Abstract
The dietary calcium requirement of fingerling Labeo rohita (6.0 ± 0.11 cm; 1.94 ± 0.07 g) was examined using seven iso-nitrogenous and iso-caloric purified diets (350 g kg−1 crude protein; 16.72 kJ g−1 gross energy) with incremental levels of calcium (2.14, 3.12, 4.23, 5.11, 6.14, 8.23, and 11.24 g kg−1) that were hand-fed to triplicate groups of fish (20 fish per tank) to apparent satiation for 8 weeks. At the end of the feeding trial, ten fish from each replicate tank (n = 10 × 3) were sampled to obtain serum, scales, and vertebrae. The remaining fish in each tank were sampled to determine proximate chemical body composition. Live weight gain (LWG; 637.6%), protein gain (PG; 2.19 g fish−1), protein retention efficiency (29.89%), and feed conversion ratio (1.69) improved with increased dietary calcium up to 5.11 g kg−1 and then stabilized. Dietary calcium up to 5.11 g kg−1 significantly increased calcium and phosphorus concentrations of whole body, vertebrae, and scales. Fish fed a diet with calcium higher than 5.11 g kg−1 had a significant decrease (P < 0.05) in magnesium and zinc, while potassium was unaffected. Serum alkaline phosphatase activity increased (P < 0.05) with increased dietary calcium up to 5.11 g kg−1, but serum calcium and phosphorus concentrations remained unchanged. Based on broken-line regression analysis of LWG, PG, whole body, vertebrae, and scale calcium content against dietary calcium concentration, dietary calcium in the range of 5.16–5.48 g kg−1 is recommended for fingerling L. rohita.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutritionally balanced and cost-effective feed plays an important role in determining the quality and success of intensive aquaculture. A complete diet must have adequate amounts of protein, fat, carbohydrate, vitamins, and minerals. Although energy-giving nutrients play an important role, the importance of vitamins and minerals cannot be underestimated. Minerals are involved in the formation of skeletal, and other hard, tissues and also function in electron transfer, regulation of acid-base balance, production of membrane potentials, and osmoregulation (NRC 2011). Of the minerals required by fish, some, the macro-minerals, are required in large quantities; these include calcium, phosphorus, potassium, and magnesium. Calcium (Ca) is required by aquatic organisms for proper growth, bone mineralization, and other physiological functions such as blood clotting, muscle functioning, and the transmission of nerve impulses (NRC 2011). Calcium plays a key role in ionic regulation of freshwater fish because it influences biological membrane permeability and inhibits diffusive efflux and excessive loss of ions to the surrounding water (Wood and McDonald 1988). It is also essential for activating several enzymes and stimulating muscle contraction (Hossain and Yoshimatsu 2014). Approximately 99% of the body calcium of teleosts is incorporated into bone and scales, which may act as internal calcium and phosphorus reservoirs (Flik et al. 1986).
Calcium is present dissolved in water, and all or part of the calcium requirement of the fish can be met by absorption. A fish may be able to satisfy its requirement via absorption when in high-calcium water, but in low-calcium water, the contribution of calcium present in food increases (Ichikawa and Oguri 1961; Steffens 1997). Despite the importance of calcium for fish growth and health, few studies have been performed to quantify the dietary calcium requirements of freshwater fish species (Chavez-Sanchez et al. 2000; Paul et al. 2004, 2006; Shiau and Tseng 2007; Liang et al. 2012; Kandeepan 2013). A few studies have been conducted on fish held in calcium-free water (Robinson et al. 1986, 1987).
Indian major carps (IMCs) are the mainstay of freshwater aquaculture on the Indian subcontinent. IMCs are mostly produced via semi-intensive farming using feeds based on locally availability ingredients. Of the IMCs, Labeo rohita is the most popular aquaculture species. It has good market demand, fast growth, and attains the market size of 800–1000 g in a year (FAO 2009). With the introduction of intensive cultivation, there is a need to develop formulated feeds that meet all the nutritional requirements of a species (NRC 2011). Apart from some information about phosphorus and calcium requirements of L. rohita fry (Paul et al. 2006), there is a lack of knowledge about the dietary calcium requirement of fingerling L. rohita. A study was, therefore, performed to generate data relating to the calcium requirement and calcium/phosphorus ratio in the diet of fingerling L. rohita.
