Abstract
This experiment was conducted to investigate the growth performance, stress and immune responses, and welfare of juvenile turbot (Scophthalmus maximus) under three different densities (initial density 9.3, 13.6, and 19.1 kg m−2) for 120 days in a recirculating aquaculture system. Turbot were measured every 20 days to evaluate growth biometrically and sampled every month to measure biochemical parameters and mRNA levels of some stress-related genes. No significant differences were detected in the parameters and gene expression among density groups until the final sampling, except Fulton’s condition factor, lysozyme, immunoglobulin M, and complement C3. At the end of the experiment (final density 26.11, 38.22, and 52.25 kg m−2), turbot reared in the high-density (HD) group had lower body mass increase, specific growth rate, and Fulton’s condition factor, as well as higher feed conversion ratio and coefficient of variation for weight than those reared in the low-density (LD) group (P < 0.05). Fish in the HD group had higher serum cortisol, glucose, lactate, and cholesterol levels than fish in the other groups, whereas they had lower lysozyme, immunoglobulin M, and complement C3 and C4 contents (P < 0.05). Fish reared in the HD group also had higher serum chloride and osmolality levels and higher sodium-potassium adenosine triphosphatase (Na+,K+-ATPase) activity and higher Na+,K+-ATPase gene expression levels in gills compared to the other groups (P < 0.05). The mRNA levels of cytochrome P450 1A (CYP1A) and heat-shock proteins 70 and 90 (HSP70 and HSP90) were significantly upregulated, whereas glutathione S-transferase (GST) mRNA levels were significantly downregulated in the head kidney of fish in the HD group relative to fish in the other groups at the end of this trial (P < 0.05). These results indicated that overly high stocking (~50 kg m−2) density can negatively affect the growth performance, serum biochemical parameters, osmolality levels, stress-related gene expression, and overall welfare of turbot.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the first 15 years of the new millennium, the contribution of aquaculture to the world production of aquatic animals has increased from 25.7 to 46.8%, and finfish farming accounted for 67.59% of total aquaculture output of aquatic animals in 2016 (FAO 2018). To a certain extent, the development of aquaculture benefits from the development and application of intensive high-density farming models. However, it has been demonstrated that inappropriate stocking densities may impair the growth performance, behavior, health, production, and welfare parameters in fish (EFSA 2008; Ellis et al. 2010; Segner et al. 2012). High stocking density is a stressor that has suppressive effects on the immune system and disease resistance of fish (Magnadóttir 2010).
As the higher vertebrates, fish possess innate and adaptive immune systems (Zapata et al. 2006). The innate immune system is a fundamental defense mechanism of fish (Magnadóttir 2006). Fish contain a large number of innate humoral components, such as antimicrobial peptides, lysozyme, complement, interferon, pentraxins, lectins, anti-proteases and natural antibodies. The influence of crowding stress on the immune parameters of several freshwater and marine fish has been demonstrated (Montero et al. 1999; Sadhu et al. 2014; Yarahmadi et al. 2014). High stocking density could cause alteration of immune-related enzymes, proteins, or genes (Salas-Leiton et al. 2010; Ni et al. 2014; Vargas-Chacoff et al. 2014). However, little is known on the effects of stocking density on the stress and immune responses of turbot in a commercial model of a recirculating aquaculture system (RAS).
Turbot (Scophthalmus maximus) is an important cold-water aquaculture species in China and Europe. It has several characteristics to facilitate intensive commercial aquaculture production, such as fast growth rate, high feed conversion efficiency, better survival, few disease problems, high tolerance to water quality variation, and non-aggressive behavior (Lei and Liu 2010). There is a clear need for studying the influence of stocking density on commercial turbot because turbot production mainly occurs in land-based farms that use recirculation and flow-through systems (Person-Le Ruyet et al. 1991; Li et al. 2013; Liu et al. 2017a). However, it is not easy to scale up the impact of stocking density on turbot from lab-scale studies to the larger commercial environment. Furthermore, different physiological stages of fish have different tolerances to stocking density, as was confirmed in the previous studies of the effects of culture density on growth of turbot with mass of 3–70 g and 70–180 g (Aksungur et al. 2007; Baer et al. 2011; Jia et al. 2016; Liu et al. 2017a). In the next growth phase, turbot continue to gain weight quickly. Once they reach about 600 g, they are large enough to meet the mainstream demand of the Chinese market. Therefore, culture management during this growth stage (180–600 g) is especially important.
