose for 50% of the individuals (LD50) was determined by testing different A. hydrophila concentrations (106, 107, and 108 colony forming units (CFU) mL–1). The bacterial inoculum was diluted in sterile saline solution (0.85 g per 100 mL) at 1 × 108 CFU mL–1. Mortality caused by A. hydrophila was observed and recorded in each group every 12 h for eight additional days. Then, on day 91 of the experiment, 0.2 mL of A. hydrophila solution was inoculated intramuscularly in the laterodorsal right side of each fish in the experimental groups (n = 10 per tank).
Before the A. hydrophila challenge, all fish were healthy and without apparent signs of clinical symptoms of the disease. After this challenge, the fish were maintained under the same experimental conditions of feed management and water quality. Blood collection and hematological and plasmatic analysis were performed on all surviving fish on day 98, as detailed in item 2.6.
Statistical analysis
The data and results are expressed as the mean ± standard error of the mean. We analyzed the normality of the residuals of the data (Shapiro–Wilk at a at 0.05) and the homogeneity of variances (Levene at a at 0.05). The data showed homogeneous variances and were compared using a one-way analysis of variance (ANOVA) followed by the Tukey post hoc test (p < 0.05). Since the treatments were quantitative independent variables with graded levels of CLE in the diet, significant results (p < 0.05) were compared using orthogonal polynomial contrasts. All values were used to determine the linear or quadratic effects of the different treatments tested. The best model was based on the p-value and R2 value. The data of hematological and plasma analyses after the A. hydrophila challenge that did not show homogeneous variances were analyzed using a Mann–Whitney non-parametric test (p < 0.05) and did not differ between treatments.
Results
Composition of Citrus limon extract
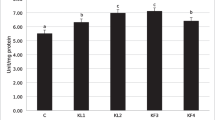
Without needing previous isolation, the nuclear magnetic resonance spectroscopic technique identifies metabolites in complex mixtures, like plant extracts. Using this technique, we can observe signals of chemical shifts characteristic of the presence of primary and secondary metabolites. The 1H NMR spectrum of the CLE revealed signals in all spectral regions (Fig. 1). Expressive signals in the aromatic region at 5.50–7.50 ppm may be associated with the presence of phenolic compounds (mainly flavonoids). On the other hand, the existence of signals in the region between 3.00 and 5.50 ppm was attributed to the presence of carbohydrates (polysaccharides), which were the major chemical constituents in the extract due to the high intensity of the signals (Fig. 1). This information is confirmed by the observation of 13C NMR spectra (Figs. 1–2S, supplementary information) where were observed the presence of less intense signals in the region of 170–100 ppm, and more intense signals in the region of 60–100 ppm, which is characteristic of the presence of carbohydrates.
Two-dimensional NMR experiments (1H-1H COSY, 1H-1H TOCSY, 1H-13C HSQC, and 1H-13C HMBC) (Figs. 3S–6S, supplementary information) were performed; however, there was extensive overlap of signals in all spectral regions, making the identification of specific metabolites in the extract complex. The results presented in the NMR analysis are in accordance with the results obtained by mass spectrometry in the negative (Fig. 2A) and positive (Fig. 2B) modes. In Fig. 2A, the presence of sugars could be observed by the ions at m/z 341 (maltose), 179 (glucose), and 161 (which is a result of the loss of water from the glucose molecule).
Growth performance
Fish supplemented with 0.4 g CLE kg diet–1 showed final weight, weight gain, and SGR significantly higher than fish supplemented with other diets (except for 0.2 g CLE kg–1) (p < 0.05). For these same variables, the control group presented values significantly lower than treatments with content between 0.2 and 1.6 g CLE kg–1 (p < 0.05). The SGR for the control group was significantly lower than the fish fed 3.2 g CLE kg–1 (p < 0.05). In addition, for final weight and weight gain, fish fed 0.2 g CLE kg–1 showed values significantly higher than fish fed 1.6 and 3.2 g CLE kg–1 (p < 0.05). The FCR was significantly higher in the control group compared to other treatments and significantly lower in the treatments 0.2 and 0.4 g CLE kg diet–1 compared to treatments containing CLE between 1.6 and 3.2 g kg diet–1 (p < 0.05) (Table 2).
Linear regression analysis showed that the dietary increase in CLE caused a proportional decrease in HSI (y = 2.919 – (0.146x); R2 = 0.10). Initial weight, initial length, HSI, and VSI did not differ between treatments (p > 0.05). There was no fish mortality before the A. hydrophila challenge (Table 2).
Biochemical, hematological, and immune analysis before the A. hydrophila challenge
Fish supplemented with 3.2 g CLE kg diet–1 had significantly higher total thrombocyte, total leukocyte, lymphocyte, and monocyte values than fish supplemented with other diets (except 0.2 g CLE kg–1 for total leukocytes and lymphocytes and 1.6 g CLE kg–1 for lymphocytes and monocytes) (p < 0.05) (Table 3).
