Abstract
The aim of the present study was to investigate the influence of dietary supplementation with Bacillus subtilis C-3102 on the productive, intestinal histomorphometry, haemato-immunological aspects, as well as evaluated the resistance of juvenile Pseudoplatystoma sp. after challenge with Aeromonas hydrophila. Inclusion levels of the commercial probiotic were set at 0 (control), 1, 2, 3 and 4% of CALSPORIN® kg feed−1 with six replicates. Blood samples were collected on day 0, 10 and 20 for hematological analysis, and on the 20th day, samples were collected for intestinal histomorphometry, zootechnical and survival analyses. The results showed that probiotic supplementation with B. subtilis significantly improved intestinal morphology in fish from groups 1% and 2% and phagocytic activity in all supplemented groups, regardless of the applied dose. In addition, on the 20th day, improvements in non-specific immunity were observed, such as an increase in the number of monocytes (groups 2, 3 and 4%), eosinophils (group 3%), leukocytes (1%) and lymphocytes (group 4%) of the fish that received the supplemented feed. It was also possible to verify that the probiotic caused significant changes in the haemogram between the sampling periods on 0, 10th and 20th day, showing activation of specialized cells of the fish’s non-specific defense mechanism. At the end of the experimental period, supplementation of B. subtilis improved the productive indexes and survival of Pseudoplatystoma sp. after bacterial challenge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental issues are increasingly present in the aquaculture production sector, where gaps related to environmental sustainability need answers from research and development groups, as they are extremely important for the sustainable advancement of the activity (Kavitha et al. 2018; Di et al. 2019; Kuebutornye et al. 2019). In this context, probiotics are emerging as environmentally friendly tools for application in aquaculture (Di et al. 2019; Kuebutornye et al. 2019). As a functional additive, probiotics directly affect the performance of aquatic organisms and have been widely used to supplement fish and shrimp diets (Liu et al. 2012; Telli et al. 2014; Kavitha et al. 2018; Di et al. 2019; Kuebutornye et al. 2019; Interaminense et al. 2019; Zhou et al. 2019).
Freshwater fish farming is the activity that most contributes to fish production in the world (FAO 2018). In this scenario, we can highlight the catfish of the genus Pseudoplatystoma sp. of high economic value, which emerge as an object of interest for aquaculture in Latin America (Campos 2005; Inoue et al. 2009). However, disease outbreaks, followed by high mortality rates and consequent economic losses, are common and are generally related to nutritional and health factors in the early stages of commercial production of Pseudoplatystoma sp. (Tavares-Dias and Martins 2017; Tavares et al. 2018). Nutrition is closely linked to the health of aquatic organisms. Thus, probiotics may, by competing for adhesion of the adhesion site in the digestive tract, nutrients and energy sources, prevent the establishment of pathogens in the intestinal epithelium, prevent injury, restore intestinal mucosa integrity and thereby improve the absorption of nutrients (Tournut 1998; Petri 2000; Kiron 2012; Kuebutornye et al. 2019) and thus may restrict the growth of undesirable microorganisms (Pal et al. 2019).
Considering all the possible nutritional and health benefits, several components have been tested to act on the intestinal mucosa aiming to manipulate the intestinal flora and morphology, to hinder the adhesion and colonization of pathogenic microorganisms to the intestinal epithelium (Tournut 1998; Boleli et al. 2000; Maiorka et al. 2002; Kuebutornye et al. 2019; Owatari et al. 2019). The benefits already observed for different fish species after ingestion of probiotics containing Bacillus spp. are diverse, among which we can highlight the increased immune response (Aly et al. 2008; Kumar et al. 2008; Dias et al. 2012; Farias et al. 2016; Nakandakare et al. 2018), growth improvement (Gatesoupe 1999; Farias et al. 2016; Kuebutornye et al. 2019), higher survival against some pathogens (Dias et al. 2012), microbiota replacement after antibiotic use and production of broad-spectrum antimicrobial substances (Green et al. 1999; Raida et al. 2003; Balcázar et al. 2006; Kuebutornye et al. 2019).
Bacteria of the genus Bacillus constitute a diverse group of gram-positive rods, characterized by their ability to produce important secondary metabolites such as antibiotics, bioinsecticides, biocins, organic acids and enzymes (Olmos 2003; Silva et al. 2013; Kuebutornye et al. 2019), which are very effective in breaking down a wide variety of carbohydrates, lipids and proteins (Sonnenschein et al. 1993). Bacillus are characterized by the formation of resistant spores that allow greater viability after feed processing (Yılmaz et al. 2020). In addition, Bacillus has high survival rates after exposure to stomach gastric acid, making them highly usable in aquaculture (Hong et al. 2005; Kuebutornye et al. 2019).
Preliminary studies exposed by Veiga et al. (2020) in a short communication demonstrated some potential effects of B. subtilis on juvenile Pseudoplatystoma sp., and based on the information, this study aimed to continue investigations on the influence of B. subtilis in the diet of Pseudoplatystoma sp., to strengthen the knowledge of its effects on non-specific immunity, haematology, productive performance, intestinal histomorphometry and resistance to Aeromonas hydrophila.
Material and methods
The fish used in this study hybrid surubim (Pseudoplatystoma reticulatum × P. corruscans) were obtained from Piraí Fish Farm, Terenos, MS, Central-Western Brazil. All procedures for fish handling and euthanasia were approved by the Ethical Committee on the Animal Use CEUA/UEMS no 014/2013, using eugenol (75 mg L−1) as an anesthetic procedure.
