Abstract
Natural compounds of Aloe vera gel are well known for their immunostimulatory properties. In this study, adjuvant effects of Aloe vera in combination with formalin-killed A. hydrophila bacterin in immunization of common carp were evaluated and compared with Freund’s adjuvant. A total of 480 juvenile carp were randomly divided into four groups. Groups 1–4 were injected on days 0 and 14 with normal saline (as control), bacterin, bacterin + Freund’s adjuvant and bacterin + Aloe vera gel, respectively. Blood samples were taken on days 14, 28 and 42. Hematoimmunological parameters including white blood cell (WBC) count, alternative complement activity, serum lysozyme and bactericidal activity, respiratory burst activity (NBT) and antibody titer were evaluated and compared among different groups. At the end of experiment, all groups were challenged with live A. hydrophila and relative percentage survival (RPS) compared among groups. According to the results, administration of both adjuvants caused a positive change in fish immune response and most of the aforementioned parameters significantly improved in vaccinated groups compare with control group (P < 0.05). Moreover, WBC count, alternative complement activity, serum lysozyme activity and NBT activity were significantly higher in Aloe adjuvant group compared with Freund’s adjuvant (P < 0.05). Finally after challenge, groups 3 and 4 showed significantly lesser mortality than groups 1 and 2. Overall, our results indicated that combination of A. hydrophila bacterin with adjuvant can improve the vaccine efficacy and resistance against A. hydrophila infection, and in comparison with traditional adjuvants, Aloe vera gel could be used as a natural adjuvant with similar or even greater positive effects on vaccination of common carp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aloe vera gel is a natural compound extracted from the leaf pulp of Aloe barbadensis. Aloe plants have long been used to treat a wide array of ailments and diseases and have wide immunostimulating effects and healing properties. It acts as a broad-spectrum immunoregulator which can activate immunocytes, regulate the release of cytokines, promote the generation of antibody and enhance the immunological function of leukocytes (Lee et al. 2001). Acemannan, the main functional component of Aloe vera gel (Lee et al. 2001), can bind to macrophages and exhibits strong tumor suppressor and adjuvant properties (Lee 1988; Peng et al. 1991). Adjuvant function of Aloe vera gel has been described in vaccination against avian viral and bacterial diseases such as Newcastle disease, Marek’s disease and bordetellosis (Sun et al. 2011b; Chinnah et al. 1992).
Aeromonas hydrophila has been recognized as an opportunistic pathogen of a wide variety of hosts which causes infectious and several diseases in fishes (Yin et al. 2009). In the last decade, the disease has caused extensive losses in cultured freshwater fishes especially warm water fishes (Xia et al. 2004). Vaccination is an economical and safe method for controlling fish diseases. Unlike chemosynthetic drugs such as antibiotics, problems such as drug resistance and environmental pollution are not associated with vaccines (Ebanks et al. 2004). Different types of vaccines have been developed against A. hydrophila (Fang et al. 2004); although some of them resulted in satisfactory protection level against this pathogen, no vaccine is commercially available against A. hydrophila (Wang et al. 2013).
Despite the importance and success of vaccination, little is known about the method by which adjuvants enhance protection especially in the field of fish immunology (Lorenzen et al. 2000; Tafalla et al. 2013; Perez et al. 2013).
Freund’s adjuvant is a well-known classical oil-based adjuvant with strong adjuvanticity effects that has been used for years in veterinary vaccines (Wanman et al. 2007). Various adverse effects including granuloma formation, necrosis and tissue impairment have been observed with Freund’s adjuvant (Jiao et al. 2010; Gjessing et al. 2012).
These problems have persuaded researchers to study about new forms of adjuvants. Among new generation adjuvants, natural compounds are very interesting because they are economical, safe and easily available in different regions (Raa 1996). Despite numerous immunostimulating benefits of Aloe vera and its products, there is little information available about the effects of Aloe especially its adjuvanticity effects as vaccine adjuvant in aquaculture (Kim et al. 1999; Alishahi et al. 2010).
Thus, in the current study the possibility of using Aloe vera gel as an injectable adjuvant in combination with A. hydrophila bacterin for improving immunization efficacy of common carp against A. hydrophila infection was evaluated and compared with Freund’s adjuvant as a positive control.
Materials and methods
Animals
A total of 480 juvenile common carp (Cyprinus carpio) weighing 50.4 ± 3.2 g are obtained from a fish carp farm (Ahvaz, Khuzestan, Iran) and adapted with laboratory conditions 2 weeks prior to the start of experiment. In the first step, the health status of the fish was examined based on physical appearance and internal organs followed by swabs from body surface, gill and liver immediately upon arrival at the laboratory and at 14-day intervals later (Austin and Austin 1989).
Then fishes were acclimatized with laboratory condition and carefully observed for any sign of disease during acclimatizing period. During adaptation and experiment period, chemo-physical parameters of water were maintained in optimum condition by means of aeration pumps, automated thermostatic heaters, biofilters and frequent water changes for all groups. Water parameters were as follows: temperature 26 ± 1 °C; dissolved oxygen 9 ± 1 mg l−1; pH 7.7 ± 0.33; NH3 < 0.01 mg l−1; NO2 < 0.1 mg l−1; and salinity 700 µSc m−1.
Fishes were divided into four groups in triplicates (40 fish in each replicate) and each group maintained in 300-L separate tank. All tanks were equipped with suitable mechanical and biological filters, and daily water changes were done at the rate of 30 % of tank volume in order to maintain the optimum water quality.
