Abstract
This study investigated the possibility of using a finishing feeding strategy and to apply a dilution model for the calculation of the content of fatty acids (FA) in farmed brook char (Salvelinus fontinalis). Four duplicate groups of fish with an initial weight 153.3 ± 4.9 g−1 were kept in a flow-through system for 135 days, during which they more than triplicated their weight. Control groups were fed the same unmodified commercial diet with 100 % fish oil (FO) or with 60 % fish oil and 40 % rapeseed oil (RO) mixture. Two groups were fed by RO diet followed by 45 (RO:45FO) and 90 (RO:90FO) days of FO diet, respectively, at the end of the growing period. The fillet FA composition at the end of the experiment corresponded with the FA composition of the lipid source in the diet for the tested groups. A significant (p < 0.05) impact on FA composition with a decreasing tendency in the representation of n-3 HUFA with a prolonged feeding period with the RO diet was observed. The application of a dilution model enabling the prediction of the content of a given fatty acid in a given time was successfully performed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal husbandry, whether on land or in water, depends on the availability of sources for feed. Two key sources of feed components in aquaculture are fish meal (FM) and fish oil (FO). Both have their origin in traditional ocean capture fishery (De Silva et al. 2011). During the last 25 years, aquaculture has been the fastest growing animal husbandry sector with an annual increase of on average 8.5 % (FAO 2012). The demands for FM and FO are constantly rising, especially in carnivorous fish culture globally. Nowadays, more than 50 % of the total production of FO is used for feed production for salmonids while the amount of produced salmonids reaches only 4 % of the total aquaculture production (FAO 2012). At the same time, there is an increasing problem with overfishing (Tacon and Metian 2009; Tacon et al. 2011), and therefore, there is a growing pressure on replacing FM and FO more sustainable plant components (Turchini et al. 2009) or other sources (Berge et al. 2013).
However, FO is well known for a high content of nutritionally beneficial n-3 highly unsaturated fatty acids (HUFA; ≥20 carbon atoms and ≥3 double bonds), including eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid (Pike and Jackson 2010; Mráz et al. 2012a). Concerning human nutrition, it has been shown that n-3 HUFA consumption has an anti-inflammatory effect, maintains cell’s homoeostasis and has a protective function in the prevention of cardiovascular diseases (Adamkova et al. 2011; Simopoulos 2002).
Vegetable oils, on the other hand, do not contain HUFA, but polyunsaturated fatty acids (PUFA) with shorter chains and monounsaturated (MUFA) (Pickova and Mørkøre 2007). In addition, most vegetable oils are rich in n-6 PUFA and low in n-3 PUFA being considered as adipogenic, atherogenic and causing inflammatory disorders (Simopoulos 2002). Subsequently, the use of, for example, rape seed oil will significantly affect the nutritional quality of farmed fish resulting in a decreased intake of n-3 HUFA.
Both the European Food Safety Authority (EFSA) and the World Health Organization (WHO) recommend the consumption of at least two portions of fatty fish per week to maintain good health. However, this recommendation is based on fish with a “normal” fatty acid (FA) composition, which is rich in n-3 HUFA, especially EPA and DHA. The recommendation for the daily intake of the individual FA is: 2 g α-linolenic acid (ALA), 10 g linoleic acid (LA) (n-3:n-6 ratio 1:5) and at least 250 mg EPA + DHA for the normal healthy population (WHO/FAO 2003; EFSA Panel on Dietetic Products 2010).
