Abstract
A 7-week feeding trial was conducted to investigate the effect of two protein hydrolysates: fish hydrolysate (FH) and shrimp hydrolysate (SH) as feed supplement on growth performance and feed utilization of juvenile striped catfish, Pangasius hypophthalmus. Three isonitrogenous (32% crude protein) and isolipidic (12% crude lipid) experimental diets were formulated: diet 1, control (no supplementation of protein hydrolysates); diet 2, supplemented with 2% FH; and diet 3, supplemented with 2% SH. Each of the diets was fed to triplicate groups of 15 juveniles (initial mean weight of 6.99 g) twice a day until apparent satiation. The results showed that both FH and SH diets exerted positive impact on the growth and feed conversion ratio (FCR) of fish, but the improvement was more pronounced in the former as fish fed with FH diet showed significantly higher (P<0.05) specific growth rate (2.20% day−1), weight gain (193.68%), and better FCR (1.23) compared to those fed with control, yet no significant difference was recorded for these metrics between the control and SH group. Besides, fish fed with SH diet had significantly higher hepatosomatic index than those fed with control, and its viscerosomatic index was the highest among all treatment groups. The present study suggested that FH is a potential feed supplement for enhancing feed utilization and growth performance of juvenile striped catfish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant protein source is one of the most viable alternatives to fish meal because it is readily available and the production is considered environmentally and economically sustainable (Hua et al. 2019; Turchini et al. 2019). However, the presence of antinutritional factors and phytic acid is one of the shortcomings of plant protein source (Chau 2012; Daniel 2018). As such, high inclusion level of plant protein sources such as soybean meal and other oilseed meals in aquafeed often leads to reduction of feed palatability and feed intake as well as potential loss in fish growth.
Generally, protein in the aquatic animal byproducts is in macromolecule form and composed of 20 amino acids bonded with peptide bonds (Hou et al. 2017). These large molecules are hydrolyzed into small peptides and amino acids via fermentation, chemical hydrolysis, or enzymatic hydrolysis during production of protein hydrolysate (Kristinsson and Rasco 2000), and thus these free amino acids contribute to better absorption in fish (Roslan et al. 2014). Protein hydrolysate produced from industrial residues has good nutritional value owing to its high protein content and essential amino acids (Roslan et al. 2014). Previous studies showed that supplementation of fish protein hydrolysates at appropriate dietary level exerted beneficial effect on growth performance, feed utilization, antioxidant activity, and immune response of fish (Ha et al. 2019; Siddik et al. 2021). It has also been suggested that protein hydrolysate could mimic the attractant effect of fish bait and stimulate the feeding of Atlantic cod, Gadus morhua (Siikavuopio et al. 2017). Therefore, the addition of protein hydrolysate is believed to be a cost-effective way to overcome the drawbacks of high inclusion of plant protein in aquafeed.
In Malaysia, striped catfish production has reached 17,649 metric tons valued at RM 114 million in 2019 (DOF 2021). The local demand for striped catfish is ascribed by the affordable price and its favorable characteristic: boneless and neutral in taste (Othman et al. 2017). However, in order to reduce feeding costs, farmers tend to practice feeding striped catfish with chicken intestine which risks jaundice disease in fish (Luu 2013). As such, the development of more cost-effective and high-performance aquafeed would greatly benefit the catfish farming industry. Therefore, the aim of this project was to determine the effects of supplementation of fish and shrimp protein hydrolysates into the plant-based diet on feed intake and subsequent impact on feed utilization and growth performance of juvenile striped catfish.
