Abstract
The aim of the present study was to determine the chemical composition of Sambucus ebulus and Actinidia deliciosa ethanolic extracts as well as their in vitro antifungal activity on the mycelial growth of the water mold, Saprolegnia parasitica. The preliminary minimum inhibition concentration (MIC) and minimum lethal concentration (MLC) were determined by the HeMP method and finally, concentrations of each extract ranging from 1 to 10% were prepared by an agar dilution method to assess the in vitro antifungal activity, quantitatively. Both herbal extracts inhibited growth of Saprolegnia hyphae in vitro. Complete in vitro growth inhibition was found at a concentration of ≥5% for S. ebulus, whereas it was not observed even at 10% concentration of A. deliciosa. Based on gas chromatography coupled with mass spectrometry (GC/MS) analysis, the main constituents of S. ebulus include monophthalate (66.17%), fatty acids (26.47%), phytol (4.25%), and acetic acid (2.11%). Using colorimetric assays, A. deliciosa contained phenolic contents at 162 mg gallic acid (GAE)/g DW and flavonoid contents at 2.31 mg quercetin (QE)/g DW. In conclusion, the results of this study indicated that S. ebulus and A. deliciosa showed some antifungal activities against S. parasitica with formerly exhibiting stronger activity (p < 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The water mold, Saprolegnia parasitica, is one of the most important species in the genus Saprolegnia that caused saprolegniosis in fish (Paul et al. 2015). It can infect different fish developmental stages (Eissa et al. 2013; Sformo et al. 2017); however, it has been shown that there is a strong association of S. parasitica with the adult stage of the salmonid samples (Sandoval-Sierra et al. 2014). It can be considered that at least 10% of the annual economic loss in salmonid aquaculture is caused by saprolegniosis (Sandoval-Sierra et al. 2014). In order to control the disease, there is an essential need to develop strategies for the effective management of saprolegniosis (Magray et al. 2019).
For a long time, malachite green was used for treatment and prevention of saprolegniosis (Minor et al. 2014). However, the potential teratogenic, carcinogenic, and mutagenic properties of malachite green had ended in banning its use in 2002 (Minor et al. 2014). The demand for alternative treatments has pushed many researchers towards investigating more safe and eco-friendly compounds (Tedesco et al. 2019) such as medicinal herbs (Caruana et al. 2012; Khemis et al. 2016; Nardoni et al. 2019; Emara et al. 2020).
Medicinal plants can be a suitable alternative to chemotherapy in aquaculture since they have been reported to possess a complex chemical composition that displays numerous bioactivities including antimicrobial (bacterial, fungus, virus, and ectoparasites) effects (Reverter et al. 2014). Moreover, plants have some other characteristics such as biodegradability, availability, ease of cultivation, and not residing in animal tissues making them popular among aquaculturists (Dawood et al. 2021).
The genus Sambucus L., belongs to the family Adoxaceae (Rezaei-Moshaei et al. 2021), and comprises eighteen species around the world (Ebadi and Hisoriev 2011). Sambucus ebulus L. also known as dwarf elder is the only species distributed in the northern part of Iran (Ebadi and Hisoriev 2011). Various extensive studies have been done about its antioxidant, anti-inflammatory, analgesic, antimicrobial, anticancer, wound healing, antidepressant, and antiparasitic activities (Jabbari et al. 2017).
Actinidia deliciosa Liang Ferguson (Green kiwifruit) belongs to the family Actinidiaceae and grows naturally in various regions (Satpal et al. 2021). Iran is the fourth largest kiwifruit producer in the world and the fruit is mainly cultivated in the northern part of the country (Maleki et al. 2017). Due to the presence of bioactive compounds in kiwifruit, it exhibits strong antimicrobial, antiviral activity, and immunomodulatory effects (Satpal et al. 2021). It is thought that kiwifruit peel contains higher amounts of phenols and flavonoids than flesh (Alim et al. 2019).
The aim of the present study was to determine the chemical composition of Sambucus ebulus leaves and Actinidia deliciosa peels ethanolic extracts and their antifungal effects against S. parasitica.
Materials and methods
Strain tested
The in vitro tests were carried out on a S. parasitica strain (Isolate KMG3, accession number: MW819780) isolated from infected rainbow trout eggs (unpublished data). Purified cultures were kept on Sabouraud dextrose agar (SDA) and re-inoculated every month. The inocula were obtained by cutting the advancing edge of the young colonies (3-day incubation at 18 °C) using a sterile scalpel.
