Abstract
Fairy shrimp is known as a nutritional food for fish and crustaceans in aquaculture. In most hatcheries, the microalga Chlorella sp. appears to be the most common, suitable, and nutritious food to feed fairy shrimp. In this study, we attempted to determine other alternative algal diets for cultivation of fairy shrimp Branchinella thailandensis. Seven experimental diets including three treatments of dried Spirulina sp. at 0.75 (S1), 1.5 (S2), and 3.0 mg dry weight individual−1 (S3); three treatments of Chlorococcum humicola at 5 × 105 (Ch1), 1 × 106 (Ch2), and 2 × 106 cells mL−1 (Ch3); and a control diet (Chlorella vulgaris at 1 × 106 cells mL−1) were fed to 5-day-old shrimp for 15 days. Evaluation of growth performance, egg production, survival percentage, and nutritional and carotenoid content of the experimental fairy shrimp revealed that Ch3 is the most suitable algal diet. Our results suggest that C. humicola is the best alternative food source for the cultivation of B. thailandensis. In addition, dried Spirulina powder is also a good choice when live algae are not available and can be used as an alternative feed in fairy shrimp cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In tropical Southeast Asia, fairy shrimp (Class Branchiopoda, Order Anostraca) usually spend their entire lives in seasonal wetlands such as rain pools, temporary ponds, water buffalo wallows, and rice paddies (Sanoamuang et al. 2000a; Rogers et al. 2013). Fairy shrimp can withstand long periods of desiccation and can remain as encapsulated embryos, totally dehydrated, and inactive for as long as at least 1 year in the dry mud on the bottom of pools. These dried eggs require water to hatch usually from the next year rain (Brendonck et al. 2008). Most fairy shrimps are non-selective filter feeders, collecting algal and detrital foods using their thoracic limbs (Brendonck 1993).
Currently, there are three known species of fairy shrimp in Thailand. The most common and widely distributed species in the country is Streptocephalus sirindhornae Sanoamuang, Murugan, Weekers, and Dumont (Sanoamuang et al. 2000b). This taxon has been recorded also in Laos, Cambodia (Rogers et al. 2013) and Yunnan and Guangxi provinces of China (Shu et al. 2015). Branchinella thailandensis Sanoamuang, Saengphan, and Murugan is less common and has so far been recorded in the northeast, north, and central regions (Sanoamuang et al. 2002; Sanoamuang unpublished data). On the other hand, S. siamensis Sanoamuang and Saengphan is extremely rare and has been recorded only in five localities in Kanchanaburi and Suphan Buri provinces (Sanoamuang and Saengphan 2006). Recent studies revealed that fairy shrimp has replaced Artemia sp. as the most common live feed for aquacultural hatcheries. B. thailandensis has rapid growth, large size (male 26.2, female 27.8 mm), high fecundity, early maturity, short life span, and high nutritional composition (Dararat et al. 2011). Regarding carotenoids, B. thailandensis has higher carotenoid content than S. sirindhornae and S. siamensis (Dararat et al. 2012). Use of adult fairy shrimp S. sirindhornae as live and dried feed has demonstrated improvement in growth and carotenoid contents of the giant freshwater prawn Macrobrachium rosenbergii (Sriputhorn and Sanoamuang 2011) and flowerhorn cichlid fish (Sornsupharp et al. 2015). Similarly, S. sirindhornae nauplii can be used as a nutritionally adequate food for M. rosenbergii postlarvae (Sornsupharp et al. 2013). This evidence hence illustrated the potential uses of fairy shrimp as a high quality diet for aquaculture.