One of the possible ways to deal with the decrease of n-3 HUFA in fish, farmed with vegetable oil, is to use a finishing feeding strategy. This is based on the use of a diet where a substantial part of FO is replaced by a cheaper and sustainable vegetable oil during the first rearing period and for the final period (several weeks) before slaughtering, a diet with 100 % FO is used to increase the final proportion of n-3 HUFA (Rasmussen et al. 2000; Bell et al. 2003; Regost et al. 2003; Torstensen et al. 2004; Bell et al. 2005; Lane et al. 2006; Mráz et al. 2012b; Thanuthong et al. 2012). For this approach, Robin et al. (2003) developed a mathematical dilution model which can predict the changes in fillet FA composition during the finishing feeding period. This model allows to predict and tailor lipid quality, as verified for other species, for example by Jobling (2004) (Atlantic salmon), Torstensen et al. (2005) (Atlantic salmon) or Mráz et al. (2012b) (common carp, Cyprinus carpio). Simultaneously, it has been shown that there is no negative influence of a substantial FO replacement by vegetable oil on fish growth, health status or mortality of many farmed fish as reviewed by Turchini et al. (2009).
The brook char (Salvelinus fontinalis) is a North American salmonid species introduced to Europe in the nineteenth century. Its popularity among Czech fish farmers is currently increasing (Svinger et al. 2013; Kopp et al. 2014) due to a high muscle quality and tolerance to lower water pH values. It is suitable for flow-through as well as recirculating aquaculture systems. Earlier studies confirmed that brook char has the potential to become as good aquaculture species as rainbow trout (Rasmussen and Ostenfeld 2000; Amin et al. 2014). Therefore, this species was chosen to test and evaluate the use of a finishing feeding strategy in the present study.
The primary aim of this experiment was to investigate how the replacement of 40 % FO in the feed followed by subsequent finishing feeding periods of different lengths influences growth, survival and fillet FA composition of brook char. A secondary aim was to evaluate the applicability of the dilution model developed by Robin et al. (2003) to predict FA composition after finishing feeding period.
Materials and methods
Diets, fish and experimental design
The finishing feeding experiment was carried out between May and September (135 days) at the fish farming facility Annín (Klatovské rybářství a.s., Czech Republic). A total of 2400 brook char juveniles (average weight 156.3 ± 4.9 g) were randomly distributed as four duplicated groups into eight concrete channels with flow-through water supply (1 × 1 × 8 m), 300 individuals per channel.
As base for the experimental feed, a commercially available complete feeding mixture with a 60 % reduced fat content (compared to the original recipe) was used. To this feed, we manually added either fish oil (FO) or rapeseed oil (RO) until saturation by spraying. The final fat content in both diets was 26 % (initial 15.5 %) (Table 1). The experiment was designed as follows: two concrete flow-through tanks served as a control group, the fish were fed with the FO diet for the whole experiment (FO group), two fish groups were fed for 45 days with the RO diet and for the rest 90 days with the FO diet (RO:90 FO group), two fish groups were fed for 90 days with the RO diet with subsequent change into the FO diet for 45 days (RO:45 FO group). The last two fish groups (second control) were fed with the RO diet for the whole 135 days (RO group). The feeding ratio was, depending on the actual water temperature, maintained between 0.8 and 1.5 % of actual biomass in the channel. Water inflow was ensured from the River Otava (49°10′29.6″N 13°30′49.3″E) and the temperature was (14.3 ± 2.8 °C) measured continually using stationary data logger (Minikin, EMS, Brno, Czech Republic). Oxygen saturation (8.9 ± 1.2 mg l−1) and the pH value (6.4 ± 0.4) were measured regularly once a day. All the other water quality indicators such as ammonia, nitrite and nitrate were controlled once a week and their values did not reach risk levels.
Sampling
Fish were sampled at the beginning of the experiment (day 0), at times of diet changes (days 45 and 90) and at the end of the experiment (day 135). At each sampling day, 33 individuals per channel were caught, and fish were weighed and measured for the purpose of modification of feeding dose and to calculate specific growth rate (SGR) and feed conversion ratio (FCR) as follows:
where W t = final weight of fish; W 0 = initial weight of fish; F = amount of feed fed; t = time (days).
