Abstract
The aim of the present study was to investigate the effect of photoperiod regime combined with thermal treatment (PTT) on maturation of three-year-old pikeperch (Sander lucioperca). To induce the onset of maturation, all treatment groups underwent a wintering phase of 3 months at 12 °C and 12-h light-to-12-h dark. Controls were maintained at 23 °C and 12-h light-to-12-h dark throughout the trial. After the wintering phase, the PTT groups experienced regimes with 8-, 10-, 12-, and 14-h light for 4 months at 14 °C referred to as photo-thermal treatment (PTT). The four PTT groups as well as the control were studied in triplicates, with 12 fish per tank. We assessed gonad development and maturation by histological analysis, sex steroid plasma concentrations (17β-estradiol, testosterone, and 11-ketotestosterone) and, at the end of the experiment, mRNA expression of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the pituitary. As expected, both male and female pikeperch initiated reproductive maturation during the wintering phase, which was confirmed through histological analysis and sex steroid measurements. During wintering, the onset of maturation in females was confirmed by the increasing diameter of ovarian follicles (217 ± 65 to 800 ± 97 µm), the developmental stages (50 % mid and 21 % late vitellogenic females), concomitant with elevated 17β-estradiol (E2) plasma concentrations reaching 2600 ± 1500 pg/mL, compared to 380 ± 230 pg/mL at the onset of the experiment. Similarly in males, maturation was indicated by peak concentrations of the androgens observed within the first 2 months of wintering (testosterone 21 ± 12 ng/mL first month, 11-ketotestosterone 5.7 ± 3 ng/mL second month). Subsequently, the photoperiod treatments after the wintering phase influenced the progression of reproductive maturation. During the PTT, follicle diameter increased irrespective of the light regime from 800 to more than 1100 µm, but the progression of the vitellogenesis was differentially modulated by the photoperiod. Already after 1 month of the PTT, 92 and 86 % of females reared at 12- and 14-h light per day were in a late vitellogenic stage. After 2 months, females with mainly atretic follicles were observed under long light conditions (14 h of light), indicating overripeness and spawning. In contrast, 82 and 72 % of the females reared at 8- and 10-h light per day were still in the final stage of vitellogenesis at the end of the experiment and thus ready to undergo final maturation. Concomitant to the histological outcomes, highest E2 concentrations were observed under long-day conditions (12, 14 h) in females with peaks of 4200 ± 3300 (12 h of light) and 6800 ± 4200 pg/mL (14 h of light). In male pikeperch, ongoing spermatogenesis was indicated by rising androgen levels especially under long-day conditions (14 h of light) reaching peak levels of 27 ± 21 ng/mL testosterone and 39 ± 91 ng/mL of 11-ketotestosterone at the end of the experiment. In all PTT, the mRNA expression of FSH and LH was significantly elevated compared to the control, confirming the activation of the hypothalamus–pituitary–gonad axis. Here, no effect between the different light regimes was detected, neither in males nor females. Thus, photoperiod revealed a slight influence on male and female pikeperch maturation when combined with an effective thermal treatment and therefore can be explored as a cheap and easy-to-handle fine-tuning tool for artificial pikeperch reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, aquaculture is the fastest growing sector in agriculture, but, to date, Europe (with few exceptions such as Norwegian salmon aquaculture) does not participate in this development. In the last decades, increasing attention has been given to the evaluation and introduction of new species for European aquaculture production. Here, candidate species are highly valuable to the market, but often facing problems with regard to the aquaculture technology, for example Burbot (Lota lota) (Barron et al. 2012; Wocher et al. 2012; Palinska-Zarska et al. 2014, 2015), Eurasian perch (Perca fluviatilis) (Abdulfatah et al. 2013; Pimakhin et al. 2015), and also pikeperch (Sander lucioperca) (Zakes 2007; Mueller-Belecke and Zienert 2008; Szczepkowski et al. 2011; Zarski et al. 2012, 2013).
In Europe, aquaculture production is mainly based on open systems such as ponds, net cages, or raceways, but due to limited resources (water, sites) required for open systems (ponds, net cages), the expansion of production in closed recirculation aquaculture systems (RAS) is envisioned for the future. These land-based systems allow a specific control over the rearing environment, but are challenged by the economic feasibility. Here, control of the production cycle is a key issue which mainly relies on the control and management of reproduction allowing the stocking independently of the season. Consequently, it is essential to identify the species-specific cues triggering reproduction. In temperate species, the reproductive cycle is usually synchronized by seasonal triggers, predominantly light and temperature (Taranger et al. 2010; Wang et al. 2010). The role of these external factors may be species-specific, and reproductive management may involve different triggers at different periods of the reproductive cycle or even a combination of different environmental cues.