In this study, juvenile turbot (initial mass ~185 g) were cultured in a common commercial RAS, and their growth performance, stress responses, osmolality levels, and immune and metabolic-related parameters were monitored to evaluate the effects of stocking density on their welfare. Results of this study will provide a reference for selecting an appropriate rearing density and enhancing the management of turbot cultured in intensive aquaculture systems.
Materials and methods
Experimental facilities and fish maintenance
The experiment was conducted in a commercial land-based RAS at Shandong Oriental Ocean Sci-Tech Co., Ltd. (Shandong, China). The RAS consisted of 10 rearing tanks and a water treatment unit which contains a filter screen, foam separation unit, biofilter, UV sterilizer, and dissolved oxygen (DO) regulating tank. The area of each tank was 30 m2 and the water level was 60 cm.
The temperature and DO in each tank varied slightly, but in all cases they were maintained at 18 ± 1 °C and 8 ± 1 mg L−1 throughout the trial, respectively. The photoperiod was maintained at 12-h light:12-h dark using artificial lighting. The nitrification function of the biofilters in the RAS was established prior to the trial. Total ammonium nitrogen (TAN) and nitrite of all tanks were monitored every 2 days and parameters were kept at TAN < 0.3 mg/L and nitrite < 0.25 mg/L.
Fish were acclimated in rearing tanks for 15 days prior to experimentation. Turbot with an average body mass of 185.42 ± 1.10 g were obtained from Shandong Oriental Ocean Sci-Tech Co., Ltd. (Shandong, China). Juvenile turbot were randomly divided into 9 rearing tanks (the tenth tank was empty) and were submitted for 120 days at three initial densities, 19.12 ± 0.16 kg m−2 (high density HD, 3100 fish per tank), 13.58 ± 0.06 kg m−2 (medium density MD, 2200 fish per tank), and 9.30 ± 0.05 kg m−2 (low density LD, 1500 fish per tank), in triplicate. All fish were fed twice daily (6:30 and 18:30 h local time) and the daily feed ration was approximately 1.0% of the tank biomass. The feed was commercial turbot diet which was produced by Ningbo Tech-Band Co., Ltd. in China (52% crude protein, 16.0% ash, 12% lipids, 12% water, and 3.0% fiber).
Growth parameters and survival
Dead fish in each tank were recorded and removed daily to evaluate the survival rate over the entire study period. At the end of the trial, 15% of the population in each tank were weighed and measured for standard length to evaluate growth biometrically (Garcia et al. 2013). The calculation methods of stocking density, specific growth rate (SGR), feed conversion ratio (FCR), Fulton’s condition factor (K), coefficient of variation for weight (CVw), and fin index (FI) were according to our previous study (Liu et al. 2017b).
Fish sampling and biochemical parameter assays
Fish were starved for 24 h prior to being collected for these analyses. Each month, 20 fish from each tank were randomly sampled. They were anesthetized with MS222 (200 ppm, Sigma Diagnostics Inc., St. Louis, MO, USA), and blood samples were collected from caudal vessels using a syringe without an anticoagulant. Samples were chilled at 4 °C for 4 h and then centrifuged (10 min at 4 °C, 1600g) to separate the serum. Among them, five fish from each tank were used for sampling of gill and head kidneys. A small piece (1 × 1 cm) of the second gill arch from the upside that was sampled from each fish was flash-frozen in liquid nitrogen and stored at − 80 °C for later RNA extraction. Meantime, five pieces (1 × 1 cm) of the gill tissue were collected and stored at − 20 °C for assays of Na+, K+-ATPase activity and protein content. Head kidneys of each turbot were sampled with clean surgical scissors and pointed tweezers to avoid contamination and then were flash-frozen in liquid nitrogen and stored at − 80 °C for later RNA extraction.