Ethics statement
In the present study, S. parasitica recovered from infected fish eggs was used and ethical approval was not required for the study. According to US National Institutes of Health, Policy on Humane Care and Use of Laboratory Animals (PHS) applies to offspring of egg-laying vertebrates only after hatching (Bartlett and Silk 2016).
Plant material preparation
A. deliciosa peel and S. ebulus leaves were obtained from the north of Iran (Mazandaran and Golestan provinces, respectively), air-dried in a dark place, ground by a kitchen blender, and stored at 4 °C.
Preparation of plant extracts
The pulverized plant samples were added to distilled water (1:1 masses per volume) and subjected to boiling for 10 min. After filtration, the filtrate was mixed with 70% ethanol and remained for 1 week in a dark place with intermittent shaking. The ratio of sample to solvent was 1: 9 (volume per volume). After that, the supernatant was filtered through a filter paper Whatman No. 1 (Whatman Ltd., England). The extracts were concentrated at 55 °C and then were maintained in a closed and dark container (Salama et al. 2018).
GC-MS analysis
Constituents of S. ebulus ethanolic extract were analyzed by GC-MS as previously reported (Khosravi et al. 2018). A 6890 N Agilent gas chromatograph coupled to a 5975 C Agilent mass-selective detector (Agilent Technologies, Avondale, PA, USA) with a 7683 Agilent auto sampler was employed and 1.0 µL of the sample was injected in the split less mode at 250 °C into a 30 m × 0.25 mm × 0.5 μm DB-5 MS capillary column and operated by MSD Chemstation Software (Agilent Technologies). The temperature program used for the chromatographic separation was as follows: 50 °C for 2 min, the temperature increase at 25 °C.min −1 to 100 °C and hold for 2 min, then temperature increase at 5 °C.min −1 to 290 °C where it was finally held for 5 min. The carrier gas was helium (99.999%) and was kept at a constant flux of 1.0 mL.min −1. The mass spectrometer was run in the electron impact ionization mode and the energy of the electrons was retained at 70 eV. After injection of sample to GC/MS, some unknown peaks were detected. The impact mass spectra of these obtained peaks were searched for in our computer library (Khosravi et al. 2018).
Determination of total phenolic and flavonoid contents
The phenolic contents of A. deliciosa were assessed using the Folin-Ciocalteu method described previously with minor modification (Ainsworth and Gillespie 2007). In brief, 25 µl of the extract was added to 95 µl of 2n Folin-Ciocalteu reagent and then 300 µl sodium carbonate solution was added. The final volume reached 1800 µl using distilled water and then incubated at 37 °C for 45 min. The absorbance of the solution was calculated at 758 nm by spectrophotometry and expressed as mg gallic acid equivalents (GAE)/g dry weight sample.
Flavonoid contents of A. deliciosa were determined using aluminum chloride in a colorimetric method; the result was derived from the calibration curve of quercetin (QE) and expressed as mg QE /g dry weight sample (Aryal et al. 2019).
In vitro assays
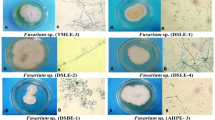
Tests were performed following the HeMP (Stueland et al. 2005) and agar dilution (Ali et al. 2019) methods with some modifications. For the HeMP method, agar plugs (5 mm) with actively growing mycelia were inoculated in the center of yeast extract glucose chloramphenicol (YGC) agar plate (8 cm) and incubated at 18 °C for 1 day. The sterilized hemp seeds were then placed in a circle around the Saprolegnia colony and the agar plates were incubated at 18 °C for another 2 days. Then, the Saprolegnia-colonized hemp seeds were transferred with sterile forceps into wells of a 48-well flat-bottom tissue culture plate (NEST, China) and 1 mL of each test extract was added. Stock solutions at 30% of herbal extracts were diluted into sterile distilled water (SDW) to obtain concentrations ranging from 0.1 to 10%, to find a MIC value, and from 0.5 to 30% to find a MLC value expressed as the lowest concentration, at which fungal growth was not noticeable in two replicates. All concentrations were tested twice. SDW without extract and malachite green at 10 ppm were set as controls. In order to ensure good growth of Saprolegnia, 2% GY broth (D (+)-glucose monohydrate 10 g, yeast 2 g, potassium dihydrogen orthophosphate (KH2PO4) 2.04 g, and (disodium hydrogen phosphate anhydrous) Na2HPO4 0.596 g (in 1 L of distilled water and autoclaving) (Willoughby and Pickering 1977) were included in each test concentration. To find MIC, Saprolegnia-colonized hemp seeds were exposed to different herbal extract concentrations for 48 h. To find the MLC, they were kept in contact with the solutions for 60 min, followed by rinsing of the exposed hempseeds in water and the transfer of the seed to pure water. The wells on the tissue culture plates were examined by the use of a stereomicroscope (Olympus, Japan) 48 h after the start of the exposure. The mycelial growth of Saprolegnia was graded as 0 for complete inhibition of the growth and 1 for a partial or full growth of the mycelium on the hemp seeds (Stueland et al. 2005) (Fig. 1).