Experimental design
Water quality monitoring was performed daily at 08:00 with the aid of a handheld YSI Professional Plus multiparameter equipment. The environmental conditions during the experiment remained at a temperature of 24.8 ± 1.8 °C, dissolved oxygen 4.9 ± 0.4 mg L–1, pH 6.9 ± 0.3 and conductivity 193.0 ± 15.0 μS cm−1, within the recommended comfort range for the species (Sipaúba-Tavares et al. 1995).
Three hundred juveniles surubim, trained to receive dry food, with total length and weight of 15.9 ± 0.8 cm and 24.5 ± 3.6 g, respectively, were used. The fish were distributed in 30 experimental units with 40 L volume (10 fish per unit) with continuous water flow and aeration. Before starting the experiment, an external visual check was performed on the fish’s body, to verify the absence of lesions or changes in the pigmentation on the skin and gills. The fish remained for 10 days in the acclimatization period, until they got used to the daily handling and feeding routine.
The experimental design was randomized with four treatments and a probiotic-free control group, each with six replications. Four increasing levels of probiotic composed by B. subtilis were added to the experimental diet: 1%, 2%, 3% and 4% CALSPORIN® kg feed−1. The probiotic was included in the diet using oily medium (2% soybean oil) according to Nakandakare et al. (2013), for better adherence of the product to feed pellets. An extruded commercial feed 40% crude protein (2–4 mm pellets) was used for carnivorous fish. The fish were fed twice a day until satiety at 7:00 am and 5:00 pm during the experimental period of 20 days. After the inclusion of the probiotic, a sample of the feed from each treatment was submitted to microbiological analysis at the Microbiology Laboratory “Comércio e Indústria Uniquímica Ltda”–Diadema, São Paulo, to quantify the B. subtilis concentration present in the feed. For this, a sample of 1 g of feed from each treatment was macerated and inserted in a Falcon tube containing 50 mL of diluent to be homogenized. Subsequently, 1 mL of the sample was serially diluted in Falcon tubes containing 9 mL of diluent solution until the concentration of the dilution reached a count of 30–300 microorganisms per 1 mL. After the final dilution, an aliquot with a volume of 1 mL was sown in a Petri dish containing specific culture medium and incubated in a bacteriological incubator at 37 °C for 48 h. After incubation, B. subtilis colonies were confirmed and counted (Table 1).
Animal management and growth performance
To evaluate the growth performance of the fish, biometrics were performed to obtain weight and length data at the beginning and end of the experimental period. The zootechnical indices evaluated were apparent feed conversion (AFC) = feed intake/weight gain; weight gain (WG) = final weight − initial weight; biomass gain (BG) = final biomass − initial biomass; protein efficiency ratio (PER) = ratio between the biomass gain and crude protein intake (CPI), where CPBI corresponds to the percentage of crude protein (CP) of the experimental feed multiplied by the total feed intake; feed intake (FI) = average feed intake per fish during the experimental period; specific growth rate (SGR) = natural log of final weight (g) − natural log of initial weight (g) / experimental period (days) × 100; and survival = the final number of fish / the initial number of fish × 100.
Haematological parameters
For haematological analysis, the blood was collected and analysed at three different times: at the beginning of the experiment (0 day—basal collection), on the 10th day, and on the 20th day. Blood samples were withdrawn from 12 fish per treatment by puncturing the caudal vessel using 3% EDTA emulsified disposable syringes and needles.
The haematological parameters evaluated were hematocrit percentage by the microhematocrit method according to Goldenfarb et al. (1971), hemoglobin according to Collier (1944), red blood cells count (RBC) in a Neubauer chamber. From hematocrit, hemoglobin and erythrocyte amount data, the mean corpuscular volume (MCV) and the mean corpuscular hemoglobin concentration (MCHC) were calculated. Duplicate blood smears were made for each fish and stained with May Grünwald-Giemsa-Wright (Tavares-Dias and Moraes 2003) for differential leukocyte count, total thrombocyte count and white blood cell count (WBC) (Hrube and Smith 1998).
Phagocytic activity
At the end of the probiotic supplementation period, six fish from each experimental group were anesthetized in a solution containing eugenol and then injected intraperitoneally with the Saccharomyces cerevisiae yeast solution (9000 cell mm−3) according to Ranzani-Paiva et al. (2008).
After 2 h of yeast incubation, the animals were euthanized. Then, a ventral incision was made, where the peritoneal cavity was washed with 1.5 mL of phosphate-buffered saline solution (PBS—137 mM NaCl, 2.7 mM KCl, 20 mM Na2HPO4, 5 mM KH2PO4, pH 7.4). The fluid containing the inflammatory cells (macrophages) was aspirated with Pasteur pipette and then centrifuged at 1500g for 5 min. After centrifugation, the supernatant was discarded, and the remainder of the precipitate was deposited between the slide and coverslip and observed under phase-contrast microscopy for phagocyte count.
Phagocytic capacity (PC) and phagocytic index (PI) of phagocytes were calculated according to the methodology of Silva et al. (2002, 2005), where phagocytic capacity (PC) is the percentage of a particular cell type that is phagocytizing, expressed by the equation PC = (number of phagocytes / total counted phagocytes) × 100, and phagocytic index (PI) is the average number of yeast within phagocytic cells where 100 cells are counted, whose formula is PI = total number of yeast within phagocytes / total number of active phagocytes.