Serum concentrations of chloride (Cl−), potassium (K+), sodium (Na+), glucose, total cholesterol (TCH), and triglycerides (TG) were determined with an automatic biochemical analyzer (Hitachi 7600-110, Tokyo, Japan). The activity of sodium-potassium adenosine triphosphatase (Na+,K+-ATPase) in gill tissue was measured using a Na+,K+-ATPase activity colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The serum alkaline phosphatase (ALP) and cortisol levels were measured with commercial kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China). The levels of serum complement components C3 and C4 were assayed by commercial kits (Elikan, Wenzhou, Zhejiang, China). Immunoglobulin M (IgM) and lactate levels were measured using a commercially available ELISA kit (mlbio, Shanghai, China). Lysozyme (LZM) activity was measured using a turbidimetric assay according to Björnsson et al. (2012). All analyses were conducted in triplicate.
Gene expression analysis
Total RNA from gills and head kidneys were extracted using a fast pure RNA kit according to the manufacturer’s instructions (Takara, Dalian, China). The concentration of RNA was measured using a GeneQuant 1300 device (GE Healthcare Biosciences, Piscataway, NJ, USA). The first-strand cDNA was synthesized using the PrimeScript RT Reagent Kit (Takara, Dalian, China) with 2 μg of total RNA according to the manufacturer’s instructions.
The quantitative RT-PCR was carried out in an ABI PRISM 7500 Detection System (Applied Biosystems, Foster City, CA, USA). Five genes were chosen to detect their expression under different density groups, including heat shock-protein 70 (HSP 70), HSP 90, Cytochrome p450 family 1 subfamily A (CYP1A), glutathione s-transferase (GST), and sodium-potassium adenosine triphosphatase (Na+,K+-ATPase). β-actin was chosen as a reference gene for internal standardization. The primers used for amplification and gene expression analyses were shown in Table 1. Each reaction was carried out in a total volume of 20 μL, containing 10 μL of SYBR® Premix Ex TaqTM (Perfect Real Time) (Takara), 0.4 μL of ROXII, 4 μL of cDNA template (10-fold diluted), 0.4 μL of each primer (10 μmol L−1), and 4.8 μL of ddH2O. The PCR program was set to run for 95 °C for 10 s, 40 cycles of 95 °C for 5 s, and 60 °C for 34 s. The melting curve was used to confirm that a single product was amplified and detected and to check for the absence of primer-dimer artifacts. Each sample was run at least in triplicate along with the internal control gene. The gene expression relative to controls was determined by the 2-∆∆CT method (Livak and Schmittgen 2001).
Statistical analysis
Means and standard deviations (SD) were calculated for each measured parameter. All data were presented as mean ± SD. A one-way analysis of variances was used to test the statistical differences among tanks with the three densities (SPSS 18.0 statistical package, SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
Effect of stocking density on growth performance and survival rate
No difference in mean mass from days 0 to 100 was found among all density groups, whereas it was lower in the HD group than that in other two groups on day 120 (P < 0.05, Table 2). There was no significant difference for standard length among all density groups at any time point (Table 2). Survival rates were very high in all tanks with no significant differences among all density groups (Table 2). The stocking density increased gradually with increasing culture time (Table 2). The final densities (day 120) were 26.11 ± 0.06, 38.22 ± 0.18, and 52.25 ± 0.20 kg m−2 for the LD, MD, and HD groups, respectively.
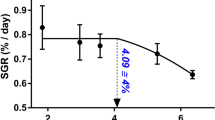
At the end of the experiment, turbot in the HD group had significantly lower SGR compared with those in the LD and MD groups (Fig. 1a) but greater FCR (P < 0.05, Fig. 1b). K did not differ significantly among all density groups before day 100; however, it was significantly higher in the LD group than that in the HD group at and after day 100 (P < 0.05, Fig. 1c). CVw increased with higher stocking density and was significantly higher in the HD group compared with that in the LD group (Fig. 1d). FI for caudal fins and lateral fins varied little and did not differ significantly among treatment groups (Fig. 1e and f).