Since 2005, attempts had been made to study several biological and biochemical aspects of S. sirindhornae and B. thailandensis in laboratory cultures using green alga Chlorella sp. as their main foods (Saengphan et al. 2005; Boonmak et al. 2007; Plodsomboon and Sanoamuang 2007; Saengphan and Sanoamuang 2009; Dararat et al. 2011, 2012; Saejung et al. 2011, 2014a, b; Sriputhorn and Sanoamuang 2007, 2011; Plodsomboon et al. 2012; Sornsupharp et al. 2013, 2015). Although B. thailandensis can be fed with other types of food such as rice bran, Spirulina powder, and yeast, green alga Chlorella sp. seem to be the most common, suitable, and nutritious food for the shrimps (Saengphan et al. 2005; Sriputhorn and Sanoamuang 2007; Saengphan and Sanoamuang 2009). There has been interest in finding more nutritious and practical foods to feed the fairy shrimps in laboratory cultures. In this study, dried Spirulina powder and the green microalga Chlorococcum humicola were selected as alternative diets for B. thailandensis. Since dried Spirulina sp. containing high protein, essential amino acids, fatty acids, and carotenoid pigments (Soeder 1980; Saleha et al. 2011) is commercially available, it can be applied when live feed production is undersupplied. In addition, C. humicola has a high concentration of six major carotenoids including violaxanthin, astaxanthin, lutein, zeaxanthin, α-carotene, and β-carotene (Sivathanu and Palaniswamy 2012; Yuan et al. 2002). Thus, the aims of the present study were to determine the appropriateness of Spirulina powder and C. humicola as alternative foods for the cultivation of B. thailandensis in laboratory cultures. We evaluated the growth, egg production, survival percentages, and nutritional and carotenoid contents of the fairy shrimp under laboratory conditions.
Materials and methods
Experimental algal diets
Three species of microalgae, Chlorococcum humicola, dried Spirulina sp., and a control Chlorella vulgaris, were used for this experiment. Stock cultures of C. humicola (TISTR 8461) and C. vulgaris were obtained from the Center of Excellence for Marine Biotechnology, Chulalongkorn University and the Thailand Institute of Scientific and Technological Research (TISTR). Both live algae were cultured in BG11 medium (Stanier et al. 1971) with continuous aeration and illumination at 3000 lx (light/dark, 24:0) at 25–28 °C. When cell concentration reached 5 × 106 cells mL−1 for C. humicola and 12 × 106 cells mL−1 for C. vulgaris, the algae were harvested and diluted to three concentrations, i.e., 5 × 105, 1 × 106, and 2 × 106 cells mL−1 prior to use. The experimental system with each constant concentration of the algae was maintained throughout the experiment. Biomass and relationship of dry weight and algal cell concentration were determined by filtering known volumes of algal cells through a pre-weight GF/C filter, dried at 60 °C overnight in an oven and measured using a four-decimal analytical balance (Sartorius BP 210S). Spirulina powder was purchased from the Green Diamond Co., Ltd., Thailand. For each Spirulina powder treatment, Spirulina powder was weighted and an average dry weight was calculated from three replicates.
Experimental animals
Eggs of B. thailandensis were hatched and the nauplii were fed with C. vulgaris at 1 × 106 cells mL−1 following the method described by Saengphan et al. (2005) until the animals reached 5 days old (average initial body length of 10.57 ± 0.33 mm, n = 35). For each treatment, ten individuals (five males and five females) of the shrimp were randomly selected and reared in a 1.5-L plastic container containing 1 L dechlorinated tap water (conductivity 288–462 μs cm−1, temperature 29–30 °C, and pH 7.0–7.3) with slight aeration. The experiment consisted of a control treatment (B. thailandensis fed with 1 × 106 cells mL−1 C. vulgaris, according to the examination by Saengphan and Sanoamuang 2009) plus six treatments including B. thailandensis fed with dried Spirulina sp. at 0.75 (S1), 1.5 (S2), 3.0 mg dry weight individual−1 (S3) and with C. humicola at 5 × 105 (Ch1), 1 × 106 (Ch2), 2 × 106 cells mL−1 (Ch3). The algal foods were fed to the shrimp twice a day for 15 days with three replicates for both the control and treatments. Dissolved oxygen was measured using a Hanna Oxy-Check HI 9147 DO meter. Nitrite, unionized ammonia, and total ammonia nitrogen concentrations were determined by spectrophotometric methods according to Strickland and Parsons (1972) and Bower and Holm-Hansen (1980), respectively. Water in the experimental units was removed daily and replaced with freshly prepared water. Total body lengths (from head to ceracopod) of the shrimp were measured by a Vernier Caliper every 5 days. Survival rates were determined at day 15. All deposited eggs from five females in each treatment were collected and counted daily until day 15 to determine egg production. Finally, the fairy shrimps were reared for mass cultures for 20 days at the optimal concentrations of feeding for each alga (Ch3, S3, and the control), and they were collected for nutritional proximate and carotenoid analyses.