Materials and methods
Experimental diets
Seven iso-nitrogenous and iso-energetic (350 g kg−1 crude protein; 16.72 kJ g−1 gross energy) feeds (C1-C7) were prepared with calcium lactate (Loba Chemie, India) replacing cellulose to obtain graded concentrations of calcium. The dietary range of calcium was based on requirements reported for other fish species (NRC 2011; Antony Jesu Prabhu et al. 2016). Analyzed dietary calcium concentrations were 2.14, 3.12, 4.23, 5.11, 6.14, 8.23, and 11.24 g kg−1 (Table 1). Dietary phosphorus concentration was fixed at 6.56 g kg−1, based on information from Musharraf and Khan (2019). Based on previous studies with L. rohita, dietary protein was fixed at 350 g kg−1 (Renukardhyay and Varghese 1986; Khan 1991; Debnath et al. 2012), with casein and gelatin serving as dietary protein sources. Feeds were prepared as described by Abdel-Hameid et al. (2017); ingredients were mixed to form a dough which was forced through a 2-mm die to obtain feed strands that were dried in hot air at 40 °C, crumbled and sieved and then stored in a freezer in small sealed polythene bags.
Fish husbandry and experimental setup
L. rohita fingerlings were obtained from the hatchery of College of Fisheries, G. B. Pant University of Agriculture and Technology, Pantnagar. These fingerlings were dipped in KMnO4 solution (1:3000) as a prophylactic measure. Fishes were then transferred to indoor plastic lined cylindrical fish tanks (1.22 m in diameter, 0.91 m in height; water volume 600 L) and acclimated to the laboratory conditions and basal diet (350 g kg−1 crude protein) for 2 weeks.
Acclimatized L. rohita fingerlings (6.0 ± 0.11 cm; 1.94 ± 0.07 g) were randomly distributed to 21 polyvinyl circular tanks (volume 70 L; water volume 55 L; fitted with flow-through (1–1.5 L min−1) system) in triplicate at the stocking density of 20 fish per tank for seven dietary treatment levels. Crumbled experimental diets were hand-fed near to satiation three times daily at 0800, 1200, and 1600 h. Fecal matter was removed before every feeding. The unconsumed feed was collected, dried, and weighed to calculate the feed intake. To monitor the growth performance, anesthetized fish (MS-222; 100 mg L−1) of each experimental group was fortnightly weighed on a top-loading electronic balance (Precisa 120A; 0.1 mg sensitivity; Oerlikon AG, Zurich, Switzerland). The duration of the feeding trial was 8 weeks.
Water quality parameters
The water quality parameters such as temperature, total alkalinity, dissolved oxygen, total ammonia nitrogen, free carbon dioxide, and pH were evaluated daily as per the APHA (1992) standard methods and were found to range between 25.2–28.3 °C, 66.1–75.2 mg L−1, 7.2–7.6 mg L−1, 0.27–0.35 mg L−1, 6.2–9.1 mg L−1, and 7.1–7.4, respectively. The calcium and phosphorus concentrations of the rearing water were found in the range of 18.65–24.23 mg L−1 and 0.07–0.14 mg L−1, respectively, during the feeding trial.
Sample collection and chemical analysis
Fishes were deprived of the feed for 24 h before sampling. At the start of feeding trial, 40 fish were anesthetized (MS-222; 200 mg L−1), sacrificed, and pooled to determine the initial body composition. At the end of feeding trial, ten fish from each replicate tank were randomly collected, anesthetized with 100 mg L−1 of MS-222, and the blood samples were collected from the caudal vein of fish using a 2-mL syringe. The collected blood samples from each replicate tank were pooled in Eppendorf tubes, clotted, and then centrifuged at 3000×g for 10 min at 4 °C. Serum was separated and stored at − 20 °C until used. After blood collection, these fishes were sacrificed for obtaining the scales and vertebrae samples as per the methods detailed in our previous study (Musharraf and Khan 2019). Scales were gently removed through direct scrape from the anterior lateral sides of fish, cleaned using distilled-deionized water, and then dried at 105 °C. Subsequently to the scales collection, fish were cooked in a microwave oven for about 5 min to remove the vertebrae. Vertebrae samples were lightly scrubbed, cleaned, oven dried for about 2 h at 105 °C, and then solvent extracted and dried at 60 °C. Scales and vertebrae samples were then ground separately for mineral analysis. Remaining ten fish from each replicate tank (n = 10 × 3) were sampled to determine the final body composition.