a Specific growth rate (SGR). b Feed conversion ratio (FCR). c Changes in Fulton’s condition factor (K). d Coefficient of variation for weight (CVw). e, f Fin index (FI) of turbot reared in three experimental groups for 120 days. Data are presented as mean ± SD. A different letter at the same sampling time indicates significant differences among the three stocking densities (LD, low density; MD, medium density; and HD, high density; P < 0.05)
Effect of stocking density on stress, immune, and metabolic-related parameters in serum
At the end of the trial, turbot in the HD group had significantly higher cortisol levels than fish in the other two groups (P < 0.05, Fig. 2a). Beginning on day 90, the LZM content was lower in the HD group than that in the LD and MD groups, and LZM content was in the order LD > MD > HD at the end of the experiment (P < 0.05, Fig. 2b). The IgM level was also lower in the HD group than in the other groups beginning at day 90 (P < 0.05, Fig. 2c). C3 content followed the same pattern as LZM content, and C4 content was significantly higher in the LD group than in the other two groups at the end of the experiment (P < 0.05, Fig. 2e and f). The contents of glucose, lactate, and cholesterol in the HD group were significantly higher than those in the other two groups at day 120 (P < 0.05, Fig. 2g–i). However, ALP and triglyceride levels did not differ among the different stocking density groups throughout the experiment (Fig. 2d and j).
Changes of stress-, immune-, and metabolic-related parameters in serum of turbot reared in three experimental groups for 120 days. Data are presented as mean ± SD. A different letter at the same sampling time indicates significant differences among the three stocking densities (LD, low density; MD, medium density; and HD, high density; P < 0.05)
Effect of stocking density on serum ion concentrations, osmolality levels, and in the activity and expression of Na+,K+-ATPase
At the end of the trial, serum Cl− levels were significantly lower in the LD group than in the other two groups (Fig. 3a). Compared with the LD and MD groups, fish in the HD group had a significantly higher serum osmolality level, Na+,K+-ATPase activity, and Na+,K+-ATPase gene expressions level in gill tissue at day 120 (Fig. 3d–f). Serum Na+ and K+ levels were not influenced by increasing stocking density (Fig. 3b and c).
Changes of ions (a, b, c), osmolality (d) levels in serum and Na+,K+-ATPase activity (e), and gene expressions levels (f) in gill of turbot reared in three experimental groups for 120 days. Data are presented as mean ± SD. A different letter at the same sampling time indicates significant differences among the three stocking densities (LD, low density; MD, medium density; and HD, high density; P < 0.05)
Effect of stocking density on stress-related gene expression in the head kidney
Stress-related genes showed no difference in expression among the three stocking densities in the first 3 months. However, at day 120, tissues from fish in the HD group exhibited higher mRNA levels of CYP1A, HSP90, and HSP70 and lower mRNA levels of GST compared with tissues from fish in the other two groups (Fig. 4a–d).
The effect of stocking density on gene expression levels of GST (a), CYP1A (b), HSP70 (c), and HSP90 (d) in the head kidney of turbot reared in three experimental groups for 120 days. Data are presented as mean ± SD. A different letter at the same sampling time indicates significant differences among the three stocking densities (LD, low density; MD, medium density; and HD, high density; P < 0.05)
Discussion
High stocking densities may adversely affect the growth potential of fish (Iwama 2007). After 120 days, high stocking density reduced turbot growth in the present study. The HD group had lower mean weight and SGR and higher FCR than the other two groups, which was also reported for flatfish populations and many other fish species, including Atlantic salmon (Salmo salar) (Liu et al. 2015), rainbow trout (Oncorhynchus mykiss) (Larsen et al. 2012), rockfish (Sebastes schlegelii) (Hwang et al. 2014), and Senegalese sole (Solea senegalensis) (Sánchez et al. 2013). Condition factor (K) can reflect the fish state of energy storage, which is associated with fish welfare (Dennis and Bulger 1995). In this experiment, high stocking density resulted in decreased K value in turbot, which was consistent with that found in Atlantic salmon (Oppedal et al. 2011) and rainbow trout (Wagner et al. 2010). Increasing CVw, often related to high-density farming conditions, has been considered an indicator of inter-individual competition among fish groups (North et al. 2006; Merino et al. 2007). Compared with the other two groups, a higher CVw was found in the HD group at the end of the experiment, which indicates that large variation of fish weight existed in the HD group. This result was similar to that reported in turbot of small experimental RAS (Irwin et al. 1999).