Intestinal histomorphometry
On the 20th day, biological material was collected for intestinal analysis. Histological slides were made for subsequent histomorphometry analysis of the intestinal epithelium of fish at the Veterinary Pathology Laboratory, UFMS. Twelve animals from each treatment were used.
The fish were euthanized, dissected and submitted to a ventral longitudinal incision for organ exposure. Approximately 3-cm sections were obtained from the midgut, cut longitudinally with microsurgical scissors. Afterward, the samples were fixed open with the internal organ wall facing upwards on a Styrofoam holder, dipped in Bouin’s fixative solution for 6 h and then transferred to 70% alcohol. Then, the samples were dehydrated in increasing series of alcohol, diaphanized in xylol and included in paraffin, to obtain cross-sections with the aid of microtome in 5 μm thickness. From each intestine, sample had made five slides containing five serial sections each, which were stained with hematoxylin-eosin (H & E) and analysed under a light microscope. The material was analysed at the Veterinary Pathology Laboratory of the Federal University of Mato Grosso do Sul, under a Zeiss optical microscope, coupled to a microcomputer with a computer program Motic Images Plus 2.0 ML.
For intestinal histomorphometric analyses, 25 intestinal villi per animal were measured, totaling 300 villi per treatment (Schamber 2008; Silva et al. 2010) in a computer program Motic Images Plus 2.0 ML.
Bacterial challenge
The bacterial challenge was performed at the end of the probiotic supplementation period. The fish were anesthetized in eugenol, and then, the bacterium A. hydrophila was inoculated intraperitoneally at a concentration of 2 × 108 CFU mL−1 according to Silva et al. (2012). A total of 90 animals were challenged, with 18 fish per treatment. Mortality and clinical signs of bacteriosis were observed and recorded every 24 h.
The Aeromonas hydrophila strain (ATCC 7966) used in the bacterial challenge was supplied by the Marine Shrimps Laboratory, Department of Aquaculture, Federal University of Santa Catarina. This strain was obtained during a bacterial outbreak with high mortality in a fish farm and was isolated from symptomatic surubim, presenting haemorrhagic septicemia. To prepare the material used in the experimental challenge, A. hydrophila bacteria were sown in 50 mL BHI (brain heart infusion) and incubated for 24 h in a bacteriological incubator at 30 °C. Then, it was centrifuged at 400g for 20 min, the supernatant was discarded and the pellet resuspended in sterile saline (0.65% sodium chloride) to maintain bacterial concentration. For each gram of fish, 0.01 mL of this bacterial suspension was used. Moribund and dead fish were removed from the tanks daily. To calculate the relative percent survival (RPS), the formula RPS = (1 − (% mortality / % mortality control)) × 100 was used, according to Amend (1981).
Statistical analysis
The experiment was structured presenting a logical ordering between the treatments that can be expressed as a function of each other. Regression analysis was used to verify the variation of y as a function of x. Data were submitted to analysis of variance (ANOVA) using Statistica 13.0 (Statsoft Inc., Tulsa, USA). Tukey’s test was used to compare means, with significance level of 5%. Data transformations were used according to pertinence.
Results
Growth performance
During the experimental period, the fish readily accepted diets containing probiotic B. subtilis and showed no behavioral changes. Weight gain (WG) was statistically different (p < .05) in fish in groups 1%, 2%, 3% and 4% when compared with fish in control group. Feed intake (FI) was lower in fish from the control group (p < .05) when compared with fish from other treatments. Among the four levels of inclusion of the probiotic, feed intake remained the same. Likewise, the biomass gain (BG) was statistically different (p < .05) between the groups. The fish in the 3% and 4% group obtained greater biomass gain when compared with the fish in the control group and similar to the other supplemented groups. In the other productive indexes, there were no statistical differences between the treatments (Table 2).
Haematological parameters
A statistical difference (p < .05) was observed in the haematological parameters of the fish between the treatments within the sampling periods, as well as between the sampling periods.
The most expressive results were observed on the 20th day of supplementation, where a number of monocytes in the fish of the 2%, 3% and 4% groups were statistically different when compared with the control group fish. Eosinophils were statistically higher in fish in the 3% group when compared with the other groups. Leukocyte numbers were statistically higher in fish in group 1% when compared with fish in control group and lymphocyte number was higher in fish in group 4% when compared with fish in group 2% and 3%, but similar to fish in control and 1% groups. Glucose was statistically different in fish in the 4% group when compared with the other groups. It was also possible to observe a significant difference (p < 0.05) in the number of erythrocytes, hemoglobin and MCHC between the supplemented and non-supplemented fish.
The probiotic caused significant changes (p < .05) in the haemogram between the sampling periods on days 0, 10 and 20. The comparisons over time showed an activation of specialized cells of the fish’s nonspecific defense mechanism. The number of erythrocytes, neutrophils, eosinophils, basophils, lymphocytes, monocytes, special granulocytic cells and leukocytes was statistically different in the collection on the 10th when compared with the collection in the basal period; however, it was the same when compared with the collection on the 20th (Table 3).