At each sampling day, 10 randomly chosen fish were killed and filleted. The left fillet with skin was use for laboratory analyses of lipid content and composition. Samples were packed, marked, frozen in liquid nitrogen and stored at −80 °C until analysis.
Lipid analysis
Samples were thawed and the whole fillet with skin was cut into approximately 2 cm large pieces and homogenized in a kitchen-sized food processor. Lipid extraction was carried out with hexane–isopropanol (3:2, v:v) following the method of Hara and Radin (1978) with slight modifications described in Zajic et al. (2013). Lipids were methylated with the use of borontrifluorid–methanol complex (BF3) as described in Appelqvist (1968) and the obtained FA methyl esters were analysed with a gas chromatograph (Trace Ultra FID, Thermo Scientific, USA) equipped with a flame ionization detector and PVT injector, using a BPX 70 silica capillary column (SGE, Austin, TX, USA), length 50 m, id 0.22 mm and film thickness 0.25 µm (Fredriksson Eriksson and Pickova 2007). Helium was used as carrier gas at a flow rate of 1.2 ml per min and nitrogen was used as make-up gas. The peaks were identified by comparison with the standard mixture GLC-68D (Nu-Check Prep, Elysian, MO, USA) and other authentic standards. For calculation of the absolute amount of individual FA, an internal standard (21:0) (Nu-check Prep, Elysian, USA) was used.
Dilution model
The obtained results of fillet lipid composition were later compared against predicted values calculated by the dilution model designed by Robin et al. (2003):
where Pi T is the predicted percentage of taken FA at time T; Pi F is the percentage of taken FA at time T in the fillet of control fish (from FO group); Pi 0 is the percentage of taken FA in the lipid of treated fish at the beginning of the finishing feeding period; Q T is the value of fish weight (kg) multiplied by the lipid content (%) in the fillet of treated fish at time T; Q 0 is the value of fish weight (kg) multiplied by the lipid content (%) in the fillet of treated fish at the beginning of the finishing feeding period.
Statistical analysis
Results were processed in STATISTICA CZ, version 12.0 software package. All data are presented as mean ± standard deviation. Differences among fillet FA compositions were determined using one-way ANOVA by analysis of variance and Tukey’s HSD test.
A regression analysis was performed to evaluate the differences within each experimental group during the experiment. Differences with a p level <0.05 were considered statistically significant.
Results
Table 2 shows the production data of the experiment. There was a normal mortality during the experiment which did not exceed 4 % in all channels. The growth of the fish did not differ between the groups, indicating that the 40 % substitution of fish oil by rapeseed oil did not affect the growth of the brook char. Similarly, no influence of dietary oil on weight variability was shown. There are a slightly lower value of SGR and marginally increased FCR in FO group compared to the others, but these differences are not statistically substantiated. On average, all fish more than tripled their weight over the duration of the experiment.
The fillet lipid content at the beginning of the experiment was 5.25 ± 1.23 %. At the time of the first diet change (t − 45) it increased in all groups, however there was significantly lower value found in RO:45FO and non-significantly in RO:90FO and RO (Fig. 1) compared to the FO control group.
There were no differences between the groups at the time of the second diet change (t − 90) as well as at the end of the experiment (t − 135). However, there was a slightly lower fat content found in the finishing feeding groups (9.92 ± 1.64 % in the RO:45FO group and 9.74 ± 1.79 % in the RO:90FO group, respectively) compared to the control groups (10.3 ± 2.66 % in the FO group and 10.35 ± 1.70 % in the RO group, respectively) at the end of the trial.