For the agar dilution method (Ali et al. 2019), the extracts were added at 1, 2.5, 5, and 10% concentration to sterilized molten SDA held at 45 °C. SDA without herbal extracts was used as a negative control treatment. Then, the plates were inoculated with Saprolegnia-colonized agar plugs (5 mm) and/or hemp seeds and the mean radial growth of Saprolegnia hyphae was calculated after 3 days of incubation at 18 °C. All the concentrations were examined in triplicates. All chemicals used, unless otherwise stated, were purchased from Merck Company (Darmstadt, Germany).
Statistical analysis
One-way ANOVA followed by Duncan’s comparison test was performed using SPSS version 15 for differences in radial growth of Saprolegnia at different herbal extract concentrations. Differences between treated and controls were evaluated at a p value < 0.05.
Results
GC-MS analysis
A total of seven components were identified from GC-MS of S. ebulus leaf extract that together constitute 99% of the compound. The main constituents in the extract were monophthalate (66.17%), palmitic acid (13.83%), isovaleric acid (6.28%), α-linoleic acid (5.24%), phytol (4.25%), acetic acid (2.11%), and pentanoic acid (1.12%) (Table 1).
Total phenolic and flavonoid contents
A. deliciosa ethanolic extract contained total phenolic (TP) contents at 162 mg (GAE)/g DW and total flavonoid (TF) content at 2.31 mg (QE)/g DW.
In vitro assay
Based on the HeMP method, the ethanolic extract of S. ebulus showed an antimycotic activity with MIC and MLC values of 2.5% and 20%, respectively. Regarding A. deliciosa, none of the tested doses had a fungicidal effect; nevertheless, it appeared clearly to have a fungistatic effect with an observed MIC corresponding to a dose of 5%.
We also tested the effect on mycelial growth quantitatively by using the agar dilution method. Compared to the control group, the growth of S. parasitica was inhibited by S. ebulus in a dose-dependent manner (Fig. 2). In this way, treatment with 1% concentration resulted in an incomplete but statistically significant (p <0.05) inhibition of mycelial growth. A concentration of 2.5% gave a reduction of close to 57% (p < 0.05) and at 5% concentration of S. ebulus, no hyphal growth was observed (Figs. 2 and 3). On the contrary, the radial growth of S. parasitica did not show statistically significant differences comparable with the control group up to 10% concentration of A. deliciosa (p >0.05) (Fig. 2). Ten percent concentration yielded an inhibition of 68% (p < 0.05) (Figs. 2 and 4).