Phagocytic capacity
The inclusion of the probiotic B. subtilis in the diet of Pseudoplatystoma sp. increased phagocytic capacity (p < .05) in fish in the 4% group when compared with fish in the control and 1% group. However, it was similar to fish in groups 2% and 3%. There was no significant difference (p > 0.05) between the treatments for the phagocytic index (Fig. 1).
Intestinal histomorphometry
In the analysis of intestinal histomorphometry, the results showed significant differences (p < .05) between the groups. Villi height (μm) was higher in fish in group 2% when compared with fish in groups 1%, 3% and 4%, but it was similar to fish in the control group. In the measures of Villi width (μm), the fish in the 2% group was statistically greater when compared with the fish in the control group and similar to the other supplemented groups. Villi thickness (μm) was statistically higher in fish in the 1% group when compared with the other treatments (Fig. 2 and Table 4).
Intestinal histology analysis of juvenile Pseudoplatystoma sp. after 20 days of dietary supplementation with probiotic B. subtilis. A The intestinal morphology of the fish in the 2% group where the highest villi height was observed (long villi and lamina propria—arrow). B Morphometry of the fish in the control group that presented similar villi height to the fish in the 2% group (inner muscular layer—arrow). C It shows the intestinal morphology of the fish from the 2% group that presented greater villi width (external transverse muscular layer—arrow). Finally D we can observe the intestinal morphology of the fish in the 1% group that presented greater villi thickness (brush border—arrow). HE (bar = 200 μm)
Bacterial challenge
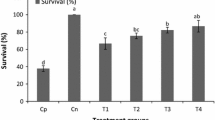
Significant differences (p < .05) were observed in survival rates between the groups in the first 24 h of exposure to the pathogen. The fish in the 3% group had higher survival rates when compared with the fish in the control groups, 1% and 4%; however, it was similar to the fish in the 2% group. In subsequent periods in 48, 72, 96 and 120 h of exposure to A. hydrophila, mortality was observed in all groups, but without significant differences. After 120 h, no mortality was observed.
In absolute numbers, after 120 h of exposure to the pathogen, the fish in group 4% had the lowest percentages of survival (61.1%), followed by fish in groups 1% and 2% (72.2%). Fish not supplemented had a survival rate of 77.8%, while fish in the 3% probiotic kg of feed−1 group had a survival rate of 83.3 (Fig. 3).
Percent survival of juvenile Pseudoplatystoma sp. supplemented for 20 days with probiotic B. subtilis at 1%, 2%, 3% and 4% kg feed−1 and control (0%) group after challenge with Aeromonas hydrophila. Different letters indicate significant differences (p < .05) in survival rates between the groups at the respective analysis times
Discussion
The present study verified the influence of dietary supplementation containing B. subtilis C-3102 for juveniles Pseudoplatystoma sp. The results obtained in the present study offer a starting point for new productive perspectives, including the haemato-immunological and sanitary aspects, as well as, important information about the influence of the probiotic on the intestinal morphology of Pseudoplatystoma sp.
Probiotic bacteria have been shown to contribute to fish growth. The literature proves that Bacillus species have probiotic properties and, when administered to fish, increase growth and improve the feed intake. Generally, the fact is related to the improvements conferred by the probiotic in the intestinal morphology, increasing the activity of digestive enzymes and, consequently, they can increase the digestive capacity of the feeds offered to fish. This is one of the reasons for choosing Bacillus as probiotic, because they are capable of this modulation in the digestive tract of fish (Dawood et al. 2019; Kuebutornye et al. 2019; Olmos et al. 2020).
The diets containing the probiotic showed different responses in growth performance and feed intake parameters of the present research. Likewise, Veiga et al. (2020), when offering a diet containing B. subtilis to Pseudoplatystoma sp. for 10 days, observed increases in biomass gain in fish supplemented with 3% probiotic per kilo of feed. Similarly, in the present research, fish that received the inclusion of 3% probiotic in the diet showed improvements in production performance.
According to Olmos et al. (2020) B. subtilis produce enzymes capable of improving the assimilation of nutrients, favoring the metabolism of carbohydrates, lipids and proteins in aquatic animals. Probiotic bacteria can facilitate the digestion of dietary proteins, as enzymes produced by probiotic bacteria can complement protease activity by increasing food digestibility (Ochoa-Solano and Olmos-Soto 2006; Kuebutornye et al. 2019). In this context, the catfish Pseudoplatystoma sp. as a carnivorous species (Honorato et al. 2015) could obtain productive improvements by including B. subtilis in its diet, since the digestion of alternative protein sources, such as those of vegetable origin, could be optimized. Replacement of even a small fraction of dietary protein may represent significant financial effectiveness and significantly lower environmental impact on large-scale production (Martino et al. 2002).
Haematological analyses may reflect the sanitary state of the fish. Complete fish blood cell counting is an important and powerful diagnostic tool, as well as a component of a valuable database. It can be used to monitor the health status of fish in response to changes related to nutrition, water quality and disease and in response to therapeutic methods (Fazio 2019). In the present study, three specific moments were decisive in the blood assessment of fish and, in this way, different haemograms were made. It is noted that in the present study, even without the influence of the treatment applied to the fish, the blood parameters may show marked variations, as observed in the basal data. If we observe the haemogram of the basal samples, there were differences in the number of erythrocytes between the groups. This fact may indicate that some stressful factor affected the fish prior to collection and reflecting in the other haematological indexes of the basal collection.