Nutritional proximate analysis
Proximate analysis of algae and B. thailandensis samples were determined according to the official standard methods of AOAC (AOAC 2012). To determine moisture content, samples were dried at 125 °C using a hot air oven until constant weight and the moisture-free dried weight was compared with the initial dry weight. Crude protein (N × 6.25) was analyzed using acid digestion by Kjeldahl methods. Crude lipid was analyzed using a Soxhlet extractor. For ash analysis, samples were dried in the crucible for 5 h at 550 °C and cooled in a desiccator; ash weight was a subtract of weight before and after furnace. Carbohydrate was estimated by subtracting the biomass with moisture, crude protein, crude lipid, and ash.
Carotenoid analysis
The algal and fairy shrimp samples were analyzed for carotenoid contents using high-performance liquid chromatography (HPLC). Three replicates of the samples were weighed and extracted by grinding with 1 mL absolute methanol in a 1.5-mL Eppendorf tube following by centrifuge at 5000 rpm 15 min for three times. Supernatant was collected and made up the final volume of 5 mL using methanol. All extraction procedures were done in a low light condition and kept cool in ice baths. Total carotenoids were measured at 452 nm using a spectrophotometer (Genesys 10 UV scanning thermo spectronic). Carotenoid composition was analyzed using a Nova-Pack C18 HPLC column (3.9 × 150 mm 4 μm, Waters, Ireland) and detected at 452 nm using a Waters 487 UV-Vis detector. The isocratic mobile phase was acetonitrile, dichloromethane, methanol, and water (79.9, 10, 10, 0.1% v/v −1, respectively). Chromatography was performed in reverse phase at 1.0 mL min−1 flow rate with 25 μL injection volume. Peaks of carotenoids were identified by retention time of carotenoid standards. Total carotenoids and carotenoid composition were calculated according to Strickland and Parsons (1972) and Jeffrey et al. (1997).
Statistical analysis
The variance of the data is given as a standard error (S.E.) of the mean of three replicates. Data was analyzed using one-way analysis of variance (ANOVA). Significant differences at P < 0.05 were discovered by Duncan’s new multiple range tests.
Results
Growth performance of fairy shrimp
The body lengths of B. thailandensis fed with the experimental algae for 15 days are shown in Table 1. There was a significant difference in body lengths at the time of the experiment (P < 0.05). The body lengths of the shrimp fed diets with Ch3, S3, and Ch2 were significantly longer than that of the control, Ch1, S1, and S2 during the entire experiment. At the end of the experiment, the longest body length of 22.62 ± 0.51 mm was examined in the shrimp fed with Ch3 diet.
Survival percentage and total numbers of eggs (per female for whole life span) of the shrimp fed with the seven algal treatments are presented in Table 2. At the end of the feeding trial, survival rates of the shrimp fed with the seven algal diets ranged from 36.67 to 70.00%. The shrimp fed with the Ch3 diet had the highest survival rate of 70.00%, which was significantly different from that fed with the control (53.33%), the S3 (56.67%), and the Ch1 (36.67) (P < 0.05). Comparative data showed that at day 15, the shrimp fed with the Ch3 diet had the highest egg production of 2954 ± 28.42 eggs, which was significantly different from that fed with the control (1405 ± 7.81 eggs) and the other treatments (P < 0.05).