Materials and methods
Experimental diets
Three isonitrogenous (32.08–32.67% crude protein) and isolipidic (12.43–12.50% crude lipid) experimental diets were formulated as shown in Table 1. Apart from protein hydrolysate, the ingredient composition of all diets was similar to each other. The experimental diets were designated as 2% fish hydrolysate (FH), 2% shrimp hydrolysate (SH), and a control (no added FH or SH) where the FH or SH diets were incorporated with 20 g kg−1 FH or SH, respectively. The two protein hydrolysates were provided by a local supplier. The amino acid composition of the protein hydrolysates is listed in Table 2. Soybean meal was the major dietary protein source, contributing 75% of the total crude protein in the experimental diets, while Danish fish meal contributing to another 25% of total crude protein; corn starch was included as the carbohydrate source. Carboxymethyl cellulose was included as a binder agent and α-cellulose served as a filler. All the pre-weighed dry ingredients were mixed homogenously in a Hobart mixer. Then, the oil was thoroughly mixed with the ingredient mixture and distilled water was added. The resultant moist dough was screw-pressed through a 2-mm die using a locally assembled meat mincer, and the formed feed pellets were fan-dried at room temperature to about 10% moisture. All diets were stored in airtight polyethylene bags at −20°C until use. All dietary ingredients and experimental diets were subjected to proximate analysis. In brief, the dry matter of the sample was analyzed by using oven-drying method; the sample was dried in the oven at 103°C until a constant weight achieved (AOAC 1997). For ash determination, sample was ashed in the furnace at 550°C for 5 h (AOAC 1997). Kjeldahl method was used for determination of protein content; sample was digested with concentrated H2SO4 and catalyst; the product then undergone distillation with the presence of distilled water and 32% NaOH. The distillate formed was titrated with 0.1 N HCl and the volume of HCl needed for titration was recorded (AOAC 1997). For determination of fiber, the pre-dried defatted sample was digested by boiling with 0.13 M H2SO4 and 0.23 M NaOH sequentially, and then washed with distilled water using Fibrebag system filtration. The dried digested sample was then ashed in the furnace at 600°C for 4 h and weighed (AOAC 1997). For determination of lipid, a modified Folch et al. (1957) method was adopted. Samples were soaked overnight with chloroform and methanol (2:1), and a polytron homogenizer was used to homogenize the sample. The homogenized sample was then filtered using Buchner funnel. The filtrate was transferred to separating funnel and distilled water was added. The separating funnel was then shaken to break the emulsion and left to allow the separation of two layers to occur. The lower layer of the extracts was collected, dried at 40°C until constant weight, and weighed. The nitrogen-free extract of sample was calculated by subtracting the composition of ash, protein, lipid, and fiber with the composition of dry matter.
Tank set-up and feeding experiment
The fish rearing was conducted in the Aquaculture Facilities (AQF) in Universiti Tunku Abdul Rahman (UTAR) Kampar, Perak (4°20′25.7″N 101°08′07.6″E). Juvenile striped catfish were purchased from a local hatchery in Malim Nawar, Malaysia. Upon arrival at AQF, all fish were acclimatized in a stocking tank and fed with 2 mm commercial freshwater fish feed (Cargill Feed Sdn. Bhd.) for 3 weeks. One week before the feeding trial, the commercial freshwater fish feed was switched to the experimental control diet for conditioning. Feed was given at 10% of the fish body weight per day during these periods. At the commencement of the feeding trial, nine groups of 15 juvenile striped catfish with an average weight of 6.99 ± 0.01 g were randomly selected, weighed, and stocked into series of nine cylindrical fiberglass tanks (570 L each). Flow through system was set up and filtered water was flowed through each of the tanks continuously with a flow rate of 0.25 L min−1. All tanks were provided with an individual air stone for continuous aeration as to maintain dissolved oxygen (DO) level between 6.45 and 7.87 mg L−1. Each of the experimental diets was randomly assigned to triplicate groups of fish. Fish were hand-fed twice a day at 0800 and 1700 h until apparent satiation for 7 weeks and feed consumption was recorded daily. Water qualities were monitored on a weekly basis throughout the feeding trial. The water pH and temperature were measured using Hanna handheld pH meter; DO was measured using Eutech DO meter while total ammonia nitrogen (TAN); nitrite and nitrate were measured using API freshwater master test kit. Throughout the experiment, the average water pH level of tanks was ranged 7.47–7.61; the average temperature was 28.61°C; TAN was averagely lower than 0.2 ppm; and no nitrite and nitrate were detected in all treatment tanks.
Sample collection and proximate analysis
At the termination of the feeding trial, all fish were individually weighed and their total length measured. Four fish were randomly selected from each tank and anesthetized with 0.05% of tricane methane sulphonate (MS 222). The four sampled fish were then culled and their blood was collected into heparinized tubes by severing the caudal peduncle. The capillary tubes were micro-centrifuged at 4000 × g for 5 min using a micro-hematocrit centrifuge (Kubota 3220) and the relative volume of the packed red blood cells was measured to determine the percent hematocrit value. The four sampled fish were dissected to remove various tissues for analysis. Liver, viscera, and intraperitoneal fat were excised and weighed for the determination of hepatosomatic index (HSI), viscerosomatic index (VSI), and intraperitoneal fat index (IPF), respectively, liver and gastrointestinal tract counted as viscera. HSI, VSI, IPF, and condition factor were applied to determine the nutritional status of the fish. These body indices were calculated as a percentage of organ or tissue to the whole-body weight of individual fish.