Inhibition of S. parasitica growth on Sabouraud dextrose agar (SDA) following treatment with different Sambucus ebulus (S. ebulus) and Actinidia deliciosa (A. deliciosa) ethanolic extract concentrations. Different superscript letters indicate the existence of statistical difference among groups (p < 0.05)
Discussion
In the present study, the hyphal growth of S. parasitica was inhibited completely by S. ebulus at 5% concentration, and the growth inhibition was dose-dependent (p < 0.05). This finding is in agreement with the study of Rodino et al. (2015), who observed mycelial growth inhibition to 50 and 84% in Phytophthora infestans (the oomycete), when using S. ebulus fruit hydroalcoholic extract at the concentration of 2 and 4% in the medium, respectively. Also, Shahiri Tabarestani et al. (2017) reported that different concentrations (10, 20, and 30%) of alcoholic extracts of S. ebulus leaves significantly inhibited the mycelial growth and formation of sclerotia of the Macrophomina phaseolina (the agent of soybean charcoal rot). Interestingly, the chemical composition of S. ebulus leaves extracts in our study was similar to Shahiri Tabarestani et al. (2017) results and in both works, phthalates were the most constituents (concentration >50%). Phthalates are synthetic chemical compounds used as plasticizers (Kumari and Kaur 2021). However, in recent years, several natural phthalates were extracted from microorganisms, algae, and plants (Kilani-Feki et al. 2011), and their antifungal activity was demonstrated by many authors (Harikrishnan et al. 2010; Akpuaka et al. 2012). Phthalates were found to alter the activity of various enzymes including purified yeast glucose 6-phosphate dehydrogenase (Ohyama 1977) and PINK1, a serine/threonine kinase in human neuroblastoma cells (Xu et al. 2021). These enzymes were also reported from S. parasitica, e.g., glucose 6-phosphate dehydrogenase (SPRG_0526) and protein-serine/threonine kinase (SPRG_10674). The observed inhibitory activity of S. ebulus in the present study may be attributable to alteration in the activity of such enzymes, but it needs to be investigated more detail in the future.
There are numerous reports referring to antifungal properties of fatty acids (Liu et al. 2008; Pohl et al. 2011). In the current study, palmitic acid (13.83%) was found to be the most prevalent fatty acid in the ethanol extracts of S. ebulus leaves followed by isovaleric acid (6.28%) and α-linoleic acid (5.24%). These fatty acids were also present in the alcoholic extracts of S. ebulus leaves used by the study of Shahiri Tabarestani et al. 2017, but in different ratios (8.24, 4.33, and 7.78, respectively). Also, Shah et al. (2021) identified palmitic acid as the second highest compound (11.95%) in the Thymus linearis leaf extract with a potent anti-oomycetes activity against S. parasitica. The extract showed mycelial growth inhibition to 54.45 ± 0.9% at 320 ppm and complete inhibition (100%) at 5120 ppm (Shah et al. 2021). According to Pohl et al. (2011), antifungal fatty acids elicit profound effects on the cell membrane, causing an increase in membrane permeability, which will result in leakage of the cytoplasm and cell death.
Phytol, an acyclic diterpene alcohol, was another compound with a considerable amount (4.25%) in S. ebulus leaf extract. Its antimicrobial activity was proven against several bacterial and fungal strains (Inoue et al. 2005; Pejin et al. 2014). For example, Inoue et al. (2005) reported that it could disrupt the cell membrane of Staphylococcus aureus, resulting in potassium ions discharging from the cells, and causing the bacterium to shrink. Recently, based on molecular docking, phytol was identified to target a number of proteins in S. parasitica such as plasma membrane ATPase, host target protein-1 and TKL protein kinase, and V-type protein ATPase (Shah et al. 2021).
Moreover, it was found that acetic acid has an antifungal effect on S. parasitica (MIC at 250 ppm) (Tedesco et al. 2019). In the present study, the concentration of acetic acid in the ethanolic extract of S. ebulus was 2.11% (Table 1). The amount of acetic acid in our work (i.e., 2.5% concentration of S. ebulus leaf extract) was calculated and equaled to 527.5 ppm which was about twice the MIC recorded for acetic acid for S. parasitica (Tedesco et al. 2019). Therefore, it seems that in our experience, the antimycotic activity of S. ebulus against S. parasitica could be originated from phthalates, fatty acids, phytol, and acetic acid. However, more detailed studies are needed to clarify which bioactive molecules are responsible for the observed activity.