Group 1 received normal saline instead of vaccine and served as negative control, group 2 received bacterin without any adjuvant (positive control), group 3 received bacterin combined with Freund’s adjuvant, and group 4 received bacterin combined with Aloe vera gel as adjuvant.
Bacterial strain
Lyophilized A. hydrophila code no: AH-04 (approved by Institute of Aquaculture, Uni. Sterling, Scotland; isolated from diseased silver carp) kindly donated by Dr. Soltani (Department of fish disease, Faculty of Veterinery, University of Tehran) was used as bacterial stock. The bacterial strain was previously identified and proved using standard morphological, physiological, molecular and biochemical plate and tube tests.
Vaccine preparation and immunization
A. hydrophila was cultured in tryptic soy broth (TSB) media for 48 h at 25 °C, and then, 1 % formalin (v:v) (Merck, Germany) was added to bacterial suspension and mixture kept in 4 °C for 24 h. Finally, inactivated bacteria collected by centrifuging of media in 4000g for 30 min and the supernatant were discarded, and the pellet was re-suspended in sterile normal saline. The last stage was repeated for three times in order to make sure that the formalin and culturing media have been removed thoroughly. Complete inactivation of prepared bacterin was tested and confirmed by its subcultures in TSB for 48 h at 25 °C. Bacterin stock concentration was adjusted to 109 CFU ml−1 using McFarland turbidity meter (Sun et al. 2011a; Bastardo et al. 2012).
Freeze-dried powder of Aloe vera leaf inner gel (Alavi Food and Drug Industries, Shiraz, Iran) was mixed with normal saline in a ratio of 100 mg ml−1 and sterilized by passing through 0.22-µm filter.
Bacterial test of Aloe vera gel was performed using TSB before use. 100 µl of filtered Aloe vera gel added to 5 ml of sterile TSB medium and incubated in 30 °C for 7 days. After incubation period, TSB medium turbidity was observed a sign of bacterial growth (Carter 2008). No bacterial growth was observed.
Same amounts of Aloe vera solution and bacterin stock (i.e., 50:50 v:v ratio) were totally mixed by shaking solution vigorously for 5 min using vortex mixer (Habeeb et al. 2007; Sun et al. 2011b).
Freund’s adjuvant (Baharafshan co, Iran) and bacterin were mixed in a ratio of 50:50 (v:v), then vortexed and homogenized according to manufacturer instruction. Equal ratio of sterile normal saline was added to bacterin instead of adjuvant for group 3. Thus, the final concentration of vaccine solutions for all vaccinated groups was about 5 × 108 CFU ml−1 as described previously (Bastardo et al. 2012).
Fishes were anesthetized by 50 ppm of MS222 (Western Chemicals Inc. Tricaine-S) and intraperitoneally vaccinated with 0.1 ml of different formulated vaccines according to group. Vaccination was repeated 14 days after first injection as same as described previously except application of incomplete Freund’s adjuvant instead of complete one in second injection of group 3 (Evensen et al. 2005; Sun et al. 2011b).
Sampling
On days 14, 28 and 42 of study, fish were anesthetized with 50 ppm of MS222 and blood samples were collected from the caudal vein of nine fishes (three from each replicate). Sera were obtained by centrifuging blood in 3500 rpm and stored in −80 °C. During the experiment after collection of blood samples, fish were euthanized and internal organs and peritoneal cavity were examined by the observation and gross pathology.
White blood cell count (WBC)
White blood cell count (WBC) was calculated in a Neubauer counting chamber as described by Schaperclaus et al. (1991).
Alternative complement activity
The activity of the alternative complement pathway was assayed using sheep red blood cells (SRBC) as targets (Ortuno et al. 1998). SRBC were washed in phenol red-free Hank’s balanced salt solution (HBSS) containing Mg2+ and EGTA and re-suspended at 3 % in HBSS. Aliquots (500 µl) of test serum as complement source, diluted in HBSS, were added to 500 µl of SRBC to give final concentrations of 10, 5, 2.5, 1.25, 0.625, 0.313, 0.1565 and 0.078 %. After incubation for 1 h at 22 °C, the samples were centrifuged at 800g for 5 min at 4 °C to remove non-lysed erythrocytes. The relative hemoglobin content of the supernatants was assessed by measuring their optical density at 540 nm in a spectrophotometer. The values of maximum (100 %) and minimum (spontaneous) hemolysis were obtained by adding 500 µl of distilled water or HBSS to 500 µl samples of SRBC, respectively. The degree of hemolysis (Y) was estimated, and the lysis curve for each specimen was obtained by plotting Y/1 − Y against the volume of serum added (ml) on a log–log scaled graph. The volume of serum producing 50 % hemolysis (ACH50) was determined, and the number of ACH50 unit ml−1 was obtained for each experimental group.
Serum lysozyme activity
The lysozyme activity was measured using photoelectric colorimeter equipped with attachment for turbidity measurement (Biophotometer, Eppendorf, Germany). A series of dilutions of standard lysozyme from hen egg white (Sigma) mixed with Micrococcus lysodeikticus (Schroeter) (Sigma) suspension was prepared for establishing the calibration curve. 10 µl of standard solutions or serum was added to 200 µl of micrococcus suspension (35 mg of Micrococcus dry powder/95 ml of 0/02 M phosphate buffer + 5.0 ml of 1 M NaCl solution). The changes in the extinction were measured at 546 nm by measuring the extinction immediately after adding the solution which contained the lysozyme (start of the reaction) and after a 20 min incubation of the preparation under investigation at 40 °C (end of the reaction). The lysozyme content is determined on the basis of the calibration curve and the extinction measured (Ellis 1990).