Proximate composition of experimental diets and final and initial body samples were analyzed following the standard methods (AOAC 2005). Moisture was estimated in a hot air oven at 102 ± 1 °C (Yorko Instruments, New Delhi, India), crude protein (N × 6.25) was analyzed by the Kjeldahl method using an automatic analyzer (Kjeltec Tecator™ Technology 2300, Hoganas, Sweden), crude fat was estimated by the ether-extraction method using a Socs Plus SCS 4 (Pelican Equipments, Chennai, India), and crude ash was estimated by incineration in a muffle furnace at 550 °C for 12 h (S.M. Scientific Instrument Pvt. Ltd., Jindal Company, New Delhi, India). Gross energy content was estimated using a Gallenkamp ballistic bomb calorimeter CBB 330 010L (Gallenkamp, Loughborough, UK). Calcium and phosphorus concentrations of the dietary ingredients, test diets, scales, vertebrae, whole body, serum, and rearing water and potassium, magnesium, and zinc concentrations of the scales, vertebrae, and whole body samples were analyzed by the inductively coupled plasma atomic emission spectrometer (ICP-AES, model: IRIS INTREPID II XSP DUO; Thermo Electron Corporation, Beverly, MA, USA) after acid digestion of the samples as per the method adopted by Liang et al. (2014). Serum alkaline phosphatase (ALP) activity was assayed as per the method adopted by Apines et al. (2003). Briefly, 15 μL diluted serum sample was added to 1.0 mL of 16.7 mM p-nitrophenyl phosphate in 0.5 M 2-amino-2 methyl-1 propanol buffer at pH 10.4 (Sigma Chemical Co., St. Louis, MO, USA). Following incubation at 37 °C for 30 min, 2.0 mL of 0.25 N NaOH was added to stop the reaction. Killed enzyme blanks were prepared by adding 2.0 mL of 0.25 N NaOH directly to the sample followed by the addition of the buffered p-nitrophenyl phosphate. The optical density of the samples and blanks was determined in a spectrophotometer at 405 nm against p-nitrophenol standards (Sigma Chemical Co.).
Evaluation of growth performance
The different nutritional indices were determined as follows:
Statistical analyses
Shapiro-Wilk test was used to test the normality of the data, while Levene’s test was used to test the equality of variances before conducting one-way analysis of variance (ANOVA; Sokal and Rohlf 1981). Differences among treatment means were determined by Tukey’s honestly significant difference (HSD) test at a P < 0.05 level of significance. Optimal dietary calcium requirement of fingerling L. rohita was determined using the broken-line regression model (Robbins et al. 2006). All the statistical analyses were performed using the Origin software (version 6.1; Origin Software, San Clemente, CA, USA).
Results
Growth performance and conversion efficiencies
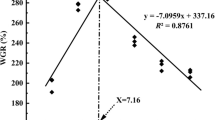
All fish survived for the full duration of the trial. Growth and feed utilization improved as dietary calcium concentrations increased from 2.14 to 5.11 g kg−1, but there were no further improvements thereafter (Table 2). Broken-line analysis of live weight and protein gain against increasing concentrations of dietary calcium indicated the optimal requirements at 5.19 and 5.22 g kg−1 of diet, respectively (Figs. 1 and 2).
Whole body composition
Whole body moisture declined consistently (P < 0.05), while crude protein increased with increasing levels of calcium up to 5.11 g kg−1 diet (C4) and then stabilized. Whole body fat did not changed (P > 0.05) in fish fed all the diets. Crude ash responded positively (P < 0.05) up to 5.11 g calcium kg−1 of diet (C4) and then levelled off (Table 3).