Stress is one of the factors that can make fish susceptible to disease, causing changes in the innate immune responses that occur at the cellular level in fish. In aquaculture, stressors are part of the activity characteristics, where handling, water quality, transport and stocking densities can affect fish physiology and health (Kuebutornye et al. 2019). The use of a probiotic based on B. subtilis was able to provide a stress reduction in Clarias gariepinus catfish, making it an important tool to mitigate the negative effects of cultivation systems (Romanova et al. 2020).
The haematological results found in the present study are similar to those found by Mouriño et al. (2016), when they performed a hematological approach of the hybrid surubim Pseudoplatystoma reticulatum × P. corruscans. At the time of the research, they investigated bacterial strains with probiotic potential isolated from fish intestines. Compared with control fish, fish supplemented with probiotic W. cibaria showed an increase in RBC. In contrast, Veiga et al. (2020), when supplementing the diets of Pseudoplatystoma sp. with B. subtilis for 10 days, did not observe significant differences for haematocrit, hemoglobin, erythrocytes, MVC, MCHC, glucose, monocytes, basophils, eosinophils and special granulocytic cell.
Unlike in the present study, haematological evaluation on the tenth day revealed a higher number of leukocytes circulating in the blood of fish. The probable answer to this contrast observed between the two studies may be the difference in the average water temperature in the different studies, where Veiga et al. (2020) observed that the water temperature remained at an average of 26.2 ± 2.9 °C, while in the present study, it was close to 24.8 ± 1.8 °C. The thermal comfort temperature for surubim is close to 28 °C (Campos 2005). Thus, it is very likely that, in the present study, the probiotic triggered a more consistent non-specific response on the tenth day of the experiment due to possible thermal stress due to the lower temperature.
The results of the haemogram of the present study showed that the probiotic supplementation was able to activate some leukocyte lineages in a representative manner between the groups. The cells that support the fish’s non-specific immune system are circulating in the bloodstream and immune responses can be triggered at any time in response to physical injuries or inflammatory processes. In addition, the phagocytic activities of B lymphocytes, granular eosinophilic cells present in the intestinal mucosa and gills can respond to bacteria and parasites (Ranzani-Paiva and Silva-Souza 2004; Kiron 2012).
The phagocytic process and cellular metabolism end up resulting in the production of reactive oxygen species that can be harmful when the production of lipid peroxidation exceeds the antioxidant capacity of cells or tissues. In these cases, Bacillus spp., such as probiotics, can produce antioxidant enzymes, for example superoxide dismutase and glutathione, to effectively eliminate free radicals. Whether in serum or mucus, Bacillus spp. can modulate antioxidant activities (Kuebutornye et al. 2019). The inclusion of B. subtilis in juvenile Pseudoplatystoma sp. diets provided higher phagocytic activity in all treated groups. Immune responses involving phagocytosis are assisted by non-specific immune cells such as monocytes/macrophages, neutrophils, among others. Phagocytosis is one of the main mechanisms used to remove pathogens and cellular debris (Kiron 2012). Thus, we can infer that the probiotic treatment had a positive effect on the fish immune system, increasing the innate defense capacity of the animals that received the additive at different inclusion levels.
The gut is a multifunctional organ directly involved in nutrition, so it is strongly related to fish immunity (Sugita et al. 1998; Kiron 2012). Cell renewal through mitosis or loss of cells at the base of the villi is caused by the development of the intestinal mucosa, increasing the density and height of the epithelial cells (Maiorka et al. 2002). In these cases of cell renewal, through increased cell proliferation, nutrient digestion and absorption are maximized with greater weight gain (Boleli et al. 2000).
In the present study, we observed a reduction in villi height at the highest levels of inclusion of B. subtilis (3% and 4%), suggesting that the gradual increase in the probiotic levels did not cause the development of the intestinal mucosa. These results corroborate with Veiga et al. (2020), who in a short communication reported that the probiotic B. subtilis improved the intestinal absorption surface of the hybrid Pseudoplatystoma corruscans × P. reticulatum. On the occasion, the researchers found the highest villi height in the inclusion content at 1% and the lowest heights in the highest probiotic inclusion levels.
The beneficial effects of probiotic B. subtilis on intestinal morphometry are related to the reduction of colonization of the intestinal epithelium by bacteria harmful to the mucosa, which act by inhibiting and competing for the adhesion sites in the enterocytes through the connection with the glycocalyx (Zhao et al. 2012; He et al. 2013; Zhou et al. 2019; Kuebutornye et al. 2020), promoting the competitive exclusion of unwanted bacteria in different segments of the intestine (Kuebutornye et al. 2019). Thus, when experimental conditions involve challenges to the immune system, it is very important to observe whether improvements in the intestinal morphology of animals will have a corresponding effect during exposure to the pathogen, as these responses are potentially related to the digestive physiology of fish when using supplements, such as probiotics.
The probiotic B. subtilis positively influenced the fish survival to A. hydrophila during the experimental challenge in the present study. Bacteria are natural components of the environment and, as such, are present in aquaculture environments. Probiotics have emerged as a sustainable solution to contain pathogens in aquaculture and bacteria of the Bacillus genus have been proven to more successfully combat a wide variety of fish pathogens, such as Aeromonas spp. Vibrio spp., Streptococcus spp., compared with other probiotics in aquaculture (Kavitha et al. 2018; Kuebutornye et al. 2020; Hayatgheib et al. 2020). In this way, B. subtilis is strengthened as a sustainable alternative to the use of antibiotics in aquaculture.