Cortisol, glucose, and lactate levels are considered to be good indicators of acute or chronic stress levels in fish and, thus, also can be used as welfare indicators (van de Nieuwegiessen et al. 2009; Sadhu et al. 2014). Crowding stress caused by high stocking density often result in the increase of cortisol levels in turbot (Jia et al. 2016) and other fish species such as Senegalese sole (Salas-Leiton et al. 2010), Atlantic salmon (Liu et al. 2015), and European sea bass (Lupatsch et al. 2010). Data from the current study confirmed this premise, because turbot had significantly higher cortisol levels in the HD group than those in the MD and LD groups at the final sampling day. Serum glucose levels were also the highest in the HD group, which was also reported in Asian seabass (Sadhu et al. 2014) and Atlantic salmon (Diesen Hosfeld et al. 2009). Gluconeogenesis induced by cortisol could lead to the increase of glucose levels (Pottinger 2010). Due to the chronic stress, the consumption of energy reserves and reallocation of metabolic energy are enhanced, accompanied by variations in biochemical parameters, such as increase in cholesterol and lactate (Ruane et al. 2002; Herrera et al. 2009). The results of this study showed that high stocking density induced a significant increase in serum cholesterol and lactate levels. We speculate that the elevated serum lactate level may have been due to a stress-induced increase in anaerobic activity in turbot. Cholesterol is the final product of lipid metabolism, and its increase reflects the consumption of fat in turbot under high-density conditions to meet their energy needs (Conte 2004; Di Marco et al. 2008).
Total IgM level is another characteristic indicator of stress in fish (Wenderlaar Bonga 1997; Iwama 2007). The low serum IgM level and increased serum cortisol level in fish in the HD group observed in the present study were similar to results reported for Atlantic salmon (Liu et al. 2015) and Ayu (Plecoglossus altivelis) (Iguchi et al. 2003). LZM and complement C3 and C4 levels or activities are important indexes of the innate immunity of fish, and they also reflect the response to crowding stress induced by high stocking density (Montero et al. 1999; Ortuno et al. 2001; Costas et al. 2013). Lower levels of serum LZM, C3, and C4 in the HD group compared with the other two groups suggested that turbot were stressed when reared under high-density conditions. We postulate that crowding suppressed immunity levels in turbot, as suggested by Mazur and Iwama (1993).
The responses of fish to stress are associated with the neuroendocrine system, which is critical for adaptations to osmoregulation (McCormick 2001). Prior studies have reported that crowding stress induced by high stocking density can affect the balance of ion concentrations and osmolality in fish (Vargas-Chacoff et al. 2014). Costas et al. (2008) and Herrera et al. (2009) compared the effects of different stocking densities on the physiological responses of the wedge sole (Dicologoglossa cuneata) and Senegalese sole, respectively, and found that the plasma osmolality of these two kinds of fish increased in the HD and MD groups after 22 and 63 days of culture, respectively. Similarly, an uptrend in serum Cl− and osmolality levels of turbot was observed in the HD group at the end of this experiment, indicating that high stocking density disturbed the ion balance and further led to a change in osmolality. Na+,K+-ATPase is responsible for maintaining osmotic pressure between the intracellular and external environment by driving a variety of ion transport systems (McCormick 2001). In the current study, increased stocking density enhanced Na+,K+-ATPase activity and Na+,K+-ATPase gene expression levels in the gill, which was also reported in early studies of Sub-Antarctic Notothenioid fish (Vargas-Chacoff et al. 2014) and red porgy fry (Vargas-Chacoff et al. 2011). This indicated that turbot in a stressed state can enhance enzymatic activities to maintain their internal osmotic balance (Wenderlaar Bonga 1997).
The physiological stress response often is accompanied by a variety of protein changes, such as the levels of GST, CYP1A, and the HSPs. During oxidative stress, GST can catalyze the sulfhydryl group of GSH and some electrophilic substances to protect the DNA and some proteins from damage (Arockiaraj et al. 2014). In this study, the observed downregulation of GST gene expression in the HD group indicated that crowding stress caused oxidative stress and inhibited GST synthesis in turbot. CYP1A is also involved in the metabolism of xenobiotics. As a biomarker, CYP1A is often used to evaluate the physiological stress and immune status of fish (Alak et al. 2017). Gornati et al. (2004) and Ni et al. (2014) found that crowding stress caused upregulation of CYP1A gene expression in European sea bass and juvenile Amur sturgeon (Acipenser schrenckii). In the current study, high stocking density also led to significant upregulation of the CYP1A gene in the head kidney of turbot.