In salmonids for instance, photoperiodic change is considered to be the main factor controlling the synchronization of the reproductive cycle (Bromage et al. 2001; Migaud et al. 2010). In temperate species, photoperiod has been regarded as the principal environmental determinant of reproduction, and temperature has often been considered as a secondary cue (Bromage et al. 2001; Pankhurst and Porter 2003). Yet, most of the information on the role of temperature is derived from cyprinids where rising temperatures in spring initiate gonad maturation (Stacey 1984; Pankhurst and Porter 2003).
In freshwater percids, both temperature and photoperiod factors play a key role in the regulation of the reproductive cycle (Taranger et al. 2010; Wang et al. 2010). For example, gonad maturation in Eurasian perch (P. fluviatilis) is inhibited by a continuous photophase, and optimal maturation is only achieved under a natural photoperiod (Migaud et al. 2006). At a constant photoperiod (16-h light-to-8-h dark), maturation was heterogeneous, suggesting that a decreasing photoperiod is the environmental trigger for the initiation of gonad maturation. These experimental fish were kept at ambient temperatures (15–23 °C), thereby suggesting that a distinct wintering as previously described by Migaud et al. (2002, 2004a, b) was not required for the initiation of puberty (in females the transition from previtellogenesis to vitellogenesis as discussed in Okuzawa 2002; Wuertz et al. 2007). Thus, in Eurasian perch (P. fluviatilis), natural photoperiod seems to play a key role in out-of-season reproduction, and decreasing day lengths are sufficient to induce puberty.
Nevertheless, our previous investigations found temperature to be the crucial factor either to induce puberty or to arrest gonad development in the pikeperch (Hermelink et al. 2011, 2013). Subsequently, temperature also synchronizes gonadal development in pikeperch of both sexes (Hermelink et al. 2013). As a result of these studies, we presented an optimized protocol: a wintering period at 12 °C for 3 months, followed by a slight temperature increase to 14 °C for a further 4 months to optimally support the progression of gametogenesis. Divergence of temperatures from this optimized protocol (e.g., lower temperature for wintering or higher temperatures for maturation) resulted in suboptimal maturation and, at 23 °C, resulted in a complete stagnation of gametogenesis. Both experiments were carried out under a fixed photoperiod of 12-h light and 12-h dark. However, despite the technical ease of installation, low cost, and its potential for reproductive management, the influence of the photoperiod has yet to be investigated in pikeperch.
Therefore, in the present study, the effect of the photoperiod was evaluated experimentally. Maturation was induced and supported by the optimal thermal protocol (Hermelink et al. 2011, 2013) in four experimental groups reared at day lengths of 8-h light and 16-h dark (08:16), 10-h light and 14-h dark (10:14), 12-h light and 12-h dark (12:12), and 14-h light and 10-h dark (14:10) after wintering at 12:12 in one recirculating aquaculture system (RAS). We characterized the maturational process by assessing gonadotropin gene expression in the pituitary, sex steroids in the plasma and developmental stages of the ovary.
Materials and methods
Animal rearing and experimental setup
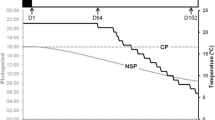
Pikeperch (aged 10–11 months) were obtained from a commercial producer (Fischerei Mueritz-Plau GmbH, Waren, Germany). Before onset of the experiment, these fish were kept at the facilities of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries for over 2 years under controlled conditions (daily artificial illumination of 12:12 and a constant water temperature of 23 °C). Prior to the experiment, 12 randomly chosen pikeperch were stocked to each of fifteen 500-L tanks of the same RAS, thus providing four photo-thermal treatment (PTT) groups and one control group (23 °C, 12:12), assessed in triplicate (Fig. 1).
Experimental setup: The effect of four different photoperiods (photo-thermal treatment at 14 °C and 08:16, 10:14, 12:12, or 14:10) on the progression of gonad maturation was studied in three-year-old pikeperch after gonad maturation was induced by wintering at 12 °C for 3 months. A fifth group was kept as a control at 23 °C and a photoperiod of 12:12
Females had an average size of 52 ± 6 cm and a mean weight of 1300 ± 440 g, and males were slightly bigger with an average size of 54 ± 7 cm and a mean weight of 1510 ± 590 g (sex of fish was assessed by histological analysis). To avoid the influence of artificial light emission from surrounding rearing units, all tanks were covered with light-tight material and illuminated artificially (Aqua medic aqualine T5 Reef White 10.000 K) for the duration of the experiment.
On commencing the experiment (day zero—2011.12.01), an optimized thermal protocol was applied to all four experimental groups (except the control group)—starting in December with 14 d of cooling down from 23 to 12 °C and a subsequent wintering at 12 °C for 3 months—according to Hermelink et al. (2011) (Fig. 1).