The fillet FA composition at the end of the experiment clearly corresponds with the FA composition of the lipid source in the diet for the tested groups. Data are presented in Table 3. There is significantly decreasing amount of total SFA in the groups with longer rapeseed oil feeding period (RO:45FO and RO, respectively). A clear effect of the presence of rapeseed oil is also seen in the total MUFA content, resulting in a higher amount of MUFA in the fish lipids with a longer RO feeding period. On the other hand, while the total PUFA content was stable in all experimental groups, there is a significant difference in the proportion of n-3 and n-6 PUFA between tested groups. With prolonged time the content of n-3 PUFA decreased in the RO group while the content of n-6 PUFA conclusively increased at the same time. Similar results have been achieved also by determination of FA as absolute amounts (mg 100 g−1 flesh). Practically all the FA identified in the brook char fillet increased their amounts during the experiment together with a rising fat content. There was a significant (p < 0.01) increase of all identified n-3 HUFA (20:4n-3, 20:5n-3, 22:5n-3, 22:6n-3) with prolonged time of feeding the fish oil-based diet. Simultaneously, representation of MUFA (specifically oleic acid; 18:1n-9) increased substantially in the RO and RO:45FO groups (p < 0.01), while it remained stable in FO and RO:90FO. Consistent in experimental groups (RO:45FO and RO:90FO) are the amounts of ALA.
The applicability of the dilution model for the prediction of a given FA in the fillet of brook char is presented in Table 4. It is evident that with a shorter finishing feeding period the accuracy of estimation is rising. There was high prediction accuracy in both tested periods (45 and 90 days) for the most of chosen FA. However, the analysed representation of ALA, EPA and DHA and n-3 HUFA, respectively, after the 45 days finishing feeding showed more accurate predictions compared to the same FA after the 90 days finishing feeding period. But, simultaneously, the coefficient of determination (R2) is very close to 1 for both 45 and 90 days of finishing feeding (R 2 = 0.9991 for 45 days finishing feeding; R 2 = 0.9978 for 90 days, respectively). It was also shown, that in case of brook char a very accurate prediction of the filet FA composition can be done based on the FA composition of the finishing diet. Figure 2 demonstrates the accuracy of this prediction for both 45 (Fig. 2a) and 90 (Fig. 2b) days of finishing feeding. The prediction accuracy increases with shortening the finishing feeding period.
Predicted values of chosen fatty acid (%) of brook char (Salvelinus fontinalis) compared to observed. The fatty acid composition of the finishing diet was used as the reference value. a prediction for the group with 45 days of finishing feeding; b prediction for the group with 90 days of finishing feeding. Each value represents the mean for 8 fish
Discussion
This experiment aimed to show whether the replacement of 40 % fish oil in the feed for brook char followed by a finishing feeding strategy has an influence on fillet FA composition. So far this strategy was tested in many economically important fish species, such as rainbow trout (Thanuthong et al. 2012), rohu (Labeo rohita) (Karanth et al. 2009), European seabass (Dicentrarchus labrax) (Mourente and Bell 2006), Atlantic salmon (Torstensen et al. 2005) or common carp (Mráz et al. 2012b), but in brook char farming only studies on partial replacement of dietary fish oil were published (Guillou et al. 1995; Simmons et al. 2011).
At the end of the experiment, fish from all groups showed nearly the same final weight but with high variability, which is suggested to be caused by territoriality and feeding aggression of adolescent brook char males (Svinger et al. 2013). Nevertheless, this variability was similar in all groups and the final average weight was not influenced (p > 0.05) by the partial replacement of fish oil. No differences between the groups were observed in survival (96.1–96.5 %). Despite the manual intervention (manipulation with lipid part) of the commercial diet, the FCR was between 0.97 and 1.01 and the SGR ranged between 0.92 and 0.99 % per day with no differences between the experimental groups. These values are in agreement with results published by Amin et al. (2014). Rasmussen and Ostenfeld (2000) showed a lower (0.81) FCR with a comparable SGR (1.00). Our results concerning growth showed a relatively high variation within the groups (approximately 20 %). However, as this variation was similar in all groups, the results of the present study indicate that the replacement of a substantial part (40 %) of fish oil by rapeseed oil in the diet has no significant influence on growth parameters, feed utilization or survival of brook char.