HSP70 and HSP90 are the most widely studied HSPs in fish, and they participate in various stress responses. After 120 days of culture, HSP70 gene expression in the head kidney of turbot in the HD group of this study was significantly increased compared with that in the LD group, which was also reported for rainbow trout (Yarahmadi et al. 2016). Similarly, HSP90 gene expression was high in the HD group of turbot. Increased HSP70 and HSP90 expression is a self-adaptive mechanism of fish, and it indicates a positive response to resist the adverse effects of stress (Roberts et al. 2010).
In conclusion, the results of this study revealed that juvenile turbot can be cultured efficiently on a commercial scale in a RAS. However, when the stocking density increased to about 50 kg m−2, the responses associated with crowding stress negatively modified the growth performance, serum biochemical parameters, osmolality levels, and head kidney stress-related gene expression of juvenile fish. We propose that the welfare of juvenile turbot cultured in a RAS would be negatively affected when reared at densities over 50 kg m−2, even though high densities do not significantly affect production safety. In this case, once the upper limit of density is achieved we recommend splitting the individuals into two units to avoid the adverse effects of crowding stress, getting an optimal balance between growth, and a better performance of the RAS. These results provide a reference for choosing a reasonable stocking density for farmed turbot and selecting more sensitive indicators to adjust crowding stress.
References
Aksungur N, Aksungur M, Akbulut B, Kutlu I (2007) Effects of stocking density on growth performance survival and food conversion ratio of turbot (Psetta maxima) in the net cages on the southeastern coast of the Black Sea. Turk J Fish Aquat Sci 7:147–152
Alak G, Yeltekin AÇ, Taş IH, Ucar A, Parlak V, Topal A, Kocaman EM, Atamanalp M (2017) Investigation of 8-OHdG, CYP1A, HSP70 and transcriptional analyses of antioxidant defence system in liver tissues of rainbow trout exposed to eprinomectin. Fish Shellfish Immunol 65:136–144
Arockiaraj J, Gnanam AJ, Palanisamy R, Bhatt P, Kumaresan V, Chaurasia MK, Pasupuleti M, Ramaswamy H, Arasu A, Sathyamoorthi A (2014) A cytosolic glutathione s-transferase, GST-theta from freshwater prawn Macrobrachium rosenbergii: molecular and biochemical properties. Gene 546:437–442
Baer A, Schulz C, Traulsen I, Krieter J (2011) Analysing the growth of turbot (Psetta maxima) in a commercial recirculation system with the use of three different growth models. Aquacult Int 19:497–511
Björnsson B, Steinarsson A, Oddgeirsson M, Ólafsdóttir SR (2012) Optimal stocking density of juvenile Atlantic cod (Gadus morhua L.) reared in a land-based farm. Aquaculture s356-357:342–350
Conte FS (2004) Stress and the welfare of cultured fish. Appl Anim Behav Sci 86:205–223
Costas B, Aragão C, Mancera JM, Dinis MT, Conceição LEC (2008) High stocking density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup 1858) juveniles. Aquac Res 39:1–9
Costas B, Aragão C, Dias J, Afonso A, Conceição LEC (2013) Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea senegalensis. Fish Physiol Biochem 39:1141–1151
Dennis TE, Bulger AJ (1995) Condition factor and whole-body sodium concentrations in a freshwater fish: evidence for acidification stress and possible ionoregulatory over-compensation. Water Air Soil Pollut 85:377–382
Di Marco P, Priori A, Finoia MG, Massari A, Mandich A, Marino G (2008) Physiological responses of European sea bass Dicentrarchus labrax to different stocking densities and acute stress challenge. Aquaculture 275:319–328
Diesen Hosfeld C, Hammer J, Handeland SO, Fivelstad S, Stefansson SO (2009) Effects of fish density on growth and smoltification in intensive production of Atlantic salmon (Salmo salar L.). Aquaculture 294:236–241
Ellis T, North B, Scott AP, Bromage NR, Porter M, Gadd D (2010) The relationships between stocking density and welfare in farmed rainbow trout. J Fish Biol 61:493–531
European Food Safety Authority (EFSA) (2008) Scientific Opinion of the Panel on Animal Health and Animal Welfare on a request from the European Commission on the Animal welfare aspects of husbandry systems for farmed trout. EFSA J 796:1–22