Mineralization in whole body, scales, and vertebrae
Calcium and phosphorus concentrations in the whole body, scales, and vertebrae increased (P < 0.05) with increase in dietary calcium levels up to 5.11 g kg−1 (C4) and then stabilized. Broken-line analysis of whole body, vertebrae, and scale calcium concentrations against varying dietary calcium levels depicted the optimal requirements at 5.16, 5.36 and 5.48 g kg−1, respectively (Figs. 3, 4, and 5). Whole body, scales, and vertebrae magnesium concentration remained unaffected (P > 0.05) up to 5.11 g kg−1 dietary calcium level and then declined significantly. Zinc concentration of whole body, scales, and vertebrae responded positively (P < 0.05) up to 5.11 g calcium kg−1 diet (C4), and thereafter, a significant decline was recorded. No significant variation was recorded in potassium concentration and calcium/phosphorus ratios of whole body, scales, and vertebrae in fish fed different dietary calcium levels (Table 4).
Biochemical composition of serum
Serum calcium, phosphorus, and calcium/phosphorus ratio were not affected (P > 0.05) with increasing levels of dietary calcium in all the treatment groups. Serum alkaline phosphatase (ALP) activity increased (P < 0.05) with increasing levels of calcium up to 5.11 g kg−1 of diet (C4) and then a constancy was recorded (Table 5).
Discussion
It is normally accepted that calcium requirement of most of the freshwater fish species is fulfilled mainly by the absorption of calcium through skin and gills and in marine fishes by drinking of seawater (Lall 2002). In this study, fingerling L. rohita fed C1 (2.14 g kg−1), C2 (3.12 g kg−1), and C3 (4.23 g kg−1) diets exhibited reduced growth, feed conversion, vertebrae, and scale mineralization indicating that these diets contained suboptimal level of calcium. These deficiency signs were also reported in many previous investigations (Paul et al. 2004, 2006; Shiau and Tseng 2007; Liang et al. 2012). However, deficiency of calcium has not been noted in some previous studies (Andrews et al. 1973; Ogino and Takeda 1976).
Although calcium concentration of the rearing water was high (18.65–24.23 mg L−1), significant gain in weight of rohu fed diets with incremental levels of calcium was recorded (Table 2), suggesting an essentiality and dietary requirement. Maximum growth and best conversion efficiencies were found at 5.11 g kg−1 dietary calcium, indicating that this calcium level is required for optimizing growth. Based on the broken-line regression analysis for mineralization response parameters, the requirement of calcium for fingerling L. rohita was determined to be in the range 5.16–5.48 g kg−1 diet. A requirement for dietary calcium, as established for fingerling L. rohita, was noted for other fish species, such as channel catfish, Ictalurus punctatus (Robinson et al. 1986) and blue tilapia, Oreochromis aureus (Robinson et al. 1987) reared in calcium-free water, and American cichlid, Cichlasoma urophthalmus (Chavez-Sanchez et al. 2000); mrigal, Cirrhinus mrigala (Paul et al. 2004); hybrid tilapia, Oreochromis niloticus × O. aureus (Shiau and Tseng 2007); and grass carp, Ctenopharyngodon idella (Liang et al. 2012) held in freshwater that contained a range of calcium concentrations. Dietary calcium does not appear to be required by all freshwater fish species, including common carp, Cyprinus carpio (Ogino and Takeda 1976); rainbow trout, Oncorhynchus mykiss (Ogino and Takeda 1978); and guppy, Poecilia reticulata (Shim and Ho 1989), when reared in water that contains calcium.
In the present investigation, it was recorded that increase in levels of dietary calcium led to increment in whole body protein and ash concentrations and decrement in the moisture concentration of fish fed diet containing calcium up to 5.11 g kg−1 (C4). Similar findings were also noted in many previous investigations (Chavez-Sanchez et al. 2000; Paul et al. 2004, 2006). The higher body protein and lower body moisture concentration in fish fed 5.11 g kg−1 (C4) dietary calcium suggest that the above level of calcium is good enough for efficient protein metabolism leading to improvement in protein retention and fish growth. However, no significant change was witnessed in the whole body fat concentration of fingerling L. rohita fed different dietary calcium levels. In contrast, body fat concentration was found to be somewhat lower in rainbow trout fed higher dietary calcium levels (Kalantarian et al. 2013). From these findings, it seems that calcium in the diet can reduce the overall fat absorption by its precipitation in intestine that resulted in its increased excretion through feces.