Fillet lipid content of experimental fish rose linearly in time depending on the growth of fish, which is a common trend (Hamilton et al. 2005; Şahin et al. 2011). After the first 45 days, a significantly lower lipid content was observed in the group fed the diet with rapeseed oil (RO:45FO; p < 0.05) with a trend of lower lipid content also in the RO:90FO and RO groups (p = 0.125) compared to the FO group fed the same diet as prior to the experiment. With time, however, these initial differences disappeared. Most likely this was due to the sudden change to a new feed. A similar situation was described in a study on common carp by Mráz et al. (2012b) who recommended to mix the feeds in order to adapt fish stock more easily to the new feed composition and probably the taste. At the end of the experiment (after 135 days), all lipid content values in all tested groups were similar. Thus, the replacement of 40 % fish oil by rapeseed oil in the feed as well as an application of the finishing feeding strategy seems to have no impact on the final fillet lipid content of brook char in the current settings.
It has been shown earlier that fillet FA composition of salmonid species is significantly influenced by the composition of dietary fat (Rasmussen and Ostenfeld 2000; Turchini et al. 2003). While there was a clearly higher content of n-3 HUFA in the FO group, the same FA decreased with time in the groups fed the diet with RO at the expense of elevated levels of both LA and ALA and MUFA. This is due to a high proportion of these FA in rapeseed oil (Pickova and Mørkøre 2007). Rapeseed oil has been previously tested as potentially suitable substitute for fish oil in similar species—Arctic char (Salvelinus alpinus) by Pettersson et al. (2009) or in Atlantic salmon by Bell et al. (2003). Due to a reduction in the intake of fish oil, it was possible to observe a trend of significant (in RO:45FO and RO groups) decreasing percentage of nutritionally less suitable SFA (Hunter et al. 2010) in the fillet lipids. Considering the dietary recommendations for daily intake of FA (EFSA Panel on Dietetic Products 2010), the contents of n-3 HUFA (including EPA + DHA) in the fillet of experimental fish at the end of the trial were still high and above the minimal recommended intake. Furthermore, the absolute amount of n-3 HUFA continually rose in time (908 ± 95, 1180 ± 222 and 1278 ± 180 mg 100 g−1 flesh after 45, 90 and 135 days, respectively) due to the increasing lipid content. This finding provides a space for even higher fish oil replacement in brook char farming (Regost et al. 2003).
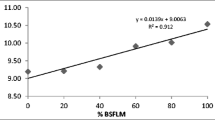
The principle of the dilution model (Jobling 2003) confirms that if the FA composition of the feed is changed, then the fillet FA composition is changed as well in time depending on the amount of newly included FA. The application has two possible ways: (1) the prediction of an amount of given FA in a given time and (2) the calculation of time required to achieve a given amount of the chosen FA. In Table 4, the high accuracy of the dilution model in the case of brook char for the chosen FA as well as for the most important n-3 FA is demonstrated. This accuracy is high enough in order to guarantee the content of given FA to consumers; nevertheless, the calculation is applicable to aquaculture as has been confirmed by several authors with similar accuracy in other species (Jobling 2004; Torstensen et al. 2005; Mráz et al. 2012b). In addition, the possibility to use the FA composition of the finishing diet as a reference value to predict the FA composition in the fillet of tested fish at the end of finishing feeding period was tested. As presented in Fig. 2, this option can be used with a high degree of reliability. Non-carnivorous fish more easily digest dietary starch and produce SFA and MUFA from its excess, so the predicted values of these FA groups are lower compared to the observed. On the other hand, the predicted values of PUFA are then higher than observed, because PUFA are diluted. It can therefore be concluded that salmonids reflect the composition of a diet into the fillet. The practical use of the results obtained in a dilution model to calculate necessary feeding periods for the production of fish with a tailored FA composition according to the consumers’ demands could be interesting for both farmers and consumers. Hypothetically, a consumer demands a guarantee that the fillets of market brook char contain at least 1.75 g 100 g−1 n-3 HUFA (250 mg−1 × 7 days). The regression equation for the relationship between the content of n-3 HUFA and time is: y = 0.0047x + 1.2541 (Fig. 3); after the calculation (y = 1.75), the farmer knows that to achieve the desired amount of n-3 HUFA, he needs to apply 105 days of finishing feeding with the FO diet after feeding a 40 % RO diet for a growing period of 30 days. If the previously used diet has lower proportions of n-3 HUFA, the model needs to be re-evaluated for the different starting FA composition.