Linear regression analysis showed that the dietary increase in CLE caused a proportional increase in plasma albumin levels (y = 2.711 + (0.161x); R2 = 0.15), respiratory burst (y = 0.246 + (0.0136x); R2 = 0.09), total leukocytes (y = 29.173 + (8.963x); R2 = 0.24), and lymphocytes values (y = 9.260 + (3.796x); R2 = 0.21). According to the quadratic regression, treatments containing between 0.2 and 1.6 g CLE kg diet–1 showed lower and higher values for erythrocytes (y = 3.403—(1.222x) + (0.422x2); R2 = 0.20) and MCV (y = 121.957 + (56.486x)—(19.911x2); R2 = 0.20), respectively. The quadratic regression analysis also verified that fish supplemented with 3.2 CLE kg diet–1 had the highest number of total thrombocytes (y = 96.662—(58.523x) + (27.272x2); R2 = 0.51) and monocytes (y = 1.340 + (0.253x) + (0.161x2); R2 = 0.39) (Table 3).
Plasma AST, glucose, triglycerides, cholesterol, total proteins levels, hematocrit, hemoglobin, MCH, MCHC, and neutrophils values did not show a regression nor a significant difference among the treatments. Basophils and eosinophils were not found (Table 3).
Biochemical, hematological, and immune analysis after the A. hydrophila challenge
There was a linear effect as the concentration of CLE in the diet increased, with a reduction in plasma total proteins (y = 0.156 + (0.0291x); R2 = 0.37) and number of neutrophils (y = 34,089.475 + (16,537.470x); R2 = 0.26). Plasma AST and glucose levels, respiratory burst, hematocrit, hemoglobin, erythrocyte, MCV, MCH, MCHC, thrombocyte, leukocyte, lymphocyte, and monocyte values did not show a regression, nor a significant difference among the treatments. Basophils and eosinophils were not found (Table 4).
Discussion
Bioactive ingredients in plant extracts are very complex, and the exact mechanisms by which phytochemicals improve growth response in fish are yet to be elucidated. We detected flavonoids and polysaccharides in the composition of CLE that must have contributed to fish development (González-Molina et al. 2010) in the present study. In addition, CLE could promote growth and stimulate juveniles’ appetites as it contains these bioactive compounds. In general, flavonoids and polysaccharides are widely known for their beneficial influences on overall performance and feeding efficiency (Ahmadifar et al. 2021; Morante et al. 2021; Souza et al. 2021). Phytochemicals and their metabolic products also provide health benefits such as improved intestinal morphometry (with consequent improvement in feed digestion and nutrient absorption) (Mohamed et al. 2021) and production of selective growth factors and fermentation substrates for beneficial intestinal microbiota while acting as selective inhibitors of harmful intestinal bacteria, thereby exerting prebiotic-like effects and contributing to growth performance in fish (Zheng et al. 2009; Van Doan et al. 2019). As the present study did not examine intestinal physiology, future studies are suggested to investigate how the plant additives may influence the gut microbiome and morphology that could link to growth performance in fish.
Polyphenolic compounds have also been reported to promote DNA, RNA, and protein synthesis, stimulate growth hormone and insulin-like growth factor 1 production and function, besides actions on the hypothalamus-pituitary–gonad axis and other anabolic effects in fish, resulting in growth increases (Citarasu 2010; Chakraborty et al. 2014; Mohamed et al. 2021). Flavonoids can decrease animal stress and enhance hepatopancreatic function (Li et al. 2019). Dietary carbohydrate utilization by fish is varied and appears to be related to the complexity of carbohydrates (Ighwela et al. 2015). They can result in a protein-sparing effect, directing the use of proteins for fish growth and reducing the elimination of nitrogenous residues, besides being their main energy source (Felix e Silva et al. 2020). In the current study, glucose and maltose were the most important carbohydrates. Among the important nutrients that provide energy to maintain the cell’s vital processes, glucose stands out as a source of carbohydrates (De Souza et al. 2021). Maltose is the least common disaccharide in nature, and when incorporated into the diet, it was efficiently utilized and contributed to body mass in Nile tilapia (Ighwela et al. 2015). Therefore, the bioactive compounds present in CLE may have contributed to improving striped catfish growth, where the 0.4 g CLE kg diet–1 treatment had the best results.
Interestingly, the effect of CLE was influenced by its concentration in the diet. Concentrations between 0.2 and 1.6 g CLE kg diet–1 demonstrated better growth results than the control group (not supplemented with CLE). Disregarding the control group, the maximum and minimum benefits of CLE for zootechnical parameters were found with 0.4 and 3.2 g kg diet–1, respectively. Only the treatment with the highest concentration of CLE (3.2 g kg diet–1) did not differ from the control group in terms of these parameters, except for FCR, whose values indicated better feed utilization. The FCR demonstrates that juveniles from the control group use food energy less effectively than the other groups for growth. In addition, adequate levels of dietary CLE (mainly 0.2 and 0.4 g kg diet–1) increased the conversion of food into muscle protein (Marchão et al. 2022). In general, the treatment 0.4 g CLE kg diet–1 showed greater stability in assessing physiological (erythrocytes and MCV) and immunological parameters (total leukocytes and lymphocytes) compared to the treatment 0.2 g CLE kg diet–1.