In teleosts, bones and scales are the main site of calcium deposition comprising of approximately 99% of the body calcium concentration (Flik et al. 1986; Hossain and Yoshimatsu 2014). Whole body, scales, and vertebrae calcium concentration have been evaluated as response parameters in many previous investigations to determine the dietary calcium requirement of fish (Shiau and Tseng 2007; Liang et al. 2012). In this investigation also, whole body, scales, and vertebrae calcium concentration have been used as reliable parameters to determine the dietary calcium requirement of fingerling L. rohita which improved up to 5.11 g kg−1 dietary calcium level. Similarly, Liang et al. (2012) observed that whole body calcium concentration and that of the above tissues of grass carp increased significantly with increasing levels of dietary calcium up to 7.99 g kg−1. Phosphorus concentration of whole body, scales, and vertebrae was also found to increase with increasing levels of dietary calcium in the fish under study, while in grass carp, phosphorus concentration increased in vertebrae only. Shiau and Tseng (2007) also observed significant improvement in calcium and phosphorus concentrations in the scales and bones of hybrid tilapia fed diets with varying doses of calcium. In this study, calcium/phosphorus ratios of whole body, scales, and vertebrae remained insignificantly different among the different treatment groups irrespective of the dietary calcium levels. Similar findings have also been reported previously (Ogino et al. 1979; Watanabe et al. 1980; Liang et al. 2012). These findings suggested that fish have the capability of balancing the calcium and phosphorus ratio by the absorption or excretion of calcium (Watanabe et al. 1980). Liang et al. (2012) suggested that different fish species maintain the calcium/phosphorus ratios steady. Furthermore, in this investigation, the level of phosphorus in trial diets was adjusted as per the requirement of fingerling L. rohita (Musharraf and Khan 2019). This may be one of the reasons for constancy in the calcium/phosphorus ratios in whole body and different tissues of L. rohita as the fish may have absorbed and utilized the dietary phosphorus to balance the calcium/phosphorus ratios.
Higher levels of dietary calcium can interfere with the availability and utilization of other essential elements in fish (Lall 2002). In this study, increase in calcium levels above 5.11 g kg−1 diet resulted in significant reduction in magnesium and zinc concentrations in the whole body, scales, and vertebrae, while potassium concentration remained unaffected. This is probably due to inhibitory effect of high dietary calcium level on magnesium and zinc, making them less available to fingerling L. rohita. In Atlantic salmon (Salmo salar), higher levels of dietary calcium supplementation resulted in reduced magnesium deposition in scales and vertebrae (Vielma and Lall 1998). Dietary calcium levels above requirement significantly decreased body magnesium concentration, and highest zinc concentration in scales and vertebrae was observed in grass carp fed on diet containing 6.24 g kg−1 calcium followed by a decrease on further increase in dietary calcium levels (Liang et al. 2012). These findings suggest that high dietary calcium levels might have a competitive inhibitory effect on these cations during intestinal absorption resulting in their reduced absorption and retention (Roy and Lall 2003).
In the present investigation, dietary calcium requirement of fingerling L. rohita obtained at 5.19 g kg−1 is higher than that reported by Paul et al. (2006) which is 1.9 g kg−1. Lower requirement of dietary calcium in Paul’s report may be because the fish were stocked in water containing high calcium concentration (50 mg L−1), while in this study, rearing water calcium concentration was lower (18.65–24.23 mg L−1). This is because of the fact that fish may uptake calcium from water to fulfil the requirement (Lall 2002). The other possibility of the difference in the dietary calcium requirements may be because the test diets did not contain higher levels of dietary calcium than recommended requirement in the investigation conducted by Paul et al. (2006). In mineral nutrition studies, growth is not considered to be a sufficient indicator to establish the requirement of mineral under question and must be substantiated by mineral analyses of the tissues (Baker 1986; Cowey 1992). In this investigation, along with growth parameters other stringent criterion such as whole body, vertebrae and scale calcium concentrations were also evaluated to assess the dietary requirement of calcium for fingerling L. rohita, and the values determined based on these parameters are close to those obtained with growth data.