It can be summarized that the dilution model is applicable in the brook char farming where 40 % of fish oil in the diet is replaced by rapeseed oil. By the application of the finishing feeding strategy, a desired declarable flesh quality could be ensured. In time of limited traditional resources for aquaculture feeds, a finishing feeding strategy could have a positive impact from many points of view. A 40 % fish oil replacement showed that the final lipid flesh quality is, regarding the widely recognized nutritional requirements, still very high. Thus, even higher fish oil replacement should be tested in brook char farming in future.
Abbreviations
- ALA:
-
Alpha linolenic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acids
- FCR:
-
Feed conversion ratio
- FM:
-
Fish meal
- FO:
-
Fish oil
- n-3 HUFA:
-
Highly unsaturated fatty acids (20:4n-3, 20:5n-3, 22:5n-3, 22:6n-3)
- LA:
-
Linolenic acid
- MUFA:
-
Monounsaturated fatty acids (16:1, 18:1n-9, 18:1n-7, 20:1n-9)
- OA:
-
Oleic acid
- PUFA:
-
Polyunsaturated fatty acids (18:2n-6, 18:3n-3, 20:2n-6, 20:3n-6, 20:4n-6, 20:4n-3, 20:5n-3, 22:5n-3, 22:6n-3)
- RO:
-
Rapeseed oil
- SFA:
-
Saturated fatty acids (14:0, 16:0, 18:0, 20:0, 22:0)
- SGR:
-
Specific growth rate
References
Adamkova V, Kacer P, Mraz J, Suchanek P, Pickova J, Kralova-Lesna I, Skibova J, Kozak P, Maratka V (2011) The consumption of the carp meat and plasma lipids in secondary prevention in the heart ischemic disease patients. Neuro Endocrinol Lett 32:17–20
Amin MN, Carter CG, Katersky Barnes RS, Adams LR (2014) Protein and energy nutrition of brook trout (Salvelinus fontinalis) at optimal and elevated temperatures. Aquac Nutr. doi:10.1111/anu.12274
Appelqvist LA (1968) Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing accumulation of lipid contaminants. Arkiv För Kemi 28:551–570
Bell J, Tocher D, Henderson R, Dick J, Crampton V (2003) Altered fatty acid compositions in Atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. J Nutr 133:2793–2801
Bell JG, Henderson RJ, Tocher DR, Sargent JR (2005) Dioxin and dioxin-like polychlorinated biphenyls (PCBs) in Scottish farmed salmon (Salmo salar): effects of replacement of dietary marine fish oil with vegetable oils. Aquaculture 243:305–314
Berge GM, Hatlen B, Odom JM, Ruyter B (2013) Physical treatment of high EPA Yarrowia lipolytica biomass increases the availability of n-3 highly unsaturated fatty acids when fed to Atlantic salmon. Aquac Nutr 19:110–121
De Silva SS, Francis DS, Tacon AGJ (2011) Fish oil in aquaculture in retrospect. In: Turchini GM et al (eds) Fish oil replacement and alternative lipid sources in aquaculture feeds. Taylor and Francis, Boca Raton, pp 1–20
EFSA Panel on Dietetic Products, N.a.A.N (2010) Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J 8:1461–1568
FAO (2012) The state of world fisheries and aquaculture. Italy, Rome
Fredriksson Eriksson S, Pickova J (2007) Fatty acids and tocopherol levels in M. Longissimus dorsi of beef cattle in Sweden—a comparison between seasonal diets. Meat Sci 76:746–754
Guillou A, Soucy P, Khalil M, Adambounou L (1995) Effects of dietary vegetable and marine lipid on growth, muscle fatty acid composition and organoleptic quality of flesh of brook charr (Salvelinus fontinalis). Aquaculture 136:351–362