Differences in the growth response to a phytochemical may be due to differences in fish species’ abilities to metabolize a particular diet substance (Chakraborty et al. 2014). As seen in our results, excessive concentrations of the herbal extract can harm fish development. This could have happened because an increase in the concentration of CLE in diets can trigger anti-lipogenic and diuretic effects (eliminate excess fluid) that limit fish growth (Beltrán et al. 2017; Rahman et al. 2019); therefore, a concentration of 3.2 g CLE kg diet–1 should be avoided in fish supplementation.
In line with our results, previous studies on adding lemon to fish diets also promoted growth in different species. This was verified with diets supplemented with dehydrated lemon peel powder for gilthead seabream (Sparus aurata) (1.5–3.0%) (Beltrán et al. 2017) and rainbow trout (Oncorhynchus mykiss) (0.5–2.5%) (Chekani et al. 2021) and with essential oil extract from lemon peels for Nile tilapia (Oreochromis niloticus) (0.75–1.0%) (Mohamed et al. 2021) and ningu (Labeo victorianus) (10.0–80.0 g kg−1) (Ngugi et al. 2017). Diets enriched with dried lemon peels for rohu (Labeo rohita) (1.0–5.0 g kg−1) (Harikrishnan et al. 2020) and with lemon pomace powder for carp (Cyprinus carp) (Laein et al. 2021) also improved weight gain and feed conversion ratio. Limonene from Citrus peels (200–600 ppm) in Nile tilapia diets increased growth and gene expression levels of insulin growth factor 1 (Aanyu et al. 2018). In contrast, there was no weight gain in diets supplemented with lemon peels (1% and 2%) for Nile tilapia and African catfish (Clarias gariepinus) (Rahman et al. 2019). Therefore, the effects of diets with lemon compounds may vary according to the experimental conditions and species studied.
Improvements in health conditions can also lead to faster growth in fish. In the present study, phytochemicals, such as flavonoids and polysaccharides in the diet provided to the striped catfish, are considered health-promoting by their antioxidant activity (capable of scavenging hydroxyl radicals, superoxide anions, and lipid peroxyl radicals) and positive modulation of the cellular and tissue redox balance (Liu 2003), and their stimulation of immunity (specific and non-specific) by modulating the functions of the immune cells related to immunity expression genes, thereby increasing antibody production (Citarasu 2010; Chakraborty et al. 2014; Gabriel and González-Redondo 2021). Flavonoids also have vasodilatory, antibacterial, antiviral, antiallergic, anti-inflammatory, and antimutagenic actions (Chakraborty and Hancz 2011). In addition, polysaccharides are an important medicinal product, playing a major role in preventing and controlling infectious microbes in aquaculture (Wang et al. 2016) because they are prebiotic substances boosting immune responses. They are widely accepted as a nutritional component for regulating intestinal microbiota and health conditions in fish (Mohan et al. 2019). Polysaccharides are in the highest capacity for transporting bio information, and monosaccharides can connect at different points to form a vast range of diverged or straight structures (Sharon and Lis 1993). In this sense, the evaluation of variations in physiological parameters is a tool that can monitor fish health and nutrition, facilitating the identification of conditions that affect growth performance (Ngugi et al. 2017; Souza et al. 2021). Therefore, the benefits of CLE for improving growth in striped catfish should be evaluated together with our results for biochemical, hematological, and immunological parameters.
In the present study, dietary supplementation of CLE showed mixed effects on these parameters. On the one hand, it did not affect hematocrit, hemoglobin, and MCHC values. We believed this could indicate that the oxygen supplementation in the tissues was not influenced by the different treatments (Tavares-Dias et al. 2007). However, to verify that the oxygen transport at the tissue level remains the same, an analysis of the oxygen consumption rate should also have been performed, which was not done in the present study, and we suggest that it be evaluated in future studies. On the other hand, an increase in erythrocytes and MCV values was also observed in the fish that received dietary CLE. Erythrocytes transport hemoglobin in the blood and an increase in MCV indicates an increase in the size of red blood cells (Presa et al. 2022). Consequently, oxygen in the tissues must have been increased in the fish fed diets supplemented with CLE, which is in line with our results for zootechnical parameters. In addition, body composition variables (HSI and VSI) and plasma AST, total proteins, triglycerides, cholesterol, and glucose levels were not influenced by diets supplemented with CLE, demonstrating that there was no change in the use of lipid, amino acid, and glycogen reserves, as well as general energetic status by the fish (e.g., HSI and VSI) (Melo et al. 2006; Souza et al. 2020; Morante et al. 2021).
Similarly, no differences were found in plasma glucose levels of African catfish, in hematological parameters of rainbow trout fed dehydrated lemon peel powder (Rahman et al. 2019; Chekani et al. 2021), nor in plasma AST, cholesterol, and total protein levels of carp fed lemon pomace powder (Laein et al. 2021). In contrast, plasma glucose levels were increased in gilthead seabream and Nile tilapia fed diets supplemented with dehydrated lemon peel powder (Beltrán et al. 2017; Rahman et al. 2019). An increase in plasma total protein levels was found in rohu receiving dried lemon peels in their diet (Harikrishnan et al. 2020). Dried lemon peels in the diet for ningu caused an increase in erythrocyte, hematocrit, hemoglobin, MCV, MCHC, and plasma total protein levels and a decrease in plasma glucose, cholesterol, and triglyceride levels (Ngugi et al. 2017). The essential oil extracted from lemon peels added to the diet of Nile tilapia increased plasma total protein levels and decreased plasma AST, cholesterol, triglycerides, and glucose levels (Mohamed et al. 2021). The responses found in our study and previous studies may even differ in some hematological and biochemical parameters. So, we believed that the greater stability of these parameters in fish fed with 0.2 to 1.6 g CLE kg diet–1 is related to better physiological homeostasis, which contributed to the growth gain observed in the juveniles of this study.