Serum bactericidal activity
Bactericidal activity was studied following procedure by Azza (2009) with slight modification. Serum samples were diluted three times with 0.1 % gelatin–Veronal buffer (GVBC2) (v/v), (pH 7.5, containing 0.5 mM ml−1 Mg2+ and 0.15 mM ml−1 Ca2+). A. hydrophila (live, washed cells) suspended in the same buffer at concentration of 105 CFU ml−1. The diluted sera and bacteria were mixed at 1:1 v/v, incubated for 90 min at 25 °C with shaker. Control group containing bacterial suspension was also included. The number of viable bacteria was then determined by counting the colonies after culturing on Trypticase soy agar (TSA) plates for 24 h at room temperature 25 °C.
Respiratory burst activity
The respiratory burst activity was measured by the reduction of nitro blue tetrazolium (NBT) by intracellular superoxide radicals (Anderson and Siwicki 1994). Briefly, 100 µl of heparinized blood from fish of each group was mixed with 100 µl of 0.2 % NBT (Merk, Germany) solution for 30 min at 25 °C. After incubation, 50 µl from the above mixture was added with 1 ml of N,N-diethylmethyl formamide (Sigma, USA) and then centrifuged at 3000g for 5 min. The optical density of the supernatant was measured at 540 nm and compared among treatments.
Anti A. hydrophila antibody titer (MAT)
The agglutination test was conducted in ‘U’-shaped microtiter plates (Swain et al. 2006). Twofold serial dilution of the 25 µl serum of fish was made with an equal volume of PBS in each well, to which 25 µl of formalin-killed Aeromonas hydrophila (107 cfu/ml) suspension was added. The plates were incubated overnight at room temperature. The titer was calculated as the reciprocal of the highest dilution of serum showing complete agglutination of the bacterial cells.
Determination of LD50
The bacterial cells (A. hydrophila AH-04) were cultured for 48 h, harvested and washed three times with sterile PBS (pH = 7.4) and centrifuged at 4000g for 15 min. Tenfold serial dilutions of the cells were then prepared into sterile PBS and total cell counts was determined using a hematocytometer. Before use, suspensions were microscopically checked by Gram staining and subculturing on TSA.
Two replicates of 10 fishes were intraperitoneally (i.p) injected with the bacterium at 101, 102, 103, 104, 105, 106, 107, 108 and 109 cells/fish at 25 °C after anesthetization by MS222 (50 mg/L), and they were kept separately in 60 L aquaria with clean water at 25 °C with aeration for over 10 days. Control groups were i.p injected with 0.1 ml/fish of sterile PBS and were kept separately. The challenge LD50 of A. hydrophila calculated according to Reed and Muench (1938). Mortality of challenged fish was recorded daily for 10 days. The cause of death was ascertained by re-isolating the infecting organism from kidney and liver of dead fish according to Soltani and Kalbassi (2001). A. hydrophila concentration for induction of 50 % mortality after 14 days (LD50) were estimated at 2.1 × 107 cells/fish.
Bacterial challenge
Thirty fish from each group (10 of each aquarium) intraperitoneally injected with the 200 µL bacterial suspension (2.1 × 107 CFU per fish = LD50) and mortality of challenged fish recorded daily for 14 days. The cause of death was ascertained by re-isolating the infecting organism from kidney and liver of dead fish on TSA media. Cumulative daily mortality curve was drawn according to Misra et al. (2006b). The relative percentage survival (RPS) of each trial was determined using the below equation as described by Amend (1981):
Statistical analysis
The experimental data were analyzed by one-way ANOVA, and means were compared using Dunkan test using SPSS (P < 0.05) statistics version 16.0 to determine whether there was significant variation among treatment groups.
Results
Results showed that most of the aforementioned immunological parameters were improved in vaccinated groups compared to control (P < 0.05). In addition, incorporation of adjuvants to vaccines caused a significant positive change in fish immune response parameters compared to control group (P < 0.05) (Table 1).
WBC
As shown in Fig. 1, WBC count was increased in vaccinated groups compare to control group on days 14, 28 and 42 of study. On day 14, the increase was significant just in Aloe vera gel adjuvant group. On day 28, all vaccinated groups showed significantlyhigher count of WBC compared to control. On day 28, the highest record obtained for Aloe group but the differences between vaccinated groups were not significant. On day 42, the highest WBC count was recorded for Aloe group which was significantly higher than control but the difference between Aloe and Freund was not significant. This positive stimulatory effect of Aloe gel on WBCs was more considerable and more persistent in comparison with Freund’s adjuvant.
Complement activity
Immunized fishes showed overall higher level of complement activity compared to control group in most of the sampling stages of the study, and this difference was more obvious in groups which received vaccine in combination with adjuvant. In other words, groups 3 and 4 had significantly higher complement activity than group 2 (P < 0.05). Moreover, Freund’s adjuvant group showed an earlier increase in complement activity on day 14 of experiment peaking on day 28, while Aloe group showed higher complement activity compare to Freund’s adjuvant group peaking later on day 28 of experiment (P < 0.05).
Serum lysozyme activity
Immunized fishes showed higher levels of serum lysozyme activity in comparison with control group on days 28 and 42 of experiment. The Aloe adjuvant group had significantly higher lysozyme activity than control group on day 28 and 42 (P < 0.05) (Table 1).
Serum bactericidal activity
As shown in Table 1, significant decrease in bacterial colonies was observed in the Aloe group on day 28 and for Aloe and Freund’s adjuvant on day 42 (P < 0.05).
NBT
NBT activity was higher in all vaccinated groups than control group on days 28 and 42 of experiment. Significant increase in NBT was seen in Aloe adjuvant vaccinated group compare to other vaccinated groups on days 28 and 42 (P < 0.05).