Hamilton MC, Hites RA, Schwager SJ, Foran JA, Knuth BA, Carpenter DO (2005) Lipid composition and contaminants in farmed and wild salmon. Environ Sci Technol 39:8622–8629
Hara A, Radin NS (1978) Lipid extraction of tissues with a low toxicity solvent. Anal Biochem 90:420–426
Hunter JE, Zhang J, Kris-Etherton PM (2010) Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr 91:46–63
Jobling M (2003) Do changes in Atlantic salmon, Salmo salar L., fillet fatty acids following a dietary switch represent wash-out or dilution? Test of a dilution model and its application. Aquac Res 34:1215–1221
Jobling M (2004) ‘Finishing’ feeds for carnivorous fish and the fatty acid dilution model. Aquacult Res 35:706–709
Karanth S, Sharma P, Pal AK, Venkateshwarlu G (2009) Effect of different vegetable oils on growth and fatty acid profile of rohu (Labeo rohita, Hamilton); evaluation of a return fish oil diet to restore human cardio-protective fatty acids. Asian-Australas J Anim Sci 22:565–575
Kopp R, Lang Š, Brabec T, Mareš J (2014) The influence of physicochemical properties of water on plasma indices in brook trout (Salvelinus fontinalis, Mitchill) reared under conditions of intensive aquaculture. Acta Vet Brno 82:427–433
Lane RL, Trushenski JT, Kohler CC (2006) Modification of fillet composition and evidence of differential fatty acid turnover in sunshine bass Morone chtysops × M-saxatilis following change in dietary lipid source. Lipids 41(11):1029–1038
Mourente G, Bell JG (2006) Partial replacement of dietary fish oil with blends of vegetable oils (rapeseed, linseed and palm oils) in diets for European sea bass (Dicentrarchus labrax L.) over a long term growth study: effects on muscle and liver fatty acid composition and effectiveness of a fish oil finishing diet. Comp Biochem Phys B 145:389–399
Mráz J, Máchová J, Kozák P, Pickova J (2012a) Lipid content and composition in common carp—optimization of n-3 fatty acids in different pond production systems. J Appl Ichthyol 28:238–244
Mráz J, Zajíc T, Pickova J (2012b) Culture of common carp (Cyprinus carpio) with defined flesh quality for prevention of cardiovascular diseases using finishing feeding strategy. Neuro Endocrinol Lett 33(Suppl 2):60–67
Pettersson A, Pickova J, Brännäs E (2009) Effects of crude rapeseed oil on lipid composition in Arctic charr Salvelinus alpinus. J Fish Biol 75:1446–1458
Pickova J, Mørkøre T (2007) Alternate oils in fish feeds. Eur J Lipid Sci Technol 109:256–263
Pike IH, Jackson A (2010) Fish oil: production and use now and in the future. Lipid Technol 22:59–61
Rasmussen RS, Ostenfeld TH (2000) Effect of growth rate on quality traits and feed utilisation of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Aquaculture 184:327–337
Rasmussen RS, Ostenfeld TH, Rønsholdt B, McLean E (2000) Manipulation of end-product quality of rainbow trout with finishing diets. Aquac Nutr 6:17–23
Regost C, Arzel J, Robin J, Rosenlund G, Kaushik SJ (2003) Total replacement of fish oil by soybean or linseed oil with a return to fish oil in turbot (Psetta maxima): 1. Growth performance, flesh fatty acid profile, and lipid metabolism. Aquaculture 217:465–482
Robin JH, Regost C, Arzel J, Kaushik SJ (2003) Fatty acid profile of fish following a change in dietary fatty acid source: model of fatty acid composition with a dilution hypothesis. Aquaculture 225:283–293