The fish immune response parameters analyzed in our study (e.g., respiratory burst, total leukocyte count, and differential leukocyte count) are commonly evaluated by other researchers (Ngugi et al. 2017; Harikrishnan et al. 2020; Mohamed et al. 2021). In the current study, before bacterial infection, CLE increased plasma albumin levels, the number of total thrombocytes, total leukocytes, lymphocytes, and monocytes, and respiratory burst, indicating an improvement of the immune defense response in juveniles. Leukocyte levels constitute the first line of defense against any invader. The increased leukocyte count results in a high proportion of monocytes and lymphocytes, indicating a primary innate immune response against diseases (Harikrishnan et al. 2020). Respiratory burst activity is associated with the cytokine release and inflammatory response (Ngugi et al. 2017). Thrombocytes connect the innate immune system with the adjustment system and involve great potential for phagocytosis (Oliveira et al. 2019). Plasma albumin levels and the previously mentioned parameters are also indicators of immunity status in fish (Laein et al. 2021). Therefore, the elevated immunological parameters in striped catfish fed CLE may be due to the defense system’s enhancement, which also contributed to improving growth performance.
The benefits of using CLE in a fish diet can be attributed to its bioactive compounds acting in immune strengthening. Flavonoids and polysaccharides in herbal extracts are known to support the inhibition or suppression of the oxidation process (Kenari et al. 2014), promote immune function, and attenuate the inflammatory response (Wang et al. 2016; Gabriel and González-Redondo 2021), making them proficient candidates for the development of immunomodulators in aquaculture. Flavonoids such as those found in CLE may contribute to immune defenses and have antibacterial activity, including an aggregating effect on bacterial cells, which reduces the surface area of the population and limits oxygen consumption and nutrient absorption (Ahmad et al. 2015).
The increased values of these parameters point toward the improved immunological status of fish, as was previously determined with other diets with added C. limon. An improvement in humoral and cellular immunity and expression of some immune-related genes was verified in gilthead seabream fed dehydrated lemon peel (Beltrán et al. 2017). Increased plasma albumin and total leucocyte values, lysozyme activity, phagocytic activity, and other immunological parameters occurred in ningu and rohu fed dried lemon peels added to their diets and in Nile tilapia that received essential oil extracts from lemon peels in their diet (Ngugi et al. 2017; Harikrishnan et al. 2020; Mohamed et al. 2021). These alterations were similar to the diet of rainbow trout fed dehydrated lemon peel powder after crowding stress (Chekani et al. 2021). In addition, diets supplemented with other Citrus species have also shown promising results in promoting fish growth and immunity, as detailed in a recent review by Kesbiç et al. (2022). This reinforces the importance of Citrus as a food additive capable of improving fish development and health.
Our findings before the challenge with A. hydrophila indicate an increase in the defense mechanisms of juveniles against bacterial infections due to the increase in phagocytic activity (respiratory burst), thrombocytes, and leukocytes. Despite this, the different treatments of the present study did not influence the survival rate of fish after infection by A. hydrophila. Except for a reduction in plasma total proteins levels and number of neutrophils, the addition of CLE in the fish diet did not cause significant changes in the metabolic, physiological, and immune profiles of striped catfish after bacterial inoculation. Therefore, the benefits we found for growth promotion, immunity enhancement, and physiological homeostasis of striped catfish fed CLE before microbial challenge were insufficient to improve their survival after infection by A. hydrophila.
In contrast to our study, in Nile tilapia and African catfish supplemented with diets with dehydrated lemon peel, there was a decrease in mortality after infection by A. hydrophila (Rahman et al. 2019). Dried lemon peels in diets for ningu and rohu reduced mortality caused by A. hydrophila and Aeromonas sorbia, respectively (Ngugi et al. 2017; Harikrishnan et al. 2020). In addition, dietary supplementation with many plant polysaccharides reduced mortality in crucian carp (Carassius mmuneus) and grass carp (Ctenopharyngodon mmune) after a challenge with A. hydrophila (Kenari et al. 2014; Gou et al. 2018). Another study (in vitro) verified that lemon peel essential oil induced a more potent antibacterial effect in six fish pathogens, including A. hydrophila (Öntaş et al. 2016). In our study, although CLE improved the immunity of striped catfish, it did not increase their survival and, in general, did not change the biochemical, hematological, and immunological parameters after the antimicrobial challenge.
Conclusion
CLE presented flavonoids and polysaccharides as the main classes of chemical constituents, influencing the beneficial effects of CLE after the 90-day supplementation period on fish growth and health. Including 0.4 g CLE kg diet–1 in fish feed is recommended for improving the growth performance and immune resistance of striped catfish in intensive culture. Despite this, diets with CLE were not effective in improving the survival of juveniles after infection by A. hydrophila.