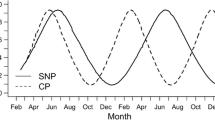
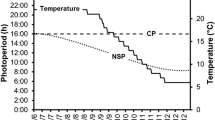
Following completion of the wintering period in March, the temperature was adjusted to 14 °C which has been identified as optimal temperature to support the maturation of pikeperch according to Hermelink et al. (2013). The rise in temperature from 12 to 14 °C was established within 1 week and maintained for an additional 4 months. During these 4 months, the four different experimental groups were exposed to either 8, 10, 12, or 14 h of light per day to evaluate the effect of the photoperiod on gonad maturation (Fig. 1). For all respective groups (except the control fish and the fish of the 12:12 group), the illumination time was adjusted on a daily basis after wintering to finally establish the designated day length within the first month. The experimental photoperiods were chosen according to natural conditions observed between December and May, when the onset of maturation (in females transition from previtellogenesis to vitellogenesis), maturation (in females vitellogenesis), and final maturation takes place in wild stocks (Fig. 2). Fish were fed on a commercial trout diet (DAN-EX 1750, Dana Feed/Biomar: 16 % carbohydrates, 17 % fat, and 50 % protein) at a daily ratio of 0.5 % of whole body mass, adjusted after each sampling point (monthly, see below).
Photoperiod during the natural reproductive cycle of the pikeperch assigned to the transition from previtellogenesis (puberty), vitellogenesis (maturation), and final maturation and ultimately spawning. The dotted lines identify the experimental photoperiod assessed here (8-, 10-, 12-, and 14-h day length), assigned to the histological stage
Sampling procedure
All fish were sampled at day zero and then again at the end of the experiment in early July (2012.07.04). During the 3-month wintering phase, 5 ± 1 fish from each tank of the PTT groups and 8 ± 1 fish from the control were sampled after each month. During the 4-month PTT, 8 ± 1 fish from each of the treatment and control tanks were sampled after each month (the number of female and male pikeperch sampled at the eight different sampling points is given in Tables 1, 2). If a fish died during anesthesia or sampling, it was excluded from the analysis.
Prior to sampling, fish were anesthetized in a MS222-bath (50 mg/L) for two to 3 min until loss of eye reflex and tail fin movement. Total length was taken to the nearest 0.5 cm from the tip of the snout to the end of the longest lobe of the tail fin. Wet weight was measured to the nearest g.
Blood was taken from the caudal vein using heparinized 2-mL syringes and immediately transferred to 2-mL Safe-Lock Tubes (Eppendorf) and centrifuged for 5 min (8000g). The cell-free plasma was separated in 400 µL aliquots, shock-frozen in liquid nitrogen, and stored at −80 °C for further analysis. Follicle samples were taken by introduction of a flexible catheter (Ø 2.2 mm) into the genital porous according to Steffens et al. (1996), Mueller-Belecke and Zienert (2008) and Zakes and Demska-Zakes (2009). Average follicle size was measured for 20 randomly chosen follicles of the largest cohort per female using an Olympus RX50 microscope equipped with an Olympus XC50 digital camera. Subsequently, follicles were transferred to Bouin’s solution for histological preparation. After the examination, fish were allowed to completely recover in aerated tanks before they were returned to their respective treatment tank.
After 7 months, at the end of the experiment, all fish were killed for the final sampling with an overdose of MS222-bath (250 mg/L). The pituitary was dissected, shock-frozen in liquid nitrogen, and stored at −80 °C for gene expression analysis of the gonadotropins FSH and LH.
Gonad histology
Sex was determined upon biopsy. After fixation in Bouin’s solution for 12 h, samples were dehydrated in a graded series of ethanol and embedded in paraffin (Paraplast, Roth, Germany). Subsequently, at least 40 slides were cut at approximately 5 μm from each sample and stained with hematoxylin–eosin. The staging of female gonads was performed as described in Hermelink et al. (2011, 2013) on the basis of Selman et al. (1993) and Lubzens et al. (2010). Individuals were classified as pre, early, mid, late vitellogenic or atretic if more than 50 % of the follicles fulfilled the characteristics of the respective stage according to Table 3. For each sample, 20 randomly chosen follicles of four different histological slides were examined. Due to the applied noninvasive sampling technique, the amount of tissue obtained from male pikeperch was only sufficient for the determination of the sex but not for a reasonable staging assessing testis stages (e.g., spermatocysts, spermatids, sperm) quantitatively.