While fish growth performance was analyzed in regard to weight gain and specific growth rate (SGR), feed utilization efficiency was determined by FCR. These parameters were measured using the formula below:
Statistical analysis
All data were presented as mean ± standard error (SE) and subjected to Shapiro-Wilk test for normality test and a one-way analysis of variance (ANOVA) using SPSS 19.0 to determine if significant differences occurred among dietary treatments. Differences among means were determined by Duncan’s multiple-range test and considered to be significant at the level of 0.05.
Results
Growth performance and survival rate
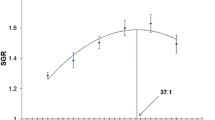
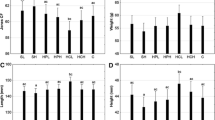
The fish given the FH feed had significantly (P<0.05) higher final weight (20.54 ± 0.25 g), weight gain (193.68 ± 3.84 g), and SGR (2.20 ± 0.03 g) than the control, but there were no significant (P>0.05) differences for these metrics between the FH and SH treatments (Table 3). The survival rate of fish was high with the mean value of 98.52%.
Biological indices and feed utilization
In respect to biological indices, fish fed SH diet had the highest HSI (1.54%) which was significantly higher than those fed with control (1.28%) but not significantly differ from those fed with FH diet (1.35); VSI was found highest in fish fed SH diet (6.22%) which was significantly higher than other two treatment groups (4.39–4.71%); yet, no significant difference was noticed among treatment groups in terms of IPF. All the experimental fish was recorded to have a similar condition factor with the value of 0.75 ± 0.01. Besides, significant difference was observed in the hematocrit level as fish fed with control diet (46.67 ± 0.17%) showed significantly higher hematocrit than the other two diets (44.78–45.72%). Total feed intake was approximately 26% higher in fish fed with FH (16.60 ± 0.41 g fish−1) and SH (16.87 ± 1.99 g fish−1) diets as compared to control group (13.17 ± 1.89 g fish−1), although no significant difference was detected among the treatment groups. In regard to FCR, fish fed the FH diet showed the lowest value (1.23 ± 0.01) which was significantly lower from those fed the control (1.42 ± 0.06) but not significantly differ from fish fed SH diet (1.30 ± 0.06).
Discussion
Feed utilization and growth performance
In the present study, fish fed with FH and SH diets recorded higher feed intake than those fed with control diet, despite no significant differences recorded. This is in line with previous studies that fish appetite is enhanced by protein hydrolysate which acts as feed stimulant that improves the feed palatability and attracts fish for food ingestion (Kolkovski et al. 2000; Siikavuopio et al. 2017). With the higher feed intake, fish fed with FH and SH diets showed improved growth rate. Previous studies reported that small molecular weight fraction of protein hydrolysate conduced to better digestibility of the juvenile red seabream, Pagrus major (Bui et al. 2014; Khosravi et al. 2015); olive flounder, Paralichthys olivaceus (Khosravi et al. 2015); and pike silverside, Chirostoma estor (Ospina-Salazar et al. 2016). It is suggested that the low-molecular-weight peptides in FH and SH diets were easier to be absorbed by the fish in comparison with control diet. In other words, the supplementation of FH and SH might enhance the nutrient uptake and lead to a rapid weight gain of juvenile striped catfish. Moreover, the growth improvement was more profound in FH diet-fed fish; this is likely due to the different composition of free amino acids and short-sized peptides in varied types of protein hydrolysates (Klompong et al. 2009; Chalamaiah et al. 2012; Taheri et al. 2013). To be more specific, protein hydrolysate solubility is influenced by the hydrophilicity of its amino acids (Kristinsson and Rasco 2000), and SH used in the study contained higher percentage of hydrophobic amino acids such as alanine, leucine, and valine, which resulted in inferior solubility than that of FH. It is noteworthy that fish fed with FH diet showed significant improved FCR than control, implying that the amino acid content in FH enhances the feed utilization of juvenile striped catfish and thus resulted in better growth performance, but this needs further investigation.