Water quality data of the fairy shrimp cultures fed with different algal concentrations are presented in Table 3. The total ammonia nitrogen and unionized ammonia concentrations recorded from the dried Spirulina treatments (S2 and S3) were significantly higher than that of the control, Ch1, Ch2, Ch3, and S1 (P < 0.05). Dissolved oxygen in the treatments fed with S2 and S3 diets were significantly lower than that of the control, Ch1, Ch2, Ch3, and S1 (P < 0.05). The other parameters of the six algal treatments showed no statistical difference with the control diet.
Proximate analysis of algae and fairy shrimp
Comparisons of the proximate compositions of the three algal diets and the fairy shrimp fed with the optimal concentrations of the three algal diets are given in Table 4. The dried Spirulina powder (S3) had the highest protein content of 61.03 ± 0.15%, which was significantly higher than that of the control (5.86 ± 0.04%) and Ch3 (6.12 ± 0.09%) (P < 0.05). The control had higher protein and lipid than Ch3. However, results from proximate analysis showed that B. thailandensis fed with Ch3 had the highest protein content of 3.60 ± 0.01%, which was significantly higher than that of S3 (2.57 ± 0.07%). Lipid in B. thailandensis fed with Ch3 (0.49 ± 0.02%) also significantly higher than that of the control (0.25 ± 0.02%) and the S3 treatment (0.19 ± 0.04%).
Carotenoid content in algae and fairy shrimp
Carotenoid content analysis of the three algal diets (Table 5) demonstrated that S3 and Ch3 had 2.84 ± 0.10 mg g−1 total carotenoid contents which was significantly higher than the control (1.34 ± 0.04 mg g−1) (P < 0.05). HPLC analysis revealed that lutein and β-carotene were the major carotenoids in S3 (0.49 ± 0.02 and 0.33 ± 0.01 mg g−1 of total carotenoids) while the major carotenoids in the Ch3 and control were lutein (0.70 ± 0.06 and 0.54 ± 0.02 mg g−1) and zeaxanthin (0.61 ± 0.02 and 0.38 ± 0.02 mg g−1).
Carotenoid compositions of the fairy shrimp fed with the optimal concentrations of three algal diets are presented in Table 6. The total carotenoids in B. thailandensis fed with the control, Ch3, and S3 were not significantly different (P > 0.05). Analysis of individual carotenoids showed that the shrimp fed with all the three diets contained four types of carotenoids: astaxanthin, lutein, canthaxanthin, and β-carotene. Predominant carotenoids in the shrimp fed with the three diets were canthaxanthin (0.021–0.031 mg g−1) and astaxanthin (0.004–0.015 mg g−1).
Discussion
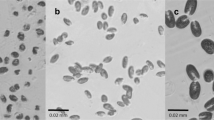
The current study demonstrated that Ch3 was the most suitable algal diet to feed B. thailandensis. Feeding the fairy shrimp with Ch3 provided significant higher body length, survival rate, and egg production than that fed by S3 and the most commonly used diet, C. vulgaris (control treatment). The suitability of Ch3 and the control diets as foods of the fairy shrimp may be due to their small cell sizes, which are less than 10 μm in diameter. Our observations under a microscope revealed that the average sizes of C. vulgaris and C. humicola were 4.22 ± 0.51 and 8.89 ± 1.99 μm. A study of gut content analysis by Selvarani (2009) has shown that C. humicola was found in the guts of immature and mature stages of the fairy shrimp S. dichotomus. Thus, this study confirmed the suitability of C. humicola as an appropriate fairy shrimp diet. However, this alga should not be fed to the nauplii stages of B. thailandensis because the alga size is larger than the nauplii’s mouth. Moreover, continuous and vigorous aeration is required to suspend C. humicola cells in the water column. One of the advantages of using C. humicola is the algal cells can be collected from culture medium by simple sedimentation. Concentrated cells can be easily concentrated and stored in cool condition prior to use.