Calcium is present in blood either as protein bound, combined with citrate or as free ion. This free calcium fraction is the physiologically important fraction which constitute half of the calcium fraction (Hanssen et al. 1991). In the present investigation, no significant changes were recorded in the serum calcium and phosphorus concentrations, and calcium to phosphorus ratio of fingerling L. rohita irrespective of the different levels of dietary calcium, suggesting the occurrence of calcium homeostasis. Similar results on calcium and phosphorus concentrations and calcium to phosphorus ratio in serum were also reported in hybrid tilapia (Shiau and Tseng 2007) and grass carp (Liang et al. 2012). In teleost fishes, serum calcium levels are regulated within narrow limits as the calcium absorption from water usually balances the losses that occur due to diffusion and through urine (Perry et al. 1992; Shiau and Tseng 2007; Liang et al. 2012). This may be the reason for the consistency in the serum calcium concentration in this study. Increased alkaline phosphatase (ALP) activity is an indicator of increase in osteoblastic activity (Kaplan 1972). It is actively involved in the absorption and retention of essential minerals, particularly calcium and phosphorus for bone mineralization (Coleman 1992). Serum ALP activity significantly enhanced with increment in calcium levels up to 5.11 g kg−1 diet, and the highest ALP activity was noted at this level. This indicates to a more efficient use of the dietary calcium in whole body, vertebrae, and scale mineralization in this study. Similar responses of serum ALP activity were also recorded in hybrid tilapia (Shiau and Tseng 2007) and grass carp (Liang et al. 2012).
Conclusion
Present study indicated the essentiality of including adequate amount of calcium in diet for optimizing growth potential and maximizing mineral deposition in whole body, scales, and vertebrae of fingerling L. rohita. Broken-line analyses of LWG, PG, whole body, vertebrae, and scale calcium concentration against dietary calcium levels indicated the calcium requirement at 5.19, 5.22, 5.16, 5.36, and 5.48 g kg−1 of diet, respectively. Hence, dietary calcium requirement ranging between 5.16 and 5.48 g kg−1 in diets with 6.5 g kg−1 phosphorus is optimum and recommended for fingerling L. rohita (in water with at least 22 mg calcium L−1).
References
Abdel-Hameid NAH, Zehra S, Khan MA (2017) Dietary copper requirement of fingerling Channa punctatus (Bloch) based on growth, feed conversion, blood parameters and whole body copper concentration. Aquac Res 48:2787–2797. https://doi.org/10.1111/are.13112
Andrews JW, Murai T, Campbell C (1973) Effects of dietary calcium and phosphorus on growth, food conversion, bone ash and haematocrit levels of catfish. J Nutr 103:766–771. https://doi.org/10.1093/jn/103.5.766
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquac 8:172–219. https://doi.org/10.1111/raq.12090
AOAC, Association of Official Analytical Chemists (2005) Animal feed. In: Horwitz W, Latimer GW Jr (eds) Official methods of analysis of official analytical chemists international, 18th edn. Association of Official Analytical Chemists, Arlington, pp 1–52
APHA, American Public Health Association (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association, Washington, DC 1268 pp
Apines MJS, Satoh S, Kiron V, Watanabe T, Fujita S (2003) Bioavailability and tissue distribution of amino acid chelated trace elements in rainbow trout, Oncorhynchus mykiss. Fish Sci 69:722–730. https://doi.org/10.1046/j.1444-2906.2003.00679.x
Baker DH (1986) Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients. J Nutr 116:2339–2349. https://doi.org/10.1093/jn/116.12.2339