Biological indices
Fish fed with SH diet had significantly larger liver relative to the total body weight than those given with control diet, and the bigger liver was the reason the fish fed with SH diet had significantly larger VSI than those fed with FH and control diets. Besides, the supplementation of FH and SH in the diet did not significantly influence the water quality. However, SH diet tended to increase the pH and TAN of tank water. Therefore, further studies are required to elucidate the interaction of protein hydrolysates with the culture water and the subsequent impact on the fish physiological condition. It has been reported that high inclusion level of plant protein in diet resulted in detrimental effects on red sea bream as the plant protein consisted of imbalance amino acid profile (Khosravi et al. 2015). In the present study, control diet was not supplemented with protein hydrolysate and the dietary protein was largely contributed by the soybean meal; therefore, it is suggested that fish fed with control diet were not utilizing the feed efficiently. Furthermore, the fish fed with FH and SH diets were found to have significantly lower hematocrit level than that of control indicating the supplementation of protein hydrolysate could improve fish health and performance. It has been previously reported that higher hematocrit level of fish denoted high quantity of erythrocytes are released from the spleen into the circulation (Fazio et al. 2015) as more red blood cells are triggered to improve the oxygen carrying capacity and to support the physiological function of fish under high energy demand condition (Shah and Altindag 2004; Eslamloo et al. 2014). Moreover, Wendelaar Bonga (2011) suggested that the hematocrit level was affected by the environmental factors and nutritional status. In light of the above, it is evident that FH is a better feed supplement than SH for juvenile striped catfish.
Conclusions
In overall, supplementation of protein hydrolysates in fish feed increased the feed intake and improved the FCR and growth performance of the fish with more profound improvement shown by FH. In conclusion, supplementation of protein hydrolysates could overcome the shortcoming of plant-based diets, and FH is a potential feed supplement for juvenile striped catfish for better growth performance and feed utilization.
References
AOAC (Association of Official Analytical Chemists) (1997) In: Cunniff PA (ed) Official methods of analysis of the AOAC International, 16th edn. AOAC International, Arlingation, VA, USA
Bui HTD, Khosravi S, Fournier V, Herault M, Lee K-J (2014) Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture 418-419:11–16. https://doi.org/10.1016/j.aquaculture.2013.09.046
Chalamaiah M, Kumar BD, Hemalatha R, Jyothirmayi T (2012) Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem 135(4):3020–3038. https://doi.org/10.1016/j.foodchem.2012.06.100
Chau TD (2012) Evaluation of locally available feed resources for striped catfish (Pangasianodon hypophthalmus). In: PhD thesis. Swedish University of Agricultural Sciences, Sweden
Daniel N (2018) A review on replacing fish meal in aqua feeds using plant protein sources. International Journal of Fisheries and Aquatic Studies 6(2):164–179
DOF (Department of Fisheries) (2021) Annual Fisheries Statistics. https://www.dof.gov.my/dof2/resources/user_29/Documents/Buku%20Perangkaan%20Tahunan%20Perikanan/Perangkaan_Perikanan_2019_Jilid_1.pdf. Accessed 16 Jan 2021
Eslamloo K, Akhavan SR, Fallah FJ, Henry MA (2014) Variations of physiological and innate immunological responses in goldfish (Carassius auratus) subjected to recurrent acute stress. Fish & Shellfish Immunology 37(1):147–153. https://doi.org/10.1016/j.fsi.2014.01.014
Fazio F, Ferrantelli V, Fortino G, Arfuso F, Giangrosso G, Faggio C (2015) The influence of acute handling stress on some blood parameters in cultured sea bream (Sparus aurata Linnaeus, 1758). Italian Journal of Food Safety 4(1):4–6. https://doi.org/10.4081/ijfs.2015.4174