RNA extraction and reverse transcription
Extraction of total RNA from pituitaries was performed using the RNeasy extraction kit (Qiagen) including on-column DNase digestion according to Trubiroha et al. (2009). The total RNA concentration was evaluated by UV absorption spectrometry with a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific). The integrity of the RNA was assessed as the RNA integrity number (RIN) for a randomly chosen set of RNA samples on RNA 6000 Nano LabChips with an Agilent 2100 bioanalyzer (Agilent Technologies). The measured RIN values were always higher than 8.5 confirming RNA quality for subsequent RT-qPCR analysis (Fleige and Pfaffl 2006). Reverse transcription was carried out in a two-step reaction using AMV-RT (Finnzymes). First, 1 µg of total RNA was incubated with 7.5 pmol oligo-dT primer (5′-CCTGAATTCTAGAGCTCA(T)17-3′) at 70 °C for 3-min primer annealing (20 µL final volume), followed by cooling on ice to stabilize hybrid molecules for 10 min. Subsequently, reverse transcription was carried out after the addition of 15 nmol dNTP (each), 10 U AMV-RT, and 3 µL 10× reaction buffer (250 mM Tris HCl, 500 mM KCl, 20 mmol/L DTT, 50 mM MgCl2, pH 8.3) with a final volume of 30 μL at 36 °C for 60 min and then at 94 °C for 3 min. A negative RT control, where the AMV-RT had been replaced by RNase-free water, was included to rule out genomic DNA contamination of the samples.
Gene expression analysis by real-time quantitative PCR (qPCR)
Expression of FSHβ as and LHβ-subunits using ribosomal protein L8 (rpL8) as a housekeeping gene was carried out as described in Hermelink et al. (2011). Relative quantification assays were performed with a Mx3005 qPCR Cycler (Stratagene) using hot start polymerase (Platinum, Invitrogen) and SYBR Green as fluorescent dye in a 20-µL reaction volume (2 µL diluted cDNA, 7.5 pmol of each primer, 1× Taq buffer, 2 mM MgCl2, 1.7 nmol of each dNTP, 0.1 µL of 200-fold diluted SYBR Green I solution, ROX reference dye, 1 U polymerase). Cycling protocol comprised an initial denaturation and activation of the hot start polymerase at 95 °C for 7 min, followed by 40 amplification cycles of 95 °C denaturation for 20 s, 62 °C primer annealing for 25 s, and 72 °C extension for 25 s. PCRs were always run in duplicate. The expression was determined by the comparative C T method (∆∆C T) according to Pfaffl (2001) with regard to a calibrator sample (pooled pituitary cDNA) compensating for run-to-run variations and corrected for PCR efficiencies of the target genes. Upon validation of even expression in the samples, rpL8 was used as a housekeeping gene. Specificity of the assays was confirmed by direct sequencing and controlled by melting curve analysis.
Steroid analysis
Steroid determination was carried out by hormone-specific ELISA (Cayman) as previously described (Hermelink et al. 2011, 2013). In summary, 100 µL of plasma was extracted twice with 1 mL of diethyl ether (Roth) in a glass vial for 1 min. Afterward, the samples were frozen for 30 min at −80 °C, the liquid organic phase was then transferred into a new vial, and the remaining phase was then extracted for a second time as described above and pooled with the first one. Diethyl ether was allowed to evaporate over night at room temperature.
Following the manufacturer’s instructions, samples were re-dissolved and diluted in assay buffer. The concentrations of all hormones (17β-estradiol—E2, testosterone—T, 11-ketotestosterone—11-KT, 17,20β-progesterone—17,20-P) were determined in duplicates using a standard dilution series. Absorption of the assays was measured with an Infinite M200 microplate reader (Tecan) at 412 nm.
Data analysis
Data are presented as mean ± SD of n samples. For the statistical analysis, data were analyzed for normality (Kolmogorov–Smirnov, passed if >0.05) and homogeneity of variances (log-transformed if necessary). Subsequently, parametric Tukey–Kramer multiple comparison or nonparametric Dunn’s multiple comparison test was carried out (p < 0.05). Statistical analysis was performed using the software packages SPSS 14.0 and GraphPad Prism 4.03.
Results
Gonad development
In female pikeperch, 3 months of wintering and subsequent 4 months of photo-thermal treatment (PTT) induced vitellogenesis and supported maturation. This was revealed by increasing follicle diameter (Fig. 3) and ongoing ripening of the gonads, in contrast to the control fish reared continuously at 23 °C (Fig. 4). In noncontrol females, follicle diameter increased continuously from the first day of wintering onwards (mean diameter measured at day zero was 217 ± 65 µm), revealing successful induction of vitellogenesis with clearly vitellogenic follicles (800 ± 97 µm) after wintering (Fig. 3). After only 3 months, at the