Antibody titer
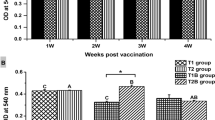
As shown in Fig. 2, antibody titer was significantly increased in all immunized groups from the day 14 of experiment. The level of antibody was higher in groups which received adjuvant compared to bacterin without adjuvant and this difference was significant on days 14 and 42. No significant difference observed among groups 3 and 4 (P > 0.05).
Side effects
Some fishes immunized with vaccine plus Freund’s adjuvant showed obvious signs of inflammation including mild ascites and tissue adhesion. In contrast, no sign of irritation and inflammation such as granulomatosis and ascites were observed in Aloe vera group. Moreover, no mortality or sign of stress observed in any of vaccinated groups during the experimental period.
Relative percentage survival (RPS)
After challenge with live bacteria, a significantly higher rate of mortality was observed in control group (66.67 ± 16.66) compared with vaccinated groups and the least mortality occurred in groups 3 (16.66 ± 16.66) and 4 (11.11 ± 9.62), (P < 0.05). RPS after challenge in Aloe and Freund’s adjuvant groups was 83.3 and 75 %, which means that the total mortality in groups 3 and 4 was significantly lower than control group (P < 0.05) (Fig. 3).
Discussion
Vaccination is a safe and helpful alternative to chemotherapy for the control of fish bacterial diseases. In vaccines, adjuvants are a crucial factor for inducing more efficient protection in host against specific pathogens (Chu 2006). Regarding considerable disadvantages of old-fashioned adjuvants especially traditional oil adjuvants in fish farms such as high cost and adverse side effects, it is important to find new more efficient adjuvants for substituting the older ones (Jiao et al. 2010; Gjessing et al. 2012). Among different compounds with probable adjuvanticity effects, natural compounds seem to be a better choice because they are generally inexpensive, easily available and safer in comparison with synthetic materials. Aloe vera is one of the most attractive natural substances that have been used for centuries due to many benefits in health and diseases, and it has been known as a potential immunostimulant in mammals and birds (Nordgren et al. 1992; Reynolds and Dweck 1999; Djeraba and Quere 2000; Lee et al. 2001).
Despite the undeniable importance of vaccination in modern animal husbandry, unfortunately the immunostimulatory mechanism of many vaccines and adjuvants is poorly understood in the field of aquaculture and aquatic animal health (Kamilya et al. 2006; Wu et al. 2014).
With due attention to this basic information, in this study the adjuvant effects of Aloe gel in Aeromonas hydrophila vaccine were evaluated and compared with traditional Freund’s adjuvant. We found that vaccinated fishes with A. hydrophila bacterin in combination with adjuvants (Freund’s adjuvant or Aloe vera gel extract) had higher levels of WBC count, alternative complement activity, serum lysozyme activity, serum bactericidal activity, NBT and antibody titer. Most of these factors are among innate part of immune system. The innate immune system of fish is considered to be the first line of defense against a wide range of pathogens and as fish are placed in lower position of evolutionary system among Animalia kingdom, innate immunity plays a greater and more important role for fish as compared with mammals. So it could be expected that improvement in these factors may generally result in a better immune response (Saurabh and Sahoo 2008).
According to the results, the highest WBC count was observed in vaccinated group + Aloe gel adjuvant followed by vaccinated group + Freund’s adjuvant. The rise in WBC count was obvious from the day 14 of experiment for vaccinated group with Aloe gel + bacterin which reached to the highest level on day 28 and last significantly higher than control group until the end of experiment. Group 3 showed a similar trend in the increase in WBC count. On day 28, all vaccinated groups showed significant difference in WBC count compared with control group. At the end of experiment (day 42), although WBC count was measured higher than control in groups 3 and 4, the difference remained at a significant level only for Aloe group when compared to control.
According to many available data from previous studies, Aloe vera has proven hematopoietic activity and one of the ways in which Aloe vera acts as an immunostimulator is through hematopoiesis and hematopoiesis-enhancing processes (Talmadge et al. 2004; Lee 2006). Similar results have been reported in cat, mice and horses which received Aloe vera-extracted compounds (Yates et al. 1992; Egger et al. 1996; Green 1996). In a previous study, oral administration of Aloe vera gel in common carp led to increase in hematopoiesis (Alishahi and Abdy 2013). Similar results were reported in tilapia, and many hematological indices including WBC count were increased under the effect of dietary Aloe vera (Gabriel et al. 2015). In contrast, although Dotta et al. 2014 reported an increase in hematocrit of Nile tilapia fed with Aloe vera, no significant increase was observed in WBC count. Regarding available data, Freund’s adjuvant can stimulate the WBC proliferation especially T cells (Freund 1956). As mentioned above, in current study we observed increase in WBC count of Freund’s group on days 14, 28 and 42; however, the difference was significant only on day 28.
Complement is one of the most important serum factors because of its activating effects on the cellular defenses. In the present study, serum alternative complement activity was significantly higher (P < 0.05) for Freund’s group on day 14 and for both adjuvant groups on days 28 and 42 compared to control group. But vaccinated group with bacterin only showed a significant difference with control group on day 28. The results of current study show that the use of both adjuvants with vaccine caused a faster increase in complement activity which lasts for a longer period of time compared to control and vaccine with no adjuvant. Similar results about effects of vaccination with adjuvant on complement activity of fish (Japanese flounder, Paralichthys olivaceus) and warm-blooded species were reported in other studies (Gupta et al. 1995; Petrovsky and Aguilar 2004; Jiao et al. 2010).