Tang et al. (2019) found that the dietary supplementation of probiotic B. subtilis affects antioxidant defenses and the immune response in grass carp exposed to A. hydrophila. The authors consider that B. subtilis can provide effective protection to fish against damage caused by A. hydrophila infection. Similarly, Zhang et al. (2019) found that B. subtilis increased intestinal apoptosis of grass carp Ctenopharyngodon idella challenged orally with A. hydrophila, suggesting that B. subtilis may play an important role in reducing intestinal damage after challenge.
As noted in the present study, as well as in the published literature, Bacillus spp. is widespread and tested on a wide variety of aquaculture species. Therefore, it becomes a viable alternative for optimizing food absorption and increased zootechnical performance, better responses to stress conditions, increased immune response and disease resistance (Kuebutornye et al. 2019).
In conclusion, the dietary supplementation with probiotic Bacillus subtilis applied in the present study in the inclusions of 1%, 2%, 3% and 4% caused significant improvements in the physiology of juvenile Pseudoplatystoma sp. and based on the data obtained in the research, we indicate the 2% and 3% inclusions as more efficient due to the improvements observed in the productive indexes, in the intestinal morphometry, as well as, in the non-specific immunity, optimizing the survival of the animals exposed to the pathogen.
References
Aly SM, Ahmed YA, Ghareeb AA, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136
Amend DF (1981) Potency testing of fish vaccines. Dev Biol Stand 49:447–454
Balcázar JL, De Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Muzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114(3–4):173–186
Boleli IC, Maiorka A, Macari M (2000) Estrutura funcional do trato digestório. In: Macari M, Furlan RL, Gonzalés EP (eds) Fisiologia aviária aplicada a frangos de corte. FUNEP/UNESP, Jaboticabal, pp 75–96c
Campos JL (2005) O cultivo do pintado. In: Baldisserotto B, Gomes LC (eds) Espécies nativas para piscicultura no Brasil. Ed. UFSM, Santa Maria, pp 327–342
Collier HB (1944) Standardization of blood haemoglobin determinations. Can Med Assoc J 50(6):550–552
Dawood MA, Koshio S, Abdel-Daim MM, Van Doan H (2019) Probiotic application for sustainable aquaculture. Rev Aquac 11(3):907–924
Di J, Chu Z, Zhang S, Huang J, Du H, Wei Q (2019) Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immunol 93:711–719
Dias DC, Furlaneto FPB, Ayroza LMS, Tachibana L, Romagosa E, Ranzani-Paiva MJT (2012) Probiotic in feeding of juvenile matrinxã (Brycon amazonicus): economic viability. Acta Scientiarum. Anim Sci 34(3):239–243
FAO (2018). The State of World Fisheries and Aquaculture 2018-Meeting the sustainable development goals. Licence: CC BY-NC-SA 3.0 IGO
Farias THV, Levy-Pereira N, de Oliveira AL, de Carla DD, Tachibana L, Pilarski F, Ranzani-Paiva MJT (2016) Probiotic feeding improves the immunity of pacus, Piaractus mesopotamicus, during Aeromonas hydrophila infection. Anim Feed Sci Technol 211:137–144
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Goldenfarb PB, Bowyer FP, Hall E, Brosious E (1971) Reproducibility in the hematology laboratory: the microhematocrit determination. Am J Clin Pathol 56(1):35–39
Green DH, Wakeley PR, Page EA, Barnes A, Baccigalupi L, Ricca E, Cutting SM (1999) Characterization of two Bacillus probiotics. Appl Environ Microbiol 65:4288–4291
Hayatgheib N, Moreau E, Calvez S, Lepelletier D, Pouliquen H (2020) A review of functional feeds and the control of Aeromonas infections in freshwater fish. Aquacult Int 28:1083–1123
He S, Zhang Y, Xu L, Yang Y, Marubashi T, Zhou Z, Yao B (2013) Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression and autochthonous bacteria of hybrid tilapia Oreochromis niloticus♀× Oreochromis aureus♂. Aquaculture 412:125–130
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Honorato CA, Ushizima TT, Santamaria FM, Flores-Quintana CI, Marcondes VM, Nascimento CA (2015) Desempenho produtivo e econômica de surubins (Pseudoplatystoma sp) alimentados com níveis de proteína e estocados em tanque-rede. Arq Bras Med Vet Zootec 67(5):1408–1414
Hrube TC, Smith SA (1998) Hematology of fish. In: Schalm’s veterinary hematology, 5th edn, pp 1120–1125
Inoue L, Hisano H, Ishikawa M, Rotta M, Senhorini J (2009) Princípios básicos para produção de alevinos de surubins (Pintado e Cachara). Embrapa Pantanal-Documentos (INFOTECA-E)