In this study, two different MICs were recorded for A. deliciosa including 5 and 10% by the HeMP and agar dilution methods, respectively. This mismatched MIC value is most likely due to different densities and growth quality of hyphae per cell culture medium in the HeMP method (Stueland et al. 2005). Moreover, here, we applied lower number of replicates in this method compared to agar dilution method (i.e., two vs. three per concentration, respectively). Such variable results were also reported by other authors (Kumar et al. 2020). However, Salama et al. (2018) found that ethanol and acetone extracts of A. deliciosa inhibited the growth of yeasts (Saccharomyces cerevisiae and Candida albicans) and the fungus Aspergillus flavus, at concentrations of 400 and 600 ppm. In the current study, compared to the study of Salama et al. (2018), rather a high MIC value was obtained. This variability may be due to specific oomycetes cell wall composition, mainly composed of cellulosic compounds along with glycans compared to other true fungi which possess chitin in their cell walls (Nardoni et al. 2019; Magray et al. 2019). Furthermore, it has been reported that the extraction techniques and solvents could influence the bioactivity of herbal extracts (Emara et al. 2020). It is not clear which bioactive fraction is responsible for the antifungal effect of A. deliciosa, but it seems that it was related to phenolic and flavonoid components. Phenolic and flavonoid compounds are natural plant secondary metabolites depicted by aromatic rings, act as reducing agents and antioxidants, and are known to have activity against microorganisms (Caruana et al. 2012; Khemis et al. 2016; Alim et al. 2019). In another study, Khemis et al. (2016) demonstrated that the ethanolic extract of Opuntia stricta (Haworth) prickly pear with high phenolic contents (147.31 mg CATE/100 g of fruit) arrested the growth of Saprolegnia sp. both in vitro and in vivo. Likewise, inhibition in the mycelial growth of P. infestans was observed when the phenolic compounds present in the extracts of the olive plant (Olea europaea L.) were added to the culture medium (Del Rı́o et al. 2003).
Based on findings of the present study, compared to A. deliciosa, S. ebulus proved to be more effective in inhibiting the growth of S. parasitica in vitro and could be of interest for controlling Saprolegnia. However, more detailed studies are needed to evaluate its possible toxic effects on different fish developmental stages and efficacy in vivo
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Akpuaka A, Ekwenchi M, Dashak D, Dildar A (2012) Gas Chromatography-Mass Spectrometry (GC/MS) analysis of phthalate isolates in n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. J Am Sci 8:146–155. Retrieved from: http://www.jofamericanscience.org/journals/amsci/am0812/022_12494am0812_146_148.pdf. Accessed 1 Jun 2021
Ali SE, Gamil AA, Skaar I, Evensen Ø, Charo-Karisa H (2019) Efficacy and safety of boric acid as a preventive treatment against Saprolegnia infection in Nile tilapia (Oreochromis niloticus). Sci Rep 9(1):1–9. https://doi.org/10.1038/s41598-019-54534-y
Alim A, Li T, Nisar T, Ren D, Zhai X, Pang Y, Yang X (2019) Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from Actinidia chinensis. Food Nutr Res 63. https://doi.org/10.29219/fnr.v63.1577
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2(4):875–877. https://doi.org/10.1038/nprot.2007.102
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 8(4):96. https://doi.org/10.3390/plants8040096
Bartlett DH, Silk SB (2016) Office of laboratory animal welfare comments. Zebrafish 13(6):563–564. https://doi.org/10.1089/zeb.2016.1344
Caruana S, Yoon GH, Freeman MA, Mackie JA, Shinn AP (2012) The efficacy of selected plant extracts and bioflavonoids in controlling infections of Saprolegnia australis (Saprolegniales; Oomycetes). Aquaculture 358:146–154. https://doi.org/10.1016/j.aquaculture.2012.06.035
Dawood MA, El Basuini MF, Zaineldin AI et al (2021) Antiparasitic and antibacterial functionality of essential oils: an alternative approach for sustainable aquaculture. Pathogens 10(2):185. https://doi.org/10.3390/pathogens10020185