Vaccination can enhance complement activity; besides, the use of adjuvants can amplify this positive enhancing effect (Jiao et al. 2010; Harikrishnan et al. 2012). There is a little amount of data available focusing on the interaction of Aloe vera and complement activity especially in fish but as shown in previous study by the authors, and oral administration of Aloe vera can stimulate complement activity in common carp (Alishahi and Abdy 2013) while in another study such positive effect on complement activity was not confirmed in rainbow trout fed with Aloe supplemented ratio (Zanuzzo et al. 2015b). On the other hand, in accordance with current study, similar results have been reported following intraperitoneal administration of herbal compounds (Alexander et al. 2010). In addition to the probable direct effect of Aloe vera gel on complement activity, the rise in serum lysozyme activity can enhance complement activity because of its opsonizing effects (Magnadottir 2006; Saurabh and Sahoo 2008). Lysozyme level or activity is an important index of innate immunity of fish. Lysozyme activity has been shown to vary depending on the various health condition including stress, infection, vaccination (Saurabh and Sahoo 2008; Harikrishnan et al. 2012). As shown in results, serum lysozyme activity had been raised in vaccinated fishes especially in combination with Aloe vera gel. Consistent with our data, some herbal active compounds and products of herbals are shown to be able to enhance innate immune parameters including lysozyme activity when administered both oral and parenteral way in different fish species (Divyagnaneswari et al. 2007; Alexander et al. 2010; Harikrishnan et al. 2011). In contrast, Kim et al. reported that although feeding rockfish with Aloe vera supplemented food does not increase serum lysozyme activity, the resistance to vibriosis was higher in Aloe-treated groups when compared with control group (Kim et al. 1999).
Serum bactericidal activity showed significant difference only on day 28 for Aloe and pm day 42 for Aloe and Freund’s groups. Various factors can affect serum bactericidal power in fish. Serum proteins such as lysozyme, complement component, immunoglobulins and antimicrobial peptides can affect serum bactericidal power in various aspects. In another study, Dotta et al. also reported that oral administration of Aloe vera extract in Nile tilapia does not significantly affect serum antimicrobial activity (Dotta et al. 2014). In contrast, Alishahi and Abdy reported an increase in serum bactericidal activity of common carp following feeding diet supplemented with 0.5 and 1 % Aloe vera extract. Plus Aloe vera extract itself possesses proven antibacterial component (Reynolds and Dweck 1999; Alishahi and Abdy 2013). In other studies, administration of some other herbal extract led to the increase in serum bactericidal activity in other fish species (Misra et al. 2006a; Divyagnaneswari et al. 2007). In two different studies, increase in serum bactericidal activity was reported after vaccination of tilapia and flounder (Kwon et al. 2006; Sun et al. 2011a) while other researcher reported no significant difference between serum bactericidal activity of vaccinated and non-vaccinated cod fish (Costa et al. 2011). This controversial information shows that the effect of vaccination and adjuvants on serum bactericidal activity is highly dependent on species, rout and term of administration.
NBT activity, an indicator of respiratory burst activity in phagocyte cells, significantly increased on days 28 and 42 for groups 3 and 4. The highest NBT activity was recorded for vaccinated group + Aloe adjuvant on day 42; this group was the only group which showed significant difference compared to control.
There are reported data showing that vaccination and the use of Freund’s adjuvant can positively affect respiratory burst activity in fish (Sakai 1999). The results of the present study showed that Aloe gel has more effect on NBT than Freund’s adjuvant.
Regarding the results of the present study and other similar studies, stimulatory effect of herbal extracts on NBT activity can be attributed to the proliferative responses of leukocytes or due to the enhancement in the respiratory burst activity and expression of interleukins (Jang et al. 1995; Peddie et al. 2002; Alishahi and Abdy 2013). As mentioned before, Aloe vera extract and acemannan have direct stimulatory effect on WBC count and proliferation as well as respiratory burst activity of macrophages (Green 1996; Stuart et al. 1997; Reynolds and Dweck 1999). On the other hand, stimulatory effect of Aloe vera on various cytokines and interleukin production has been confirmed in previous studies (Wonbel and Helderman 1988; Djeraba and Quere 2000; Abdy et al. 2016). Blood respiratory burst activity is mainly the result of white blood cells activity especially neutrophils and monocytes (Anderson et al. 1992). In current study, as WBC count was recorded higher for Aloe group, higher level of NBT activity than other groups can be explained due to the increase in WBC population.
Serum antibody titer is one of the most important immunological factors which were evaluated in this study. As expected, all vaccinated groups showed antibody response to the antigen compared to control group, but the increase in antibody level in vaccine + adjuvant groups started faster (day 14) and last significantly higher until the last stage of sampling point (day 42). In addition, no significant difference was observed in antibody titer of Aloe or Freund’s adjuvant groups in any period of sampling.
There are many documented data about the antibody response after vaccination in different fish species (Gudding et al. 1999). Observation of higher antibody level in fish which received bacterin combined with Freund’s or Aloe adjuvant was expectable due to well-known adjuvanticity and immunostimulating effects of Freund’s adjuvant and Aloe gel in human and warm-blooded animals (Freund 1956; Peng et al. 1991; Chinnah et al. 1992; Petrovsky and Aguilar 2004; Jiao et al. 2010; Lee 1988; Sun et al. 2011b).