Although the shrimp fed with the dried S3 powder had rather high growth and fecundity performances, the survival rate was low (56.67 ± 3.33%). One of the main problems with dried algae was the degradation of algal powder-released ammonia into the water through ammonification process. In this study, the treatments fed with Spirulina powder (S1, S2, and S3) had the highest total ammonia nitrogen of 0.53–1.12 mg L−1 [unionized ammonia (NH3-N) concentrations of 0.006–0.018 mg L−1] but lowest in dissolved oxygen of 3.72–4.82 mg L−1. These concentrations were higher than the standard concentration of ammonia for aquaculture, which must be less than 0.2 mg-N L−1 (Nootong et al. 2011). Moreover, microbial decomposition process also consumed oxygen so the dissolved oxygen in the treatments fed with Spirulina powder was significantly lower than that in C. vulgaris and C. humicola. Apart from acute toxicity, chronic toxicity of ammonia can also affect the animal growth (Hargreaves and Kucuk 2001), increase mortality, and possibly induce the black disease, which usually occurs in an undesired environment (Saejung et al. 2011). Hence, during the shortage of live foods in some hatcheries, use of instant foods such as dried Spirulina powder for B. thailandensis cultures can prevent the risk of production collapse. In this study, water quality was not high in values than that reported by Saengphan (2005) that presented the suitable values of water quality for fairy shrimp culture.
The lower concentration diets (Ch1, S1, and S2) significantly reduced growth and reproductive performances of B. thailandensis, compared with the control. Our results suggest that these were unsuitable food concentrations for the shrimp. Various studies have indicated that fairy shrimp can consume various types of food (Saengphan and Sanoamuang 2009; Sriputhorn and Sanoamuang 2007, 2011; Velu and Munuswamy 2007). The Spirulina powder at 0.8 mg dry weight individual−1 was the optimal concentration for nauplii (age 1–5 days) of B. thailandensis (Saengphan and Sanoamuang 2009). Some negative effects such as low egg productions and high mortality rates of the fairy shrimp S. proboscideus may be due to some toxicity of filamentous blue green algae when fed with pure S. platensis (De Walsche et al. 1991). Although rice bran and yeast could be used as substitution foods to replace C. vulgaris (Sriputhorn and Sanoamuang 2007; Saengphan and Sanoamuang 2009), most of these studies suggest that live algae are the most efficient diet for fairy shrimp cultivation. Two major reasons are that the live algae have less impact to water quality, and some algae are a good source of carotenoids (Ali and Dumont 1995; Zarattini and Mura 2004; Sriputhorn and Sanoamuang 2007; Saengphan and Sanoamuang 2009).
The studied B. thailandensis fed with the Ch3 treatment contained higher protein (3.60 ± 0.01%) than that fed with the C. vulgaris (control) at 1 × 106 cells mL−1 (3.06 ± 0.16%). Although the protein content in Ch3 (26.39%) is lower than that in the control Chlorella and S3 (Table 4), it is sufficient for growth of the fairy shrimp. Similarly, commercial feed pellet for fish contains only 20–28% of protein (FAO 2017). On the other hand, Ch3 contains relatively high carbohydrate (44.13%) which is an important energy source for animals. The concept of low-protein high-calorie diet may not yet be a suitable explanation in this study because more future researches are required. In general, physiological and environmental factors have an influence on nutritional values of planktonic organisms (Jeffries 1970). Claus et al. (1979) reported that Artemia salina larvae fed with Spirulina had a rather high protein content. Artemia showed rapid protein synthesis during their growth. When the Artemia animals were starved, protein synthesis in their bodies was still functioning but the growth rate was reduced. Among the three algae used in this study, Spirulina powder itself had the highest protein content but after feeding by B. thailandensis, only 2.57 ± 0.07% of protein content was observed in the shrimp. The possible explanation could probably relate with the decomposition of the algal powder resulting in a transformation process from organic nitrogen into inorganic ammonia-nitrogen in the water. In addition, the high concentrations of ammonia in the water are not suitable for the shrimp to grow because their bodies will contain less protein.