Del Río JA, Báidez AG, Botía JM, Ortuno A (2003) Enhancement of phenolic compounds in olive plants (Olea europaea L.) and their influence on resistance against Phytophthora sp. Food Chem 83(1):75–78. https://doi.org/10.1016/S0308-8146(03)00051-7
Ebadi AG, Hisoriev H (2011) Review on distribution of Sambucus ebulus L. in the North of Iran. Am-Eur J Agric Environ Sci 10(3):351–353
Eissa AE, Abdelsalam M, Tharwat N, Zaki M (2013) Detection of Saprolegnia parasitica in eggs of angelfish Pterophyllum scalare (Cuvier–Valenciennes) with a history of decreased hatchability. Int J Vet Sci Med 1(1):7–14. https://doi.org/10.1016/j.ijvsm.2013.04.001
Emara EK, Gaafar AY, Shetaia YM (2020) In vitro screening for the antifungal activity of some Egyptian plant extracts against the fish pathogen Saprolegnia parasitica. Aquac Res 51(11):4461–4470. https://doi.org/10.1111/are.14791
Inoue Y, Hada T, Shiraishi A, Hirose K, Hamashima H, Kobayashi S (2005) Biphasic effects of geranylgeraniol, teprenone, and phytol on the growth of Staphylococcus aureus. Antimicrob Agents Chemother 49(5):1770–1774. https://doi.org/10.1128/AAC.49.5.1770-1774.2005
Harikrishnan R, Kim MC, Kim JS, Balasundaram C, Jawahar S, Heo MS (2010) Identification and Antimicrobial Activity of combined extract from Azadirachta indica and Ocimum Sanctum. Isr J Aquacult-Bamid 62(2):85–95
Jabbari M, Daneshfard B, Emtiazy M, Khiveh A, Hashempur MH (2017) Biological effects and clinical applications of dwarf elder (Sambucus ebulus L): A review. J Evid -Based Integr Med 22(4):996–1001. https://doi.org/10.1177/2156587217701322
Khemis IB, Aridh NB, Hamza N, M’Hetli M, Sadok S (2016) Antifungal efficacy of the cactaceae Opuntia stricta (Haworth) prickly pear ethanolic extract in controlling pikeperch Sander lucioperca (Linnaeus) egg saprolegniasis. J Fish Dis 39:377–383. https://doi.org/10.1111/jfd.12356
Khosravi AR, Sharifzadeh A, Nikaein D, Almaie Z, Nasrabadi HG (2018) Chemical composition, antioxidant activity and antifungal effects of five Iranian essential oils against Candida strains isolated from urine samples. J Mycol Med 28(2):355–360. https://doi.org/10.1016/j.mycmed.2018.01.005
Kilani-Feki O, Culioli G, Ortalo-Magné A, Zouari N, Blache Y, Jaoua S (2011) Environmental Burkholderia cepacia strain Cs5 acting by two analogous alkyl-quinolones and a didecyl-phthalate against a broad spectrum of phytopathogens fungi. Curr Microbial 62(5):1490–1495. https://doi.org/10.1007/s00284-011-9892-6
Kumar S, Mandal RS, Bulone V, Srivastava V (2020) Identification of growth inhibitors of the fish pathogen Saprolegnia parasitica using in silico subtractive proteomics, computational modeling, and biochemical validation. Front Microbiol 11:2533. https://doi.org/10.3389/fmicb.2020.571093
Kumari A, Kaur R (2021) Chromatographic methods for the determination of phthalic acid esters in different samples. J Anal Chem 76(1):41–56. https://doi.org/10.1134/S1061934821010056
Liu S, Ruan W, Li J, Xu H, Wang J, Gao Y, Wang J (2008) Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 166(2):93–102. https://doi.org/10.1007/s11046-008--9124-1
Magray AR, Lone SA, Ganai BA, Ahmad F, Dar GJ, Dar JS, Rehman S (2019) Comprehensive, classical and molecular characterization methods of Saprolegnia (Oomycota; Stramnipila), an important fungal pathogen of fish. Fungal Biol Rev 33(3–4):166–179. https://doi.org/10.1016/j.fbr.2018.12.001
Minor KL, Anderson VL, Davis KS et al (2014) A putative serine protease, SpSsp1, from Saprolegnia parasitica is recognised by sera of rainbow trout, Oncorhynchus mykiss. Fungal Biol 118(7):630–639. https://doi.org/10.1016/j.funbio.2014.04.008
Maleki S, Maleki-Zanjani B, Gallego PP (2017) Kiwifruit status in Iran: management and production. In: IX International Symposium on Kiwifruit 1218, pp 39-44. https://doi.org/10.17660/ActaHortic.2018.1218.5
Nardoni S, Najar B, Fronte B, Pistelli L, Mancianti F (2019) In vitro activity of essential oils against Saprolegnia parasitica. Molecules 24(7):1270. https://doi.org/10.3390/molecules24071270
Ohyama T (1977) Effects of phthalate esters on glucose 6-phosphate dehydrogenase and other enzymes in vitro. Toxicol Appl Pharmacol 40(2):355–364. https://doi.org/10.1016/0041-008X(77)90107-7