Also the positive effects of some herbal active compounds and products of herbals on adaptive immune system of fishes have been described (Harikrishnan et al. 2011), as far as we know the present work is the first research that studies the possibility of using Aloe vera gel as an injectable adjuvant in fish and its effects on antibody response of common carp. In a similar study, Sun et al. reported an increase in antibody titer of chickens immunized by Bordetella-killed vaccine in combination with Aloe polysaccharides (Sun et al. 2011b).
Finally with due attention to the improvement in many immunological factors of vaccinated fish especially in combination with adjuvants, it was not surprising that groups 3 and 4 showed the lowest mortality rate after challenge with live bacterium. The use of adjuvant lowered the mortality rate to the <30 % of control group mortality and 50 % of vaccine without adjuvant. Considering survival rate after challenge, both Aloe and Freund’s adjuvant groups showed similar efficiency and no significant difference was seen between these two groups albeit mild irritation and inflammation were seen in fishes of Freund’s adjuvant group. In previous studies, higher resistance to bacterial infection was reported in fish fed with Aloe extract as an oral immunostimulant (Kim et al. 1999; Alishahi and Abdy 2013). According to the results of a new study, Steelhead trout (Oncorhynchus mykiss) fed with diet containing 0.5 % Aloe vera powder showed 50 % lower levels of serum cortisol after injection of Aeromonas salmonicida antigen compared to control group (Zanuzzo et al. 2015a). As cortisol hormone is known to act as an immunosuppressive agent in animals, reduction in this hormone can probably lead to an overall better immune reaction such as stronger antibody response and higher survival rate in the case which animal faces an infection.
As a final conclusion, addition of adjuvant (Freund’s or Aloe gel) to A. hydrophila vaccine improves its immunogenicity and experimental efficacy. Moreover, Aloe vera gel showed similar or even stronger and more efficient immune-stimulating effects than Freund’s adjuvant in some immunological factors which have been evaluated and reported in present study.
Our results in concomitant with easy preparation or injection and low cost of Aloe vera gel may help us to consider Aloe vera gel as an adjuvant with good potential for future application in aquaculture. Future studies are necessary to clarify the details such as molecular and cellular immunostimulatory actions of Aloe vera gel as an adjuvant in carp and other species.
References
Abdy E, Alishahi M, Tabandeh MR, Ghorbanpoor M, Jafari H (2016) Comparative effect of Freund’s adjuvant and Aloe vera L. gel on the expression of TNF-α and IL-1β in vaccinated Cyprinus carpio L. with Aeromonas hydrophila C. bacterin. Aquac Res 47:2543–2552
Alexander CP, Kirubakaran CJ, Michael RD (2010) Water soluble fraction of Tinospora cordifolia leaves enhanced the non-specific. Fish Shellfish Immunol 29:765–772
Alishahi M, Abdy E (2013) Effects of different levels of Aloe vera L. extract on growth performance, hemato-immunological indices of Cyprinus carpio L. Iran J Vet Sci Technol 5(2):33–44
Alishahi M, Ranjbar MM, Ghorbanpour M, Peyghan R, Mesbah M, Razijalali M (2010) Effects of dietary Aloe vera on specific and nonspecific immunity of common carp (Cyprinus carpio). Iran J Vet Res 4(3):85–91
Amend DF (1981) Potency testing of fish vaccines. In: Anderson DP, Hennessen H (eds) Fish biologies: serodiagnostics and vaccines. Development in biological standardization, vol 49. Karger, Basel, pp 447–454
Anderson DP, Siwicki AK (1994) Duration of protection against Aeromonas salmonicida in brook trout immuno stimulated with glucan or chitosan by injection and immersion. Prog Fish Cultur 56:258–261
Anderson DP, Moritomo T, de Grooth R (1992) Neutrophil, glass-adherent, nitroblue tetrazolium assay gives early indication of immunization effectiveness in rainbow trout. Vet Immunol Immunopathol 30(4):419–429
Austin B, Austin DA (1989) Microbiological examination of fish and shellfish. Ellis Horwood, Chichester, pp 217–488
Azza MM (2009) Antagonism of Aeromonas hydrophila by propolis and its effect on the performance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27:454–459
Bastardo A, Ravelo C, Castro N, Calheiros J, Romalde L (2012) Effectiveness of bivalent vaccines against Aeromonas hydrophila and Lactococcus garvieae infections in rainbow trout Oncorhynchus mykiss (Walbaum). Fish Shellfish Immunol 32(5):756–761
Carter SJ (2008) Cooper and Gunn’s dispensing pharmacy, 12th edn. CBS publisher, New Delhi, pp 541–572
Chinnah AD, Baig MA, Tizard IR, Kemp MC (1992) Antigen dependent adjuvant activity of a polydispersed beta-(1,4)-linked acetylated mannan (acemannan). Vaccine 10:551–557
Chu WH (2006) Adjuvant effect of propolis on immunisation by inactivated Aeromonas hydrophila in carp Carassius auratus gibelio. Fish Shellfish Immunol 21:113–117
Costa A, Leef M, Bridle AR, Carson J, Nowak BF (2011) Effect of vaccination against yersiniosis on the relative percent survival, bactericidal and lysozyme response of Atlantic salmon, Salmo salar. Aquaculture 315:201–206