Interaminense JA, Vogeley JL, Gouveia CK, Portela RS, Oliveira JP, Silva SM, Bezerra RS (2019) Effects of dietary Bacillus subtilis and Shewanella algae in expression profile of immune-related genes from hemolymph of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Fish Shellfish Immunol 86:253–259
Kavitha M, Raja M, Perumal P (2018) Evaluation of probiotic potential of Bacillus spp. isolated from the digestive tract of freshwater fish Labeo calbasu (Hamilton, 1822). Aquacult Rep 11:59–69
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Tech 173(1–2):111–133
Kuebutornye FK, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol 87:820–828
Kuebutornye FK, Abarike ED, Lu Y, Hlordzi V, Sakyi ME, Afriyie G, Wang Z, Li Y, Xie CX (2020) Mechanisms and the role of probiotic Bacillus in mitigating fish pathogens in aquaculture. Fish Physiol Biochem:1–23
Kumar R, Mukherjee SC, Ranjan R, Kayak SK (2008) Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol 24:168–172
Liu CH, Chiu CH, Wang SW, Cheng W (2012) Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish Immunol 33(4):699–706
Maiorka A, Boleli IC, Macari M (2002) Desenvolvimento e reparo da mucosa intestinal. In: Macari M, Furlan RL, Gonzales E (eds) Fisiologia aviária aplicada a frangos de corte. Jaboticabal, FUNEP/UNESP pp, pp 143–148
Martino RC, Cyrino JEP, Portz L, Trugo LC (2002) Performance and fatty acid composition of surubim (Pseudoplatystoma coruscans) fed diets with animal and plant lipids. Aquaculture 209(1–4):233–246
Mouriño JLP, do Vale Pereira G, do Nascimento Vieira F, Jatobá AB, Ushizima TT, da Silva BC, Seiffert WQ, GFA J, Martins ML (2016) Isolation of probiotic bacteria from the hybrid south American catfish Pseudoplatystoma reticulatum × Pseudoplatystoma corruscans (Siluriformes: Pimelodidae): a haematological approach. Aquacut Rep 3:166–171
Nakandakare IB, Iwashita MKP, Dias DDC, Tachibana L, Ranzani-Paiva MJT, Romagosa E (2013) Growth performance and intestinal histomorphology of Nile tilapia juveniles fed probiotics. Acta Sci Anim Sci 35(4):365–370
Nakandakare IB, Iwashita MKP, Danielle de Carla DIAS, Tachibana L, Ranzani-Paiva MJT, Romagosa E (2018) Incorporação de probióticos na dieta para juvenis de tilapias-do-Nilo: parâmetros hematológicos, imunológicos e microbiológicos. Bol Inst Pesca 39(2):121–135
Ochoa-Solano JL, Olmos-Soto J (2006) The functional property of Bacillus for shrimp feeds. Food Microbiol 23:519–525
Olmos SJ (2003) Molecular characterization and phylogenetic identification of marine microorganisms. In: X Congreso Nacional de Biotecnología y Bioingeniería. Puerto Vallarta, Jalisco, Mexico
Olmos J, Acosta M, Mendoza G, Pitones V (2020) Bacillus subtilis, an ideal probiotic bacterium to shrimp and fish aquaculture that increase feed digestibility, prevent microbial diseases, and avoid water pollution. Arch Microbiol 202(3):427–435
Owatari MS, Jesus GFA, Cardoso L, Ferreira TH, Ferrarezi JVS, de Pádua PU, Mouriño JLP (2019) Different via to apply the Gamaxine® commercial biopromoter to Nile tilapia evaluating the immune system responses to Streptococcus agalactiae Ib. Aquaculture 503:254–266
Pal RR, Baidya AK, Mamou G, Bhattacharya S, Socol Y, Kobi S, Rosenshine I (2019) Pathogenic E. coli extracts nutrients from infected host cells utilizing injectisome components. Cell 177(3):683–696
Petri R (2000) Uso de exclusão competitiva na avicultura no Brasil. II Simpósio de sanidade avícola, Santa Maria
Raida MK, Larsen JL, Nilsen ME, Buchmann K (2003) Enhanced resistance of rainbow trout, Oncorhynchus mykiss (Walbaum), against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (BIOPLUS2B). J Fish Dis 26:495–498
Ranzani-Paiva MJT, Silva-Souza AT (2004) Hematologia de peixes brasileiros. Sanidade de organismos aquáticos São Paulo: Varela pp 89–120
Ranzani-Paiva MJT, Santos AA, de Carla Dias D, Seriani R, Egami MI (2008) Hematological and phagocytic response of the fat snook, Centropomus parallelus, reared in net cages, before and after inoculation with Sacharomyces ceresivisiae. Bioikos 22(1):29–35
Romanova E, Spirina E, Romanov V, Lyubomirova V, Shadyeva L (2020) Effects of Bacillus subtilis and Bacillus licheniformis on catfish in industrial aquaculture. In E3S Web of Conferences (Vol. 175, p. 02013). EDP Sciences
Schamber CR (2008) Exigência de fósforo para a tilápia do Nilo (Oreochromis niloticus) na terminação. Universidade Estadual de Maringá (2008)
Silva JRMC, Staines NA, Hernandez-Blazquez FJ, PortoNeto LR, Borges JCS (2002) Phagocytosis and giant cell formation at 0°C by macrophage of Notothenia coriiceps. J Fish Biol 60:466–478
Silva JRMC, Porto-Neto LR, Borges JCS, Jensch-Junior BE (2005) Germicide capacity of macrophages in the Antartic fish Notothenia coriiceps (Richardson, 1844) at 0°C. Polar Biol 28(4):326–328