Paul Y, Leung WL, Hintz WE (2015) Species composition of the genus Saprolegnia in fin fish aquaculture environments, as determined by nucleotide sequence analysis of the nuclear rDNA ITS regions. Fungal Biol 119(1):27–43. https://doi.org/10.1016/j.funbio.2014.10.006
Pejin B, Savic A, Sokovic M et al (2014) Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat Prod Res 28(6):372–376. https://doi.org/10.1080/14786419.2013.869692
Pohl CH, Kock JL, Thibane VS (2011) Antifungal free fatty acids: a review. In: Méndez Vilas A (ed) Science against microbial pathogens: current research and technological advances, vol 1. Formatex Research Center, Spain, pp 61–71
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquac 433:50–61. https://doi.org/10.1016/j.aquaculture.2014.05.048
Rezaei-Moshaei M, Dehestani A, Bandehagh A, Pakdin-Parizi A, Golkar M, Heidari-Japelaghi R (2021) Recombinant pebulin protein, a type 2 ribosome-inactivating protein isolated from dwarf elder (Sambucus ebulus L.) shows anticancer and antifungal activities in vitro. Int J Biol Macromol 174:352–361. https://doi.org/10.1016/j.ijbiomac.2021.01.129
Rodino S, Butu A, Petrache P, Butu M, Dinu-Pirvu CE, Cornea CP (2015) Evaluation of the antimicrobial and antioxidant activity of Sambucus ebulus extract. Farmacia 63(5):751–754
Salama ZA, Aboul-Enein AM, Asker MS, Gaafar AA, Hanan AF, Abou-Elella F, Ahmed HA (2018) Active constituents of kiwi (Actinidia deliciosa Planch) peels and their biological activities as antioxidant, antimicrobial and anticancer. Res J Chem Environ 22(9):52–59
Sandoval-Sierra JV, Latif-Eugenin F, Martín MP, Zaror L, Diéguez-Uribeondo J (2014) Saprolegnia species affecting the salmonid aquaculture in Chile and their associations with fish developmental stage. Aquaculture 434:462–469. https://doi.org/10.1016/j.aquaculture.2014.09.005
Satpal D, Kaur J, Bhadariya V, Sharma K (2021) Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J Food Process Preserv: e15588. https://doi.org/10.1111/jfpp.15588
Sformo TL, Adams B, Seigle JC et al (2017) Observations and first reports of saprolegniosis in Aanaakłiq, broad whitefish (Coregonus nasus), from the Colville River near Nuiqsut, Alaska. Polar Sci 14:78–82. https://doi.org/10.1016/j.polar.2017.07.002
Shah TK, Tandel RS, Kumar A, Bhat RAH, Dash P, Sarma D (2021) Chemical composition, antifungal activity and molecular docking of Himalayan thyme leaf extract (Thymus linearis) against fish pathogenic oomycete Saprolegnia parasitica. Aquaculture: 736988. https://doi.org/10.1016/j.aquaculture.2021.736988
Shahiri Tabarestani M, Rahnama K, Amiri A (2017) Inhibitory effect of Sambucus ebulus extracts on growth of Macrophomina phaseolina and extraction of their bioactive. J Plant Prot 30(4):622–628
Stueland S, Heier BT, Skaar I (2005) A simple in vitro screening method to determine the effects of drugs against growth of Saprolegnia parasitica. Mycol Prog 4(4):273–279. https://doi.org/10.1007/s11557-006-0131-7
Tedesco P, Fioravanti ML, Galuppi R (2019) In vitro activity of chemicals and commercial products against Saprolegnia parasitica and Saprolegnia delica strains. J Fish Dis 42(2):237–248. https://doi.org/10.1111/jfd.12923
Willoughby LG, Pickering AD (1977) Viable Saprolegniaceae spores on the epidermis of the salmonid fish Salmo trutta and Salvelinus alpinus. Trans Brit Mycol Soc 68(1):91–95. https://doi.org/10.1016/S0007-1536(77)80157-5
Xu J, Wang L, Zhang L et al (2021) Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol 38:101776. https://doi.org/10.1016/j.redox.2020.101776
Funding
This work was supported by the research council of the University of Tehran, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Experimental design: SM, MG, ARKh. Performing the experiments: SM and MG. data curation: SM, MG, and ARKh. Writing the paper: SM, MG, and ARKh.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Gavin Burnell
Rights and permissions
About this article
Cite this article
Mirmazloomi, S., Ghiasi, M. & Khosravi, A.R. Chemical composition and in vitro antifungal activity of Sambucus ebulus and Actinidia deliciosa on the fish pathogenic fungus, Saprolegnia parasitica. Aquacult Int 30, 1037–1046 (2022). https://doi.org/10.1007/s10499-022-00835-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00835-5