References
Aanyu M, Betancor MB, Monroig O (2018) Effects of dietary limonene and thymol on the growth and nutritional physiology of Nile tilapia (Oreochromis niloticus). Aquaculture 488:217–226. https://doi.org/10.1016/j.aquaculture.2018.01.036
Ahmad A, Kaleem M, Ahmed Z, Shafiq H (2015) Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—a review. Food Res Int 77:221–235. https://doi.org/10.1016/j.foodres.2015.06.021
Ahmadifar E, Yousefi M, Karimi M, Raieni RF, Dadar M, Yilmaz S, Dawood MAO, Abdel-Latif HMR (2021) Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: an overview. Rev Fish Sci Aquac 29:478–511. https://doi.org/10.1080/23308249.2020.1818689
Baba E, Acar Ü, Öntaş C, Kesbiç OS, Yilmaz S (2016) Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 465:13–18. https://doi.org/10.1016/j.aquaculture.2016.08.023
Beltrán JMG, Espinosa C, Guardiola FA, Esteban MA (2017) Dietary dehydrated lemon peel improves the immune but not the antioxidant status of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 64:426–436. https://doi.org/10.1016/j.fsi.2017.03.042
Biller-Takahashi JD, Takahashi LS, Saita MV, Gimbo RY, Urbinati EC (2013) Leukocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Braz J Biol 73:425–429. https://doi.org/10.1590/S1519-69842013000200026
Chakraborty SB, Hancz C (2011) Application of phytochemicals as immunostimulant, antipathogenic and antistress agents in finfish culture. Rev Aquac 3:103–119. https://doi.org/10.1111/j.1753-5131.2011.01048.x
Chakraborty SB, Horn P, Hancz C (2014) Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev Aquac 6:1–19. https://doi.org/10.1111/raq.12021
Chekani R, Akrami R, Ghiasvand Z, Hossein C, Jorjani S (2021) Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 539:736597. https://doi.org/10.1016/j.aquaculture.2021.736597
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18:403–414. https://doi.org/10.1007/s10499-009-9253-7
Dawood MAO, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10:950–974. https://doi.org/10.1111/RAQ.12209
De Souza AM, Copatti CE, Campeche DFB, Melo FVST, Melo JFB (2021) Glucose tolerance in six fish species reared in Brazil: Differences between carnivorous and omnivorous. An Acad Bras Cienc 93:e20201541. https://doi.org/10.1590/0001-3765202120201541
Dung TT, Ngoc NTN, Thinh NQT, Thy DTM, Tuan NA, Shinn A, Crumlish M (2008) Common diseases of pangasius catfish farmed in Viet Nam. GAA 11:77–78
FAO (2021) Citrus fruit statistical compendium 2020. Rome. Available from https://www.fao.org/3/cb6492en/cb6492en.pdf
Felix e Silva A, Copatti CE, de Oliveira EP, Bonfá HC, de Melo FVST, Camargo ACS, Melo JFB (2020) Effects of whole banana meal inclusion as replacement for corn meal on digestibility, growth performance, haematological and biochemical variables in practical diets for tambaqui juveniles (Colossomamacropomum). Aquac. Rep 17:100307. https://doi.org/10.1016/j.aqrep.2020.100307
Felix e Silva A, Pires IC, Da Costa MM, Melo JFB, Lorenzo VP, Melo FVST, Copatti CE (2022) Antibacterial and antibiofilm activities and synergism with florfenicol from the essential oils of Lippiasidoides and Cymbopogoncitratus against Aeromonashydrophila. J Appl Microbiol 132:1802–1812. https://doi.org/10.1111/jam.15336
Freire NB, Magalhães TC, Soares RAN, Da Costa MM, Gouveia GV (2019) Nutritional interference for phenotypic biofilm quantification in Aeromonas spp. isolates containing the fla gene. Microb Pathogen 127:198–201. https://doi.org/10.1016/j.micpath.2018.11.044
Gabriel NN, González-Redondo P (2021) Review on the progress in the role of herbal extracts in tilapia culture. Cogent Food Agric 5:1619651. https://doi.org/10.1080/23311932.2019.1619651
González-Molina E, Domínguez-Perles R, Moreno DA, García-Viguera C (2010) Natural bioactive compounds of Citrus limon for food and health. J Pharm Biomed Anal 51:327–345. https://doi.org/10.1016/J.JPBA.2009.07.027
Gou C, Wang J, Wang Y, Dong W, Shan X, Lou Y (2018) Hericium caput-medusae (Bull.:Fr.) Pers. Polysaccharide enhance innate immune response, immune-related genes expression and disease resistance against Aeromonas hydrophila in grass carp (Ctenopharyngodon mmune). Fish Shellfish Immunol 72:604–610. https://doi.org/10.1016/j.fsi.2017.11.027
Güroy B, Mantoğlu S, Kayalı S, Şahin İ (2014) Effect of dietary Yucca schidigera extract on growth, total ammonia–nitrogen excretion and haematological parameters of juvenile striped catfish Pangasianodon hypophthalmus. Aquac Res 45:647–654. https://doi.org/10.1111/are.12001