Although the total carotenoids in the Spirulina powder and C. humicola were two times more than that in the control C. vulgaris, such carotenoids examined in B. thailandensis fed these algae were not significantly different (P > 0.05). However, differences in their dominant carotenoid compositions are distinguished. This work demonstrated that canthaxanthin was the most abundant carotenoid in B. thailandensis followed by astaxanthin, lutein, and β-carotene, respectively. This predominant canthaxanthin profile corresponds to that of the fairy shrimp S. dichotomus (Murugan et al. 1995; Velu et al. 2003; Velu and Munuswamy 2007). However, Dararat et al. (2012) reported that major carotenoids in B. thailandensis were astaxanthin and canthaxathin. The astaxanthin and canthaxanthin contents were higher than those found in S. sirindhornae and S. siamensis. Previous studies revealed that fairy shrimp are able to convert β-carotene from ingested foods into canthaxanthin. This is related to the increasing of canthanxanthin contents in conjunction with the depletion of β-carotene (Hsu et al. 1970; Nelis et al. 1988).
In summary, this study demonstrates that C. humicola is one of the suitable alternative food sources for the cultivation of B. thailandensis. Moreover, Spirulina powder is also a good choice when live algae are not available.
References
Ali AJ, Dumont HJ (1995) Larviculture of the fairy shrimp, Streptocephalus proboscideus (Crustacea, Anostraca): effect of food concentration and physical and chemical properties of the culture medium. Hydrobiologia 298:159–165
AOAC (2012) Official methods of analysis, 19th edn. Association of Official Analytical Chemists, Gaithersburg, Maryland
Boonmak P, Saengphan N, Sanoamuang L (2007) Biology and fecundity of two fairy shrimps, Streptocephalus sirindhornae Sanoamuang, Murugan, Weekers and Dumont and Branchinella thailandensis Sanoamuang, Saengphan and Murugan. KKU Res J 12:125–131
Bower CE, Holm-Hansen T (1980) A salicylate-hypochlorite method for determining ammonia in sea water. Can J Fish Aquat Sci 37:794–798
Brendonck L (1993) Feeding in the fairy shrimp Streptocephalus proboscideus (Frauenfeld) (Branchiopoda: Anostraca). I Aspects of the feeding biology. J Crustac Biol 13:235–244
Brendonck L, Rogers DC, Olesen J, Weeks S, Hoeh WR (2008) Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia 595:167–176
Claus C, Benijts F, Vandeputte G, Gardner W (1979) The biochemical composition of the larvae of two strains of Artemia salina (L.) reared on two different algal foods. J Exp Mar Bio Ecol 36:171–183
Dararat W, Lomthaisong K, Sanoamuang L (2012) Biochemical composition of three species of fairy shrimps (Branchiopoda: Anostraca) from Thailand. J Crustacean Biol 32:81–87
Dararat W, Starkweather PL, Sanoamuang L (2011) Life history of three fairy shrimps (Branchiopoda: Anostraca) from Thailand. J Crustacean Biol 31:623–629
De Walsche C, Mertens J, Dumont HJ (1991) Observations on temperature optimum, cyst production, and survival of Streptocephalus proboscideus (Frauenfeld, 1873) (Crustacea: Anostraca), fed different diets. Hydrobiologia 212:21–26
FAO (2017) Aquaculture Feed and Fertilizer Resources Information System. http://www.fao.org/fishery/affris/species-profiles/nile-tilapia/feed-formulation/en/), 4 August 2017