Folch JM, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Ha N, Jesus GFA, Gonçalves AFN, de Oliveira NS, Sugai JK, Pesatti ML, Mouriño JLP, Fabregat TEHP (2019) Sardine (Sardinella spp.) protein hydrolysate as growth promoter in South American catfish (Rhamdia quelen) feeding: Productive performance, digestive enzymes activity, morphometry and intestinal microbiology. Aquaculture 500:99–106. https://doi.org/10.1016/j.aquaculture.2018.10.004
Hou Y, Wu Z, Dai Z, Wang G, Wu G (2017) Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. Journal of Animal Science and Biotechnology 8:24. https://doi.org/10.1186/s40104-017-0153-9
Hua K, Cobcroft JM, Cole A, Condon K, Jerry DR, Mangott A, Praeger C, Vucko MJ, Zeng C, Zenger K, Strugnell JM (2019) The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 1(3):316–329. https://doi.org/10.1016/j.oneear.2019.10.018
Khosravi S, Bui HTD, Rahimnejad S, Herault M, Fournier V, Kim S-S, Jeong J-B, Lee K-J (2015) Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus). Aquaculture 435:371–376. https://doi.org/10.1016/j.aquaculture.2014.10.019
Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes KD (2009) Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis). J Food Sci 74(2):126–133. https://doi.org/10.1111/j.1750-3841.2009.01047.x
Kolkovski S, Czesny S, Dabrowski K (2000) Use of krill hydrolysate as a feed attractant for fish larvae and juveniles. J World Aquacult Soc 31(1):81–88. https://doi.org/10.1111/j.1749-7345.2000.tb00701.x
Kristinsson HG, Rasco BA (2000) Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr 40(1):43–81. https://doi.org/10.1080/10408690091189266
Luu TTT (2013) Investigation into jaundice in farmed catfish (Pangasianodon hypophthalmus, Sauvage) in the Mekong delta. In: Vietnam. PhD Thesis. University of Stirling, United Kingdom
Ospina-Salazar GH, Ríos-Durán MG, Toledo-Cuevas EM, Martínez-Palacios CA (2016) The effects of fish hydrolysate and soy protein isolate on the growth performance, body composition and digestibility of juvenile pike silverside, Chirostoma estor. Anim Feed Sci Technol 220:168–179. https://doi.org/10.1016/j.anifeedsci.2016.08.011
Othman MF, Hashim M, Eim YM, Amal MNA, Iksan N, Chong HG, Merican Z (2017) Transforming the aquaculture industry in Malaysia. World Aquaculture 48(2):16–23
Roslan J, Yunos KFM, Abdullah N, Kamal SMM (2014) Characterization of fish protein hydrolysate from tilapia (Oreochromis niloticus) by-product. Agriculture and Agricultural Science Procedia 2:312–319. https://doi.org/10.1016/j.aaspro.2014.11.044
Shah SL, Altindag A (2004) Hematological parameters of tench (Tinca tinca L.) after acute and chronic exposure to lethal and sublethal mercury treatments. Bull Environ Contam Toxicol 73:911–918. https://doi.org/10.1007/s00128-004-0513-y
Siddik MAB, Howieson J, Fotedar R, Partridge GJ (2021) Enzymatic fish protein hydrolysates in finfish aquaculture: a review. Rev Aquac 13(1):406–430. https://doi.org/10.1111/raq.12481
Siikavuopio SI, James P, Stenberg E, Evensen T, Sæther B-S (2017) Evaluation of protein hydrolysate of by-product from the fish industry for inclusion in bait for longline and pot fisheries of Atlantic cod. Fish Res 188:121–124. https://doi.org/10.1016/j.fishres.2016.11.024
Taheri A, Anvar SAA, Ahari H, Fogliano V (2013) Comparison the functional properties of protein hydrolysates from poultry byproducts and rainbow trout (Onchorhynchus mykiss) viscera. Iran J Fish Sci 12(1):154–169
Turchini GM, Trushenski JT, Glencross BD (2019) Thoughts for the future of aquaculture nutrition: realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N Am J Aquac 81(1):13–39. https://doi.org/10.1002/naaq.10067
Wendelaar Bonga SE (2011) Hormonal response to stress. In: Farrel AP (ed) Encyclopedia of fish physiology: from genome to environment. Academic Press, Cambridge, pp 1515–1523
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The authors declare that this project obtained ethical approval for research project involving the use of animals from the institute.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teoh, CY., Wong, YY. Use of fish and shrimp hydrolysates as dietary supplements to increase feeding and growth of juvenile striped catfish (Pangasius hypophthalmus). Aquacult Int 29, 1885–1894 (2021). https://doi.org/10.1007/s10499-021-00725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00725-2