Chavez-Sanchez C, Martinez-Palacios CA, Martinez-Perez G, Ross LG (2000) Phosphorus and calcium requirements in the diet of the American cichlid, Cichlasoma urophthalmus (Günther). Aquac Nutr 6:1–9. https://doi.org/10.1046/j.1365-2095.2000.00118.x
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441–483. https://doi.org/10.1146/annurev.bb.21.060192.002301
Cowey CB (1992) Nutrition: estimating requirements of rainbow trout. Aquaculture 100:177–189. https://doi.org/10.1016/0044-8486(92)90370-Z
Debnath D, Pal AK, Sahu NP, Yengkokpam S, Baruah K (2012) Protein requirement of Labeo rohita fingerlings based on growth and ammonia excretion. J Inland Fish Soc India 44:12–20
FAO (2009) The State of World Fisheries and Aquaculture 2009. FAO Fisheries and Aquaculture Department; Food and Agriculture Organization of the United Nations, Italy
Flik G, Fenwick JC, Kolar Z, Mayer-Gostan N, Wendelaar Bonga SE (1986) Effects of low ambient calcium levels on wholebody Ca2+ flux rates and internal calcium pools in the freshwater cichlid teleost, Oreochromis mossambicus. J Exp Biol 120:249–264
Halver JE (2002) The vitamins. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 61–141
Hanssen RGJM, Aarden EM, Venne VWPHG, Pang PKT, Wendelaar Bonga SE (1991) Regulation of secretion of the teleost fish hormone stanniocalcin: effects of extracellular calcium. Gen Comp Endocrinol 84:155–163. https://doi.org/10.1016/0016-6480(91)90075-H
Hossain MA, Yoshimatsu T (2014) Review: dietary calcium requirement in fishes. Aquac Nutr 20:1–11. https://doi.org/10.1111/anu.12135
Ichikawa R, Oguri M (1961) Metabolism of radionucleotides in fish I. Strontium-calcium discrimination in gill absorption. Nippon Suisan Gakkaishi 27:351–356. https://doi.org/10.2331/suisan.27.351
Kalantarian SH, Rafiee GH, Farhangi M, Mojazi Amiri B (2013) Effect of different levels of dietary calcium and potassium on growth indices, biochemical composition and some whole body minerals in rainbow trout (Oncorhynchus mykiss) fingerlings. J Aquac Res Dev 4:170. https://doi.org/10.4172/2155-9546.1000170
Kandeepan C (2013) Dietary calcium requirement of Oreochromis mossambicus. Int J Curr Microbiol App Sci 2:89–97
Kaplan MM (1972) Medical intelligence. Current concepts-alkaline phosphatase. N Engl J Med 286:200–202. https://doi.org/10.1056/NEJM197201272860407
Khan MA (1991) Protein and amino acid requirements and efficacy of dietary formulations in some cultivable finfish species. Ph. D thesis, Department of Zoology, Aligarh Muslim University, Aligarh, India, 120 pp
Lall SP (2002) The minerals. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic Press, San Diego, pp 259–308
Liang JJ, Liu YJ, Yang ZN, Tian LX, Yang HJ, Liang GY (2012) Dietary calcium requirement and effects on growth and tissue calcium content of juvenile grass carp (Ctenopharyngodon idella). Aquac Nutr 18:544–550. https://doi.org/10.1111/j.1365-2095.2011.00916.x
Liang JJ, Yang HJ, Liu YJ, Tian LX (2014) Dietary potassium requirement of juvenile grass carp (Ctenopharyngodon idella, Val.) based on growth and tissue potassium content. Aquac Res 45:701–708. https://doi.org/10.1111/are.12008
Musharraf M, Khan MA (2019) Dietary phosphorus requirement of fingerling Indian major carp, Labeo rohita (Hamilton). J World Aquacult Soc 50:469–484. https://doi.org/10.1111/jwas.12521
NRC, National Research Council (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC, p 376+ XVI
Ogino C, Takeda H (1976) Mineral requirements in fish–III. Calcium and phosphorus requirements in carp. Bull Jpn Soc Sci Fish 42(7):793–799. https://doi.org/10.2331/suisan.42.793