Hargreaves JA, Kucuk S (2001) Effects of diel un-ionized ammonia fluctuation on juvenile hybrid striped bass, channel catfish and blue tilapia. Aquaculture 195:163–181
Hsu WJ, Chichester CO, Davies BH (1970) The metabolism of β-carotene and other carotenoids in the brine shrimp, Artemia salina L. (Crustacea: Branchiopoda). Comp Biochem Phys 32:69–79
Jeffries HP (1970) Seasonal composition of temperate plankton communities: fatty acids. Limnol Oceanogr 15:419–426
Jeffrey SW, Montoure RFC, Wright SW (1997) Phytoplankton pigments in oceanography: guidelines to modern methods p. 661. UNESCO publishing, Paris
Murugan G, Nelis HJ, Dumont HJ, De Leenheer AP (1995) Cis-and all-trans-canthaxanthin levels in fairy shrimps. Comp Biochem Physiol B Biochem Mol Biol 110(4):799–803
Nelis H, Lavens P, Van Steenberge M, Sorgeloos P, Criel G, De Leenheer A (1988) Qualitative and quantitative changes in the carotenoids during development of the brine shrimp Artemia. J Lipid Res 29:491–499
Nootong K, Pavasant P, Powtongsook S (2011) Effects of organic carbon addition in controlling inorganic nitrogen concentrations in a biofloc system. J World Aquacult Soc 42:339–346
Plodsomboon S, Maeda-Martínez AM, Obregón-Barboza H, Sanoamuang L (2012) Reproductive cycle and genitalia of the fairy shrimp Branchinella thailandensis (Branchiopoda: Anostraca). J Crustac Biol 32:711–726
Plodsomboon S, Sanoamuang L (2007) Effect of salinity on survival of the Thai fairy shrimp’s nauplii (Branchinella thailandensis Sanoamuang, Saengphan and Murugan, 2002). J Sci Res (Section T) 6:165–173
Rogers DC, Thaimuangphol W, Saengphan N, Sanoamuang L (2013) Current knowledge of the south east Asian large branchiopod Crustacea (Anostraca, Notostraca, Laevicaudata, Spinicaudata, Cyclestherida). J Limnol 72(s2):69–80
Saejung C, Hatai K, Wada S, Kurata O, Sanoamuang L (2011) Clinical observations of black disease in fairy shrimps, Streptocephalus sirindhornae and Branchinella thailandensis, from Thailand and pathogen verification. J Fish Dis 34:911–920
Saejung C, Hatai K, Sanoamuang L (2014a) Bath efficacy of sodium hypochlorite, oxytetracycline dihydrate and chloramphenicol against bacterial black disease in fairy shrimp Branchinella thailandensis. Aquac Res 45:1697–1705
Saejung C, Hatai K, Sanoamuang L (2014b) The in-vitro antibacterial effects of organic salts, chemical disinfectants and antibiotics against pathogens of black disease in fairy shrimp of Thailand. J Fish Dis 37(1):33–41
Saengphan N (2005) Culture of the Thai fairy shrimp, Branchinella thailandensis Sanoamuang, Saengphan and Murugan, 2002 for commercial applications in Thailand. Thesis, Khon Kaen University, Thailand
Saengphan N, Sanoamuang L (2009) Effect of food concentrations on growth and survival of the fairy shrimp Branchinella thailandensis Sanoamuang, Saengphan and Murugan. Burapha Sci J (special volume): 19−28
Saengphan N, Shiel RJ, Sanoamuang L (2005) The cyst hatching pattern of the Thai fairy shrimp, Branchinella thailandensis Sanoamuang, Saengphan & Murugan, 2002 (Anostraca). Crustaceana 78:513–523
Saleha A, Dhar D, Singh P (2011) Comparative pigment profiles of different Spirulina strains. Res Biotech 2:67–74
Sanoamuang L, Murugan G, Weekers PHH, Dumont HJ (2000a) Streptocephalus sirindhornae, new species of freshwater fairy shrimp (Anostraca) from Thailand. J Crustac Biol 20:559–565
Sanoamuang L, Sanoamuang N, Saengphan N, Chusing R, Athibai S, Lekchan S (2000b) Species diversity and distribution of fairy shrimps in Thailand. Khon Kaen University, Thailand