FAO (2018) FAO yearbook. Fishery and aquaculture statistics 2016. Rome
Garcia F, Romera DM, Gozi KS, Onaka EM, Fonseca FS, Schalch SHC, Candeira PG, Guerra LOM, Carmo FJ, Carneiro DJ, Martins MIEG, Portella MC (2013) Stocking density of Nile tilapia in cages placed in a hydroelectric reservoir. Aquaculture 410-411:51–56
Gornati R, Papis E, Rimoldi S, Terova G, Saroglia M, Bernardini G (2004) Rearing density influences the expression of stress-related genes in sea bass (Dicentrarchus labrax, L.). Gene 341:111–118
Herrera M, Vargas-Chacoff L, Hachero I, Ruiz-Jarabo I, Rodiles A, Navas JI, Mancera JM (2009) Physiological responses of juvenile wedge sole Dicologoglossa cuneata (Moreau) to high stocking density. Aquac Res 40:790–797
Hwang HK, Son MH, Myeong JI, Kim CW, Min BH (2014) Effects of stocking density on the cage culture of Korean rockfish (Sebastes schlegeli). Aquaculture 434:303–306
Iguchi K, Ogawa K, Nagae M, Ito F (2003) The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 220:515–523
Irwin S, O'Halloran J, FitzGerald RD (1999) Stocking density, growth and growth variation in juvenile turbot, Scophthalmus maximus (Rafinesque). Aquaculture 178:77–88
Iwama GK (2007) The welfare of fish. Dis Aquat Org 75:155–158
Jia R, Liu BL, Han C, Huang B, Lei JL (2016) Influence of stocking density on growth performance, antioxidant status, and physiological response of juvenile turbot, Scophthalmus maximu, reared in land-based recirculating aquaculture system. J World Aquacult Soc 47:587–599
Larsen BK, Skov PV, McKenzie DJ, Jokumsen A (2012) The effects of stocking density and low level sustained exercise on the energetic efficiency of rainbow trout (Oncorhynchus mykiss) reared at 19 °C. Aquaculture 324-325:226–233
Lei JL, Liu XF (2010) Culture of turbot: chinese perspective. In: Daniels HV, Watanabe WO (eds) Practical flatfish culture and stock enhancement. Wiley-Blackwell, Iowa, pp 185–202
Li X, Liu Y, Blancheton JP (2013) Effect of stocking density on performances of juvenile turbot (Scophthalmus maximus) in recirculating aquaculture systems. Chin J Oceanol Limnol 31:514–522
Liu B, Liu Y, Wang X (2015) The effect of stocking density on growth and seven physiological parameters with assessment of their potential as stress response indicators for the Atlantic salmon (Salmo salar). Mar Freshw Behav Physiol 48:177–192
Liu B, Jia R, Zhao K, Wang G, Lei J, Huang B (2017a) Stocking density effects on growth and stress response of juvenile turbot (Scophthalmus maximus) reared in land-based recirculating aquaculture system. Acta Oceanol Sin 36:31–38
Liu B, Liu Y, Sun G (2017b) Effects of stocking density on growth performance and welfare-related physiological parameters of Atlantic salmon Salmo salar L. in recirculating aquaculture system. Aquac Res 48:2133–2144
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Lupatsch I, Santos GA, Schrama JW, Verreth JAJ (2010) Effect of stocking density and feeding level on energy expenditure and stress responsiveness in European sea bass Dicentrarchus labrax. Aquaculture 298:245–250
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Magnadóttir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379
Mazur CF, Iwama GK (1993) Handling and crowding stress reduces number of plaque-forming cells in Atlantic salmon. J Aquat Anim Health 5:98–101
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
Merino GE, Piedrahita RH, Conklin DE (2007) The effect of fish stocking density on the growth of California halibut (Paralichthys californicus) juveniles. Aquaculture 265:176–186
Montero D, Izquierdo MS, Tort L, Robaina L, Vergara JM (1999) High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol Biochem 20:53–60
Ni M, Wen H, Li J, Chi M, Bu Y, Ren Y, Zhang M, Song Z, Ding H (2014) The physiological performance and immune responses of juvenile Amur sturgeon (Acipenser schrenckii) to stocking density and hypoxia stress. Fish Shellfish Immunol 36:325–335
North BP, Turnbull JF, Ellis T, Porter MJ, Migaud H, Bron J, Bromage NR (2006) The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 255:466–479