Ogino C, Takeda H (1978) Requirements of rainbow trout for dietary calcium and phosphorus. Bull Jpn Soc Sci Fish 44(9):1019–1022. https://doi.org/10.2331/suisan.44.1019
Ogino C, Takeuchi L, Takeda H, Watanabe T (1979) Availability of dietary phosphorus in carp and rainbow trout. Bull Jpn Soc Sci Fish 45(12):1527–1532. https://doi.org/10.2331/suisan.45.1527
Paul BN, Sarkar S, Giri SS, Mohanty SN, Mukhopadhyay PK (2006) Dietary calcium and phosphorus requirements of rohu, Labeo rohita fry. Anim Nutr Feed Technol 6:257–263
Paul BN, Sarkar S, Giri SS, Rangacharyulu PV, Mohanty SN (2004) Phosphorus requirements and optimum calcium/phosphorus ratio in the diet of mrigal, Cirrhinus mrigala (Ham.) fingerlings. J Appl Ichthyol 20:306–309. https://doi.org/10.1111/j.1439-0426.2004.00541.x
Perry SF, Goss GG, Fenwick JC (1992) Interrelationships between gill chloride cell morphology and calcium uptake in freshwater teleosts. Fish Physiol Biochem 10:327–337. https://doi.org/10.1007/BF00004482
Renukardhyay KM, Varghese TJ (1986) Protein requirement of the carps Catla catla and Labeo rohita (Ham.). Proc Indian Acad Sci (Anim Sci) 95:103–107. https://doi.org/10.1007/BF03179363
Robbins KR, Saxton AM, Southern LL (2006) Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci 84:155–165. https://doi.org/10.2527/2006.8413_supplE155x
Robinson EH, LaBomascus D, Brown PB, Linton TL (1987) Dietary calcium and phosphorus requirements of Oreochromis aureus reared in calcium-free water. Aquaculture 64:267–276. https://doi.org/10.1016/0044-8486(87)90189-X
Robinson EH, Rawles SD, Yette HE, Greene LW (1986) Dietary calcium requirement of channel catfish, Ictalurus punctatus, reared in calcium-free water. Aquaculture 39:343–364. https://doi.org/10.1016/0044-8486(86)90356-X
Roy PK, Lall SP (2003) Dietary phosphorus requirement of juvenile haddock (Melanogrammus aeglefinus L.). Aquaculture 221:451–468. https://doi.org/10.1016/S0044-8486(03)00065-6
Shiau SY, Tseng HC (2007) Dietary calcium requirements of juvenile tilapia, Oreochromis niloticus × O. aureus, reared in fresh water. Aquac Nutr 13:298–303. https://doi.org/10.1111/j.1365-2095.2007.00481.x
Shim KF, Ho CS (1989) Calcium and phosphorus requirements of guppy Poecilia reticulata. Nippon Suisan Gakkaishi 55:1947–1953. https://doi.org/10.2331/suisan.55.1947
Sokal RR, Rohlf FJ (1981) Biometry. W. H. Freeman and Company, New York 859 pp
Steffens W (1997) Princıpios Fundamentales de la Alimentacion de los Peces. Acribia, Zaragoza 272 pp
Vielma J, Lall SP (1998) Phosphorus utilization by Atlantic salmon (Salmo salar) reared in freshwater is not influenced by higher dietary calcium intake. Aquaculture 160:117–128. https://doi.org/10.1016/S0044-8486(97)00300-1
Watanabe T, Murakami A, Takeuchi L, Nose T, Ogino C (1980) Requirement of chum salmon held in fresh water for dietary phosphorus. Nippon Suisan Gakkaishi 46:361–367. https://doi.org/10.2331/suisan.46.361
Wood CM, McDonald G (1988) Impact of environmental acidification on gill function in fish. Environmental Protection Agency, International Symposium, Guangzhou, pp 162–182
Acknowledgments
We are grateful to the Chairman of our department for providing laboratory facilities.
Funding
Financial assistance was provided by University Grants Commission (Grant No. F. 53-2/2013 (CU)) to Mohammad Musharraf.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Musharraf, M., Khan, M.A. Dietary calcium requirement of fingerling Indian major carp, Labeo rohita (Hamilton) based on growth performance, tissue mineralization, whole body, and serum biochemical composition. Aquacult Int 28, 1125–1139 (2020). https://doi.org/10.1007/s10499-020-00515-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00515-2