Silva LCR, Furuya WM, Natali MRM, Schamber CR, Santos LD, Vidal LVO (2010) Desempenho e morfometria intestinal de juvenis de tilápia-do-nilo alimentados com dietas suplementadas com L-glutamina e L-glutamato. R Bras Zootec 39(6):1175–1179
Silva BC, Mouriño JLP, Vieira FN, Jatobá A, Seiffert WQ, Martins ML (2012) Haemorrhagic septicaemia in the hybrid surubim (Pseudoplatystoma corruscans x Pseudoplatystoma fasciatum) caused by Aeromonas hydrophila. Aquac Res 43:908–916
Silva EF, Soares MA, Calazans NF, Vogeley JL, Do Valle BC, Soares R, Peixoto S (2013) Effect of probiotic (Bacillus spp.) addition during larvae and postlarvae culture of the white shrimp Litopenaeus vannamei. Aquac Res 44:13–21
Sipaúba-Tavares LH, Ligeiro SR, Durigan JG (1995) Variação de alguns parâmetros limnológicos em um viveiro de piscicultura em função da luz. Acta Limnol Bras 7:138–150
Sonnenschein AL, Losick R, Hoch JA (1993) Bacillus subtilis and others gram-positive Bacteria: biochemistry, physiology and molecular genetics. American Society for Microbiology, Washington
Sugita H, Hirose Y, Matsuo N, Deguchi Y (1998) Production of the antibacterial substance by Bacillus sp. strain NM 12, an intestinal bacterium of Japanese coastal fish. Aquaculture 165:269–280
Tang Y, Han L, Chen X, Xie M, Kong W, Wu Z (2019) Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under Aeromonas hydrophila challenge. Probiotics Antimicro 11(2):545–558
Tavares GC, de Queiroz GA, Assis GBN, Leibowitz MP, Teixeira JP, Figueiredo HCP, Leal CAG (2018) Disease outbreaks in farmed Amazon catfish (Leiarius marmoratus x Pseudoplatystoma corruscans) caused by Streptococcus agalactiae, S. iniae, and S. dysgalactiae. Aquaculture 495:384–392
Tavares-Dias M, Martins ML (2017) An overall estimation of losses caused by diseases in the Brazilian fish farms. J Parasit Dis 41(4):913–918
Tavares-Dias M, Moraes FR (2003) Características hematológicas da Tilapia rendalli Boulenger, 1896 (Osteichthyes: Cichlidae) capturada em “pesque-pague” de Franca, São Paulo, Brasil. Biosci J 19(1):107–114
Telli GS, Ranzani-Paiva MJT, de Carla Dias D, Sussel FR, Ishikawa CM, Tachibana L (2014) Dietary administration of Bacillus subtilis on hematology and non-specific immunity of Nile tilapia Oreochromis niloticus raised at different stocking densities. Fish Shellfish Immunol 39(2):305–311
Tournut JR (1998) Probiotics. Reunião Anual Soc Bras Zootec 35:179–199
Veiga PT, Owatari MS, Nunes AL, Rodrigues RA, Kasai RYD, Fernandes CE, de Campos CM (2020) Bacillus subtilis C-3102 improves biomass gain, innate defense, and intestinal absorption surface of native Brazilian hybrid Surubim (Pseudoplatystoma corruscans x P. reticulatum). Aquacult Int 28:1183–1193
Yılmaz S, Ergun S, Yigit M, Çelik EŞ (2020) Effect of combination of dietary Bacillus subtilis and trans-cinnamic acid on innate immune responses and resistance of rainbow trout, Oncorhynchus mykiss to Yersinia ruckeri. Aquac Res 51(2):441–454
Zhang D, Wu Z, Chen X, Wang H, Guo D (2019) Effect of Bacillus subtilis on intestinal apoptosis of grass carp Ctenopharyngodon idella orally challenged with Aeromonas hydrophila. Fish Sci 85(1):187–197
Zhao Y, Zhang W, Xu W, Mai K, Zhang Y, Liufu Z (2012) Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 32(5):750–755
Zhou S, Song D, Zhou X, Mao X, Zhou X, Wang S, Qin Q (2019) Characterization of Bacillus subtilis from gastrointestinal tract of hybrid Hulong grouper (Epinephelus fuscoguttatus × E. lanceolatus) and its effects as probiotic additives. Fish Shellfish Immunol 84:1115–1124
Acknowledgments
The authors thank the National Council for Scientific Development (CNPq) for their financial support to the project entitled “Probiotic and immunostimulant in the production of surubim” (CNPq 552395/2011-0), grant to M.L. Martins (CNPq 306635/2018-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Short term of probiotic feeding evaluation in the diet of Brazilian native catfish Pseudoplatystoma sp.
- B. subtilis causes significant improvements in non-specific fish immunity.
- Probiotic feeding increases resistance to A. hydrophila.
- Short-term supplementation leads to productive improvements in Pseudoplatystoma sp.
Rights and permissions
About this article
Cite this article
Nunes, A.L., Owatari, M.S., Rodrigues, R.A. et al. Effects of Bacillus subtilis C-3102-supplemented diet on growth, non-specific immunity, intestinal morphometry and resistance of hybrid juvenile Pseudoplatystoma sp. challenged with Aeromonas hydrophila. Aquacult Int 28, 2345–2361 (2020). https://doi.org/10.1007/s10499-020-00586-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00586-1