Oppedal F, Vågseth T, Dempster T, Juell JE, Johansson D (2011) Fluctuating sea-cage environments modify the effects of stocking densities on production and welfare parameters of Atlantic salmon (Salmo salar L.). Aquaculture 315:361–368
Ortuno J, Esteban MA, Meseguer J (2001) Effects of short-term crowding stress on the gilthead seabream (Sparus aurata L) innate immune response. Fish Shellfish Immunol 11:187–197
Person-Le Ruyet J, Baudin-Laurencin F, Devauchelle N, Me’tailler R, Nicolas JL, Robin J, Guillaume J (1991) Culture of turbot (Scophthalmus maximus). In: McVey JP (ed) CRC Handbook of mariculture, Vol. II: Finfish aquaculture. CRC Press, Boca Raton, pp 21–41
Pottinger TG (2010) Changes in blood cortisol, glucose and lactate in carp retained in anglers’ keepnets. J Fish Biol 53:728–742
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33:789–801
Ruane NM, Carballo EC, Komen J (2002) Increased stocking density influences the acute physiological stress response of common carp Cyprinus carpio (L.). Aquac Res 33:777–784
Sadhu N, Sharma SK, Joseph S, Dube P, Philipose K (2014) Chronic stress due to high stocking density in open sea cage farming induces variation in biochemical and immunological functions in Asian seabass (Lates calcarifer, Bloch). Fish Physiol Biochem 40:1105–1113
Salas-Leiton E, Anguis V, Martín-Antonio B, Crespo D, Planas JV, Infante C, Cañavate JP, Manchado M (2010) Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): potential effects on the immune response. Fish Shellfish Immunol 28:296–302
Sánchez P, Ambrosio PP, Flos R (2013) Stocking density affects Senegalese sole (Solea senegalensis, Kaup) growth independently of size dispersion, evaluated using an individual photo-identification technique. Aquac Res 44:231–241
Segner H, Sundh H, Buchmann K, Douxfils J, Sundell KS, Mathieu C, Ruane N, Jutfelt F, Toften H, Vaughan L (2012) Health of farmed fish: its relation to fish welfare and its utility as welfare indicator. Fish Physiol Biochem 38:85–105
van de Nieuwegiessen PG, Olwo J, Khong S, Verreth JAJ, Schrama JW (2009) Effects of age and stocking density on the welfare of African catfish, Clarias gariepinus Burchell. Aquaculture 288:69–75
Vargas-Chacoff L, Calvo Á, Ruiz-Jarabo I, Villarroel F, Muñoz JL, Tinoco AB, Cárdenas S, Mancera JM (2011) Growth performance, osmoregulatory and metabolic modifications in red porgy fry, Pagrus pagrus, under different environmental salinities and stocking densities. Aquac Res 42:1269–1278
Vargas-Chacoff L, Martínez D, Oyarzún R, Nualart D, Olavarría V, Yáñez A, Bertrán C, Ruiz-Jarabo I, Mancera JM (2014) Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the sub-Antarctic Notothenioid fish Eleginops maclovinus. Fish Shellfish Immunol 40:424–434
Wagner EJ, Intelmann SS, Routledge MD (2010) The effects of fry rearing density on hatchery performance, fin condition, and agonistic behavior of rainbow trout Oncorhynchus mykiss fry. J World Aquacult Soc 27:264–274
Wenderlaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Yarahmadi P, Miandare HK, Hoseinifar SH, Gheysvandi N, Akbarzadeh A (2014) The effects of stocking density on hemato-immunological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquac Int 23:55–63
Yarahmadi P, Miandare HK, Fayaz S, Caipang CMA (2016) Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 48:43–53
Zapata A, Diez B, Cejalvo T, Gutierrez-de Frias C, Cortes A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20:126–136
Funding
This work was supported by the National Key R&D Program of China (grant number 2017YFD0701701), the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2017HYZD04), and the Modern Agriculture Industry System Construction of Special Funds (CARS-47-G24).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving animals were in accordance with the guidelines and ethical standards of Chinese Academy of Fishery Sciences and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Fei, F., Li, X. et al. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquacult Int 27, 1599–1612 (2019). https://doi.org/10.1007/s10499-019-00413-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00413-2