Sanoamuang L, Saengphan N (2006) A new species of Streptocephalus fairy shrimp (Crustacea, Anostraca) with tetrahedral cysts from central Thailand. Int Rev Hydrobiol 91:250–256
Sanoamuang L, Saengphan N, Murugan G (2002) First record of the family Thamnocephalidae (Crustacea: Anostraca) from Southeast Asia and description of a new species of Branchinella. Hydrobiologia 486:63–69
Selvarani BJ (2009) Food preference of fairy shrimp Streptocephalus dichotomus (Baird) Crustacea: Anostraca. J Appl Biol Sci 16:840–844
Shu S, Maeda-Martinez AM, Rogers DC, Yang J, Chen X (2015) Morphological characterization of Streptocephalus sirindhornae (Branchiopoda: Anostraca) from South East Asia: first record of the Streptocephalidae from China. Zootaxa 3911:447–450
Sivathanu B, Palaniswamy S (2012) Purification and characterization of carotenoids from green algae Chlorococcum humicola by HPLC-NMR and LC-MS-APCI. Biomed Prev Nutr 2:276–282
Soeder CJ (1980) Massive cultivation of microalgae: results and prospects. Hydrobiologia 72:197–209
Sornsupharp B, Lomthaisong K, Dahms HU, Sanoamuang L (2015) Effects of dried fairy shrimp Streptocephalus sirindhornae meal on pigmentation and carotenoid deposition in flowerhorn cichlid; Amphilophus citrinellus (Günther, 1864) × Cichlasoma trimaculatum (Günther, 1867). Aquac Res 46:173–184
Sornsupharp S, Dahms HU, Sanoamuang L (2013) Nutrient composition of fairy shrimp Streptocephalus sirindhornae nauplii as live food and growth performance of giant freshwater prawn postlarvae. Aquac Nutr 19:349–359
Sriputhorn K, Sanoamuang L (2007) Culture of the Thai fairy shrimp (Branchinella thailandensis Sanoamuang, Saengphan and Murugan) by bioextract and yeast as food. J Sci Res (Section T) 6:369–375
Sriputhorn K, Sanoamuang L (2011) Fairy shrimp (Streptocephalus sirindhornae) as live feed improve growth and carotenoid contents of giant freshwater prawn Macrobrachium rosenbergii. Int J Zool Res 7:138–146
Stanier R, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Strickland J, Parsons T (1972) A practical handbook of seawater analysis. Bulletin 167, 2 edn. Fisheries Research Board of Canada, Ottawa, Canada
Velu CS, Munuswamy N (2007) Composition and nutritional efficacy of adult fairy shrimp Streptocephalus dichotomus as live feed. Food Chem 100:1435–1442
Velu CS, Czeczuga B, Munuswamy N (2003) Carotenoprotein complexes in entomostracan crustaceans (Streptocephalus dichotomus and Moina micrura). Comp Biochem Physiol B Biochem Mol Biol 135(1):35–42
Yuan JP, Chen F, Liu X, Li XZ (2002) Carotenoid composition in the green microalga Chlorococcum. Food Chem 76:319–325
Zarattini P, Mura G (2004) The effects of food type on length-weight growth, sexual differentiation, and survival in Chirocephalus ruffoi (Anostraca) cultured under standard conditions. J Crustac Biol 24:225–231
Acknowledgements
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster Ph.D. program No. 54124.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaoruangrit, L., Tapaneeyaworawong, P., Powtongsook, S. et al. Alternative microalgal diets for cultivation of the fairy shrimp Branchinella thailandensis (Branchiopoda: Anostraca). Aquacult Int 26, 37–47 (2018). https://doi.org/10.1007/s10499-017-0191-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-017-0191-5