Divyagnaneswari M, Christybapita D, Michael RD (2007) Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shellfish Immunol 23:249–259
Djeraba A, Quere P (2000) In vivo macrophage activation in chickens with acemannan, a complex carbohydrate extracted from Aloe vera. Int J Immunopharmacol 22:365–372
Dotta G, de Andrade JI, Tavares Gonçalves EL, Brum A, Mattos JJ, Maraschin M, Martins ML (2014) Leukocyte phagocytosis and lysozyme activity in Nile tilapia fed supplemented diet with natural extracts of propolis and Aloe barbadensis. Fish Shellfish Immunol 39:280–284
Ebanks RO, Dacanay A, Goguen M, Pinto DM, Ross NW (2004) Differential proteomic analysis of Aeromonas salmonicida outer membrane proteins in response to low iron and in vivo growth conditions. Proteomics 4:1074–1085
Egger S, Brown GS, Kelsey LS, Yates KM, Rosenberg LJ, Talmadge JE (1996) Studies on optimal dose and administration schedule of a hematopoietic stimulatory (1,4)-linked mannan. Int J Immunopharmacol 18:113–126
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, New Jersey, pp 101–113
Evensen O, Brudeseth BE, Mutoloki S (2005) The vaccine formulation and its role in inflammatory processes in fish effects and adverse effects. Dev Biol Stand 121:117–125
Fang HM, Ge R, Sin YM (2004) Cloning, characterisation and expression of Aeromonas hydrophila major adhesin. Fish Shellfish Immunol 16:645–658
Freund J (1956) The mode of action of immunological adjuvants. Adv Tuber Res 7:130–148
Gabriel NN, Qiang J, He J, Ma XY, Kpundeh MD, Xu P (2015) Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish Immunol 44:504–514
Gjessing MC, Falk K, Weli SC, Koppang EO, Kvellestad A (2012) A sequential study of incomplete Freund’s adjuvant-induced peritonitis in Atlantic cod. Fish Shellfish Immunol 32:141–150
Green P (1996) Aloe vera extracts in equine clinical practice. Vet Times 26:16–18
Gudding R, Lillehaug A, Evensen O (1999) Recent developments in fish vaccinology. Vet Immunol Immunopathol 72:203–212
Gupta RK, Rost BE, Relyveld E, Siber GR (1995) Adjuvant properties of aluminium and calcium compounds. In: Powell MF, Newman MJ (eds) Vaccine design: the subunit and adjuvant approach. Plenum Press, New York, pp 229–248
Habeeb F, Stables G, Bradbury F, Nong S (2007) The inner gel component of Aloe vera suppresses bacterial-induced pro-inflammatory cytokines from human immune cells. Methods 42:388–393
Harikrishnan R, Balasundaram C, Heo MS (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317:1–15
Harikrishnan R, Kim JS, Balasundaram C, Heo MS (2012) Vaccination effect of liposomes entrapped whole cell bacterial vaccine on immune response and disease protection in Epinephelus bruneus against Vibrio harveyi. Aquaculture 342:69–74
Jang SI, Marsden MJ, Kim YG, Choi MS, Secombes CJ (1995) The effect of glycyrrhizin on rainbow trout, Oncorhynchus mykiss, leucocyte responses. J Fish Dis 18:307–315
Jiao XD, Cheng S, Hu YH, Sun L (2010) Comparative study of the effects of aluminum adjuvants and Freund’s incomplete adjuvant on the immune response to an Edwardsiella tarda major antigen. Vaccine 28:1832–1837
Kamilya D, Maiti T, Joardar S, Mal B (2006) Adjuvant effect of mushroom glucan and bovine lactoferrin upon Aeromonas hydrophila vaccination in catla, Catla catla (Hamilton). J Fish Dis 29:331–337
Kim KH, Hwang YJ, Bai S (1999) Resistance to Vibrio alginolyticus in juvenile rockfish (Sebastes schlegeli) fed diets containing different doses of aloe. Aquaculture 180:13–21
Kwon SR, Nam YK, Kim SK, Kim KH (2006) Protection of tilapia (Oreochromis mosambicus) from edwardsiellosis by vaccination with Edwardsiella tarda ghosts. Fish Shellfish Immunol 20(4):621–626
Lee YC (1988) Mannose-binding proteins of animal origin. In: The molecular immunology of complex carbohydrates. Springer, US, pp 105–121
Lee CK (2006) Efficacy of aloe. In: New perspectives on aloe. Springer, Berlin, pp 63–167
Lee JK, Lee MK, Yun YP, Kim Y, Kim JS (2001) Acemannan purified from Aloe vera induces phenotypic and functional maturation of immature dendritic cells. Int J Immunopharmacol 1:1275–1284
Lorenzen N, Cupit PM, Einer-Jensen K, Lorenzen E, Ahrens P, Secombes CJ (2000) Protection against a viral disease in fish following injection of plasmid DNA encoding neutralising single chain antibody. Nat Biotechnol 18:1177–1180
Magnadottir B (2006) Innate immunity of fish, overview. Fish Shellfish Immunol 20:137–151
Misra CK, Das BK, Mukherjee SC, Meher PK (2006a) The immunomodulatory effects of tuftsin on the non-specific immune system of Indian Major carp, Labeo rohita. Fish Shellfish Immunol 20:728–738
Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006b) Effect of multiple injections of beta-glucan on non-specific immune response and disease resistance in Labeo rohita fingerlings. Fish Shellfish Immunol 20(3):305–319
Nordgren RM, Stewart-Brown B, Rodeberg JH (1992) The role of acemannan as an adjuvant for Marek’s disease vaccine. In: Proceedings of the XIX worlds poultry congress, Amsterdam, The Netherlands
Ortuno J, Esteban MA, Mulero V, Meseguer J (1998) Methods for studying the haemolytic, chemoattractant and opsornic activities of seabream serum. In: Barnes AC, Davidson GA, Hiney MP, Mclntosh D (eds) Methodology in fish diseases research, vol I. Fisheries Research Services, Aberdeen, pp 97–100