Şahin SA, Başçinar N, Kocabaş M, Tufan B, Köse S, Okumuş I (2011) Evaluation of meat yield, proximate composition and fatty acid profile of cultured brook trout (Salvelinus fontinalis Mitchill, 1814) and black sea trout (Salmo trutta labrax Pallas, 1811) in comparison with their hybrid. Turk J Fish Aquat Sci 11:261–271
Simmons CA, Turk P, Beamer S, Jaczynski J, Semmens K, Matak KE (2011) The effect of a flaxseed oil-enhanced diet on the product quality of farmed brook trout (Salvelinus fontinalis) fillets. J Food Sci 76:S192–S197
Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21:495–505
Svinger V, Policar T, Steinbach C, Polakova S, Jankovych A, Kouril J (2013) Synchronization of ovulation in brook char (Salvelinus fontinalis, Mitchill 1814) using emulsified d-Arg6Pro9NEt sGnRHa. Aquac Int 21:783–799
Tacon AGJ, Metian M (2009) Fishing for aquaculture: non-food use of small pelagic forage fish—a global perspective. Rev Fish Sci 17:305–317
Tacon AGJ, Hasan MR, Metian M (2011) Demand and supply of feed ingredients for farmed fish and crustaceans: trends and prospects. FAO, Rome, p 87
Thanuthong T, Francis DS, Senadheera SPSD, Jones PL, Turchini GM (2012) Short-term food deprivation before a fish oil finishing strategy improves the deposition of n-3 LC-PUFA, but not the washing-out of C18 PUFA in rainbow trout. Aquac Nutr 18:441–456
Torstensen BE, Frøyland L, Lie Ø (2004) Replacing dietary fish oil with increasing levels of rapeseed oil and olive oil—effects on Atlantic salmon (Salmo salar L.) tissue and lipoprotein lipid composition and lipogenic enzyme activities. Aquac Nutr 10:175–192
Torstensen B, Bell J, Rosenlund G, Henderson R, Graff I, Tocher D, Lie O, Sargent J (2005) Tailoring of Atlantic salmon (Salmo salar L.) flesh lipid composition and sensory quality by replacing fish oil with a vegetable oil blend. J Agric Food Chem 53:10166–10178
Turchini GM, Mentasti T, Frøyland L, Orban E, Caprino F, Moretti VM, Valfré F (2003) Effects of alternative dietary lipid sources on performance, tissue chemical composition, mitochondrial fatty acid oxidation capabilities and sensory characteristics in brown trout (Salmo trutta L.). Aquaculture 225:251–267
Turchini GM, Torstensen BE, Ng W-K (2009) Fish oil replacement in finfish nutrition. Rev Aquac 1:10–57
WHO/FAO (2003) Diet, nutrition and the prevention of chronic diseases. WHO Tech Rep Ser 916:1–150
Zajic T, Mraz J, Sampels S, Pickova J (2013) Fillet quality changes as a result of purging of common carp (Cyprinus carpio L.) with special regard to weight loss and lipid profile. Aquaculture 400:111–119
Acknowledgments
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic—projects “CENAKVA” (No. CZ.1.05/2.1.00/01.0024), “CENAKVA II” (No. LO1205 under the NPU I program).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Zuzana Linhartová and Jan Mráz/Carp pond aquaculture, product processing and quality.
Rights and permissions
About this article
Cite this article
Zajic, T., Mraz, J., Sampels, S. et al. Finishing feeding strategy as an instrument for modification of fatty acid composition of brook char (Salvelinus fontinalis). Aquacult Int 24, 1641–1656 (2016). https://doi.org/10.1007/s10499-016-0067-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0067-0