Harikrishnan R, Thamizharasan S, Devi G, Van Doan H, Kumar TTA, Hoseinifar SH, Balasundaram C (2020) Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates mmune-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol 106:675–684. https://doi.org/10.1016/j.fsi.2020.07.040
Hasan NA, Haque MM, Bashar A, Hasan MT, Faruk MAR, Ahmed GU (2021) Effects of dietary Papaveraceae extract on growth, feeding response, nutritional quality and serum biochemical indices of striped catfish (Pangasianodonhypophthalmus). Aquac Rep 21:100793. https://doi.org/10.1016/j.aqrep.2021.100793
Hayatgheib N, Moreau E, Calvez S, Lepelletier D, Pouliquen H (2020) A review of functional feeds and the control of Aeromonas infections in freshwater fish. Aquac Int 28:1083–1123. https://doi.org/10.1007/S10499-020-00514-3
Heidarieh M, Mirvaghefi AR, Sepahi A, Sheikhzadeh N, Ali A, Akbari M, Shahbazfar AA (2013) Effects of dietary Aloe vera on growth performance, skin and gastrointestine morphology in rainbow trout (Oncorhynchus mykiss). Turkish J Fish Aquat Sci 13:881–896. https://doi.org/10.4194/1303-2712-v13_2_20
Ho YY, Lin CM, Wu MC (2017) Evaluation of the prebiotic effects of citrus pectin hydrolysate. J Food Drug Anal 25:550–558. https://doi.org/10.1016/J.JFDA.2016.11.014
Hoque MS, Haque MM, Nielsen M, Badiuzzaman RMT, Hossain MI, Mahmud S, Mandal AK, Frederiksen M, Larsen EP (2021) Prospects and challenges of yellow flesh pangasius in international markets: secondary and primary evidence from Bangladesh. Heliyon 7:e08060. https://doi.org/10.1016/j.heliyon.2021.e08060
Hosseini SM, Hoseinifar SH, Mazandarani M, Paknejad H, Van Doan H, El-Haroun ER (2020) The potential benefits of orange peels derived pectin on serum and skin mucus immune parameters, antioxidant defence and growth performance in common carp (Cyprinus carpio). Fish Shellfish Immunol 103:17–22. https://doi.org/10.1016/J.FSI.2020.04.019
Ighwela KA, Ahmad AB, Abol-Munafi AB (2015) Effect of dietary maltose on growth and feed utilization of Nile tilapia (O. niloticus) fingerlings. Res J Recent Sci 3:1–5
Kenari RE, Mohsenzadeh F, Amiri ZR (2014) Antioxidant activity and total phenolic compounds of dezful sesame cake extracts obtained by classical and ultrasound-assisted extraction methods. Food Sci Nutr 2:426–435. https://doi.org/10.1002/fsn3.110
Kesbiç OS, Acar Ü, Yilmaz S, Aydin OD (2020) Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem 46:103–110. https://doi.org/10.1007/s10695-019-00700-y
Kesbiç OS, Acar Ü, Mohammady EY, Salem SMR, Ragaza JA, El-Haroun E, Hassaan MS (2022) The beneficial effects of citrus peel waste and its extract on fish performance and health status: a review. Aquac Res 53:4217–4232. https://doi.org/10.1111/are.15945
Labh SN, Sahu NP, Sahoo S, Ratna S, Shakya BL, Kumar S (2017) Growth performance and immune response of silver striped catfish Pangasianodon hypophthalmus (Sauvage, 1878) fed with Lapsi Choerospondias axillaris (Roxburgh, 1832) during intensive aquaculture. Int J Fish Aquat Stud 5:188–202
Laein SS, Salari A, Shahsavani D, Baghshani H (2021) Effect of supplementation with lemon (Citrus limon) pomace powder on the growth performance and antioxidant responses in common carp (Cyprinus carpio). J Biol Environ Sci 15:47–54
Lefevre S, Huong DTT, Ha NTK, Wang T, Phuong NT, Bayley M (2011) A telemetry study of swimming depth and oxygen level in a Pangasius pond in the Mekong Delta. Aquaculture 315:410–413. https://doi.org/10.1016/j.aquaculture.2011.02.030
Li M, Zhu X, Tian J, Liu M, Wang G (2019) Dietary flavonoids from Allium mongolicum Regel promotes growth, improves immune, antioxidant status, immune-related signaling molecules and disease resistance in juvenile northern snakehead fish (Channa argus). Aquaculture 501:473–481. https://doi.org/10.1016/j.aquaculture.2018.12.011
Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78:517S-520S. https://doi.org/10.1093/ajcn/78.3.517S
Maiti S, Saha S, Jana P, Chowdhury A, Khatua S, Ghosh TK (2021) Effect of dietary Andrographis paniculata leaf extract on growth, immunity, and disease resistance against Aeromonas hydrophila in Pangasianodon hypophthalmus. J Appl Aquac. https://doi.org/10.1080/10454438.2021.1959861
Marchão RS, Ribeiro FB, Bomfim MAD, Pereira GA, Rocha AS, Vidal LVO, Copatti CE, Melo JFB (2022) Digestible threonine requirement in tambaqui (Colossoma macropomum) diets: growth, body deposition, haematology and metabolic variables. Aquac Res. https://doi.org/10.1111/are.16051
Melo JFB, Lundstedt LM, Metón I, Baanante IV, Moraes G (2006) Effects of dietary levels of protein on nitrogenous metabolism of Rhamdia quelen (Teleostei: Pimelodidae). Comp Biochem Physiol A 145:181–187. https://doi.org/10.1016/j.cbpa.2006.06.007