Peddie S, Zou J, Secombes CJ (2002) Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet Immunol Immunopathol 86:101–113
Peng SY, Norman J, Curtin G, Corrier D, McDaniel HR, Busbee D (1991) Decreased mortality of Norman murine sarcoma in mice treated with the immunomodulator, acemannan. Mol Biother 3:79–87
Perez O, Romeu B, Cabrera O, González E, Batista-Duharte A, Labrada A, Pérez R, Reyes L, Ramírez W, Sifontes S, Fernández N, Lastre M (2013) Adjuvants are key factors for the development of future vaccines: lessons from the finlay adjuvant platform. Front Immunol 4:407
Petrovsky N, Aguilar JC (2004) Vaccine adjuvants: current state and future trends. Immunol Cell Biol 82:488–496
Raa J (1996) The use of immuno-stimulatory substances in fish and shellfish farming. Rev Fish Sci 4:229–288
Reed LV, Muench H (1938) A simple method of estimating fifty percent end points. Am J Hyg 27:493–497
Reynolds T, Dweck AC (1999) Aloe vera leaf gel: a review update. J Ethnopharmacol 68:3–37
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172:63–92
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39:223–239
Schaperclaus W, Kulow H, Schreckenbach K (1991) Hematological and serological technique. In: Kothekar VS (ed) Connaught circus, vol 1, 2nd edn. Gulab primlani, Oxonian press Pvt. Ltd, New Delhi, pp 71–108
Soltani M, Kalbassi MR (2001) Protection of Persian sturgeon (Acipenser persicus) fingerling against Aeromonas hydrophila septicemia using three different antigens. Bull Eur Assoc Fish Pathol 21(6):235
Stuart RW, Lefkowitz DL, Lincoln JA, Howard K, Gelderman MP, Lefkowitz SS (1997) Upregulation of phagocytosis and candidicidal activity of macrophages exposed to the immunostimulant, acemannan. Int J Immunopharmacol 19:75–82
Sun Y, Liu CS, Sun L (2011a) A multivalent killed whole-cell vaccine induces effective protection against Edwardsiella tarda and Vibrio anguillarum. Fish Shellfish Immunol 31:595–599
Sun Z, Wei K, Yan Z, Zhu X, Wang X (2011b) Effect of immunological enhancement of aloe polysaccharide on chickens immunized with Bordetella avium inactivated vaccine. Carbohydr Polym 86:684–690
Swain P, Dash S, Sahoo PK, Routray P, Sahoo SK, Gupta SD, Meher PK, Sarangi N (2006) Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shellfish Immunol 22:38–43
Tafalla C, Bogwald J, Dalmo RA (2013) Adjuvants and immunostimulants in fish vaccines: current knowledge and future perspectives. Fish Shellfish Immunol 35:1740–1750
Talmadge J, Chavez J, Jacobs L, Munger C, Chinnah T, Chow JT, Williamson D, Yates K (2004) Fractionation of Aloe vera L. inner gel, purification and molecular profiling of activity. Int Immunopharmacol 4:1757–1773
Wang N, Yang Z, Zang M, Liu Y, Lu C (2013) Identification of Omp38 by immunoproteomic analysis and evaluation as a potential vaccine antigen against Aeromonas hydrophila in Chinese breams. Fish Shellfish Immunol 34:74–81
Wanman CH, Tanmark N, Supamattaya K (2007) Production of killed vaccine from Streptococcus sp. and its application in sea bass (Lates calcarifer). Songklanakarin J Sci Technol 29:1251–1261
Wonbel D, Helderman H (1988) Enhancement of allo responsiveness of human lymphocytes by acemannan (carrisyn2). Int J Immunopharmacol 10:967–976
Wu N, La-Patra SE, Li J, Sunyer JO, Zhang YA (2014) Complement C5a acts as molecular adjuvant in fish by enhancing antibody response to soluble antigen. Fish Shellfish Immunol 40:616–623
Xia C, Ma ZH, Rahman MH, Wu ZG (2004) PCR cloning and identification of the β-haemolysin gene of Aeromonas hydrophila from freshwater fishes in China. Aquaculture 229:45–53
Yates KM, Rosenberg LJ, Harris CK, Bronstad DC, King GK, Biehle GA, Walker B, Ford CR, Hall JE, Tizard IR (1992) Pilot study of the effect of acemannan in cats infected with feline immunodeficiency virus. Vet Immunol Immunopathol 35:177–189
Yin G, Ardo L, Thompson K, Adams A, Jeney Z, Jeney G (2009) Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol 26:140–145
Zanuzzo FS, Urbinati EC, Nash GW, Gamperl AK (2015a) Steelhead trout Oncorhynchus mykiss metabolic rate is affected by dietary Aloe vera inclusion but not by mounting an immune response against formalin-killed Aeromonas salmonicida. J Fish Biol 87(1):43–53
Zanuzzo FS, Urbinati EC, Rise ML, Hall JR, Nash GW, Gamperl AK (2015b) Aeromonas salmonicida induced immune gene expression in Aloe vera fed steelhead trout, Oncorhynchus mykiss (Walbaum). Aquaculture 435:1–9
Acknowledgments
This work was funded by a Grant from Shahid Chamran University of Ahvaz research Council. We would like to thank Prof. Soltani (Department of Aquatic Animal Health, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran) for providing us with Aeromonas hydrophila AH-04.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdy, E., Alishahi, M., Tollabi, M. et al. Comparative effects of Aloe vera gel and Freund’s adjuvant in vaccination of common carp (Cyprinus carpio L.) against Aeromonas hydrophila . Aquacult Int 25, 727–742 (2017). https://doi.org/10.1007/s10499-016-0074-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0074-1