Mohamed RA, Yousef YM, El-Tras WF, Khalafallaa MM (2021) Dietary essential oil extract from sweet orange (Citrus sinensis) and bitter lemon (Citrus limon) peels improved Nile tilapia performance and health status. Aquac Res 52:1463–1479. https://doi.org/10.1111/are.15000
Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Seedevi P, Rajan DK (2019) Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: a review. Aquaculture 500250–263. https://doi.org/10.1016/j.aquaculture.2018.10.023
Morante VHP, Copatti CE, Souza ARL, da Costa MM, Braga LGT, Souza AM, de Melo FVST, Camargo ACS, Melo JFB (2021) Assessment the crude grape extract as feed additive for tambaqui (Colossoma macropomum), an omnivorous fish. Aquaculture 544:737068. https://doi.org/10.1016/j.aquaculture.2021.737068
Naylor RL, Hardy RW, Buschmann AH, Bush SR, Cao L, Klinger DH, Little DC, Lubchenco J, Shumway SE, Troell M (2021) A 20-year retrospective review of global aquaculture. Nature 591:551–563. https://doi.org/10.1038/s41586-021-03308-6
Nazeri S, Farhangi M, Modarres S (2017) The effect of different dietary inclusion levels of rutin (a flavonoid) on some liver enzyme activities and oxidative stress indices in rainbow trout, Oncorhynchus mykiss (Walbaum) exposed to oxytetracycline. Aquac Res 48:4356–4362. https://doi.org/10.1111/are.13257
Negi P, Jayaprakasha G (2001) Antibacterial activity of grapefruit (Citrus paradisi) peel extracts. Europ Food Res Technol 213:484–487. https://doi.org/10.1007/s002170100394
Ngugi CC, Oyoo-Okoth E, Muchiri M (2017) Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, haemato-immunological parameters and disease resistance in Juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquac Res 48:2253–2265. https://doi.org/10.1111/are.13062
Nhu TQ, Hang BTB, Bach LT, Hue BTB, Quetin-Leclercq J, Scippo M-L, Phuong NT, Kestemont P (2019) Plant extract-based diets differently modulate immune responses and resistance to bacterial infection in striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol 92:913–924. https://doi.org/10.1016/j.fsi.2019.07.025
Nhu TQ, Bich HBT, Cornet V, Oger M, Bach LT, Anh DNL, Thanh HDT, Quetin-Leclercq J, Scippo M-L, Phuong NT, Kestemont P (2020) Single or combined dietary supply of Psidium guajava and Phyllanthus amarus extracts differentially modulate immune responses and liver proteome in striped catfish (Pangasianodon hyphophthalmus). Front Immunol 11:797. https://doi.org/10.3389/fimmu.2020.00797
Oliveira CPB, Lemos CHP, Vidal LVO, Couto RD, Pereira DSP, Copatti CE (2019) Anaesthesia with eugenol in hybrid Amazon catfish (Pseudoplatystoma reticulatum × Leiarius marmoratus) handling: biochemical and haematological responses. Aquaculture 501:255–259. https://doi.org/10.1016/j.aquaculture.2018.11.046
Öntaş C, Baba E, Kaplaner E, Küçükaydin S, Öztürk M, Ercan MD (2016) Antibacterial activity of Citrus limon peel essential oil and Argania spinosa oil against fish pathogenic bacteria. Kafkas Univ Vet Fak Derg 22:741–749. https://doi.org/10.9775/kvfd.2016.15311
Pan SY, Chen SB, Dong HG, Yu ZL, Dong JC, Long ZX, Fong WF, Han YF, Ko KM (2011) New perspectives on chinese herbal medicine (zhong-yao) research and development. Evid Based Complement Alternat Med 2011:403709. https://doi.org/10.1093/ecam/neq056
Patel PP, Borichangar RV, Vanza JG (2022) Effect of garlic (Allium sativum) supplementation on growth, survival and body composition of striped catfish (Pangasianodon hypophthalmus, Sauvage, 1878). J Pharm Innov 11:642–652
Presa LS, Neves GC, Maltez LC, Sampaio LA, Monserrat JM, Copatti CE, Garcia L (2022) Acute and sub-lethal effects of nitrate on haematological and oxidative stress parameters of juvenile mullet (Mugil liza) in freshwater. Aquac Res 53:3346–3357. https://doi.org/10.1111/are.15842
Punitha SMJ, Babu MM, Sivaram V, Shankar VS, Dhas SA, Mahesh TC, Immanuel G, Citarasu T (2008) Immuno stimulating influence of herbal biomedicines on nonspecific immunity in Grouper Epinephelus tauvina juvenile against Vibrio harveyi infection. Aquac Int 16:511–523. https://doi.org/10.1007/s10499-007-9162-6
Rahman ANA, ElHady M, Shalaby SI (2019) Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 505:92–97. https://doi.org/10.1016/j.aquaculture.2019.02.051
Rosenfeld G (1947) Corante pancrômico para hematologia e citologia clínica. Nova combinação dos componentes do May-Grünwald e do Giemsa num só corante de emprego rápido. Mem Inst Butantan 20:329–334
Samavat Z, Shamsaie M, Jamili MS, Soltani M, Shekarabi SPH (2019) Determination of grapefruit (Citrus paradisi) peel extract bio-active substances and its application in Caspian white fish (Rutilus frisii kutum) diet: Growth, haemato-biochemical parameters and intestinal morphology. Aquac Res 50:2496–2504. https://doi.org/10.1111/are.14204
Sharon N, Lis H (1993) Carbohydrates in cell recognition. Sci Am 268:82–89. https://doi.org/10.1038/scientificamerican0193-82
Souza EM, De Souza RC, Da Costa MM, Pinheiro CG, Heinzmann BM, Copatti CE (2018) Chemical composition and evaluation of the antimicrobial activity of two essential oils. Bol Inst Pesca 44:e321. https://doi.org/10.20950/1678-2305.2018.321
Souza EM, De Souza RC, Melo JFB, Da Costa MM, Souza SA, Souza AM, Copatti CE (2020) Cymbopogon flexuosus essential oil as an additive improves growth, biochemical and physiological responses and survival against Aeromonas hydrophila infection in Nile tilapia. An Acad Bras Ciênc 92:e20190140. https://doi.org/10.1590/0001-3765202020190140
Souza ARL, Copatti CE, Morante VHP, Da Costa MM, Braga LGT, Souza AM, Melo FVST, Camargo ACS, Melo JFB (2021) Crude extract from yellow yam (Dioscoreacayennensis) in in-vitro Lactobacillus spp. assessment, and as a growth promoter in tambaqui juveniles (Colossomamacropomum). J Appl Aquac. https://doi.org/10.1080/104544381976347
Tavares-Dias M, Barcellos JFM, Marcon JL, Menezes GC, Ono EA, Affonso EG (2007) Hematological and biochemical parameters for the pirarucu Arapaima gigas Schinz, 1822 (Osteoglossiformes, Arapaimatidae) in net cage culture. Electron J Ichthyol 2:61–68
Van Doan H, Hoseinifar SH, Sringarm K, Jaturasitha S, Yuangsoi B, Dawood MAO, Esteban MÁ, Ringø E, Faggio C (2019) Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish Immunol 93:428–435. https://doi.org/10.1016/j.fsi.2019.07.077
Wang E, Chen X, Wang K, Wang J, Chen D, Geng Y (2016) Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol 59:196–202. https://doi.org/10.1016/j.fsi.2016.10.039
Yilmaz S (2019) Effects of dietary caffeic acid supplement on antioxidant, immunological and liver gene expression responses, and resistance of Nile tilapia, Oreochromis niloticus to Aeromonas veronii. Fish Shellfish Immunol 86:384–392. https://doi.org/10.1016/j.fsi.2018.11.068
Zhang W, Zhao XJ, Jiang Y, Zhou Z (2017) Citrus pectin derived silver nanoparticles and their antibacterial activity. Inorg Nano-Metal Chem 47:15–20. https://doi.org/10.1080/15533174.2015.1137073
Zheng ZL, Tan JYW, Liu HY, Zhou XH, Xiang X, Wang KY (2009) Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 292:214–218. https://doi.org/10.1016/j.aquaculture.2009.04.025
Zhu F (2020) A review on the application of herbal medicines in the disease control of aquatic animals. Aquaculture 526:735422. https://doi.org/10.1016/j.aquaculture.2020.735422
Acknowledgements
The authors are grateful to CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the productivity research grant awarded to Copatti, C.E. (# 304329/2021-5), Almeida, J.R.G.S. (# 313235/2021-0), Tavares-Dias, M. (# 303013/2015-0), Costa, E.V (# 304577/2020-0) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) — Finance Code 001 — for a research grant provided for Macedo, J.S. The authors thank Nor-Feed (Beaucozé, France) for supporting this study.
Funding
This study was supported by research funds from the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
J.S.M.: carried out the experiments and biometric and biochemical analyses and contributed to the results and discussion. E.V.C., F.M.A.S., L.M.D., V.L.A.S., and J.R.G.S.A.: chemical composition analysis, figures, supplementary material, writing of M&M and results about chemical composition and collaboration on the discussion. M.T.D.: hematological and immunological analyzes, revision, and collaboration on the discussion. C.E.C.: statistical analysis, revision, writing, and final text. J.F.B.M.: conception and design, revision and collaboration on the discussion. All the authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The study was conducted under the rules of conduct for the use of animals in teaching and research and current legislation; This study was carried out according to the principles adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Committee on Ethics in the Use of Animals (CEUA) of the Universidade Federal do Vale do São Francisco (UNIVASF), Petrolina, Brazil, under the protocol number 0001/270121.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Macedo, J.d.S., Copatti, C.E., Costa, E.V. et al. Effects of Citrus limon extract on growth performance and immunity in striped catfish (Pangasius hypophthalmus). Aquacult Int 31, 719–738 (2023). https://doi.org/10.1007/s10499-022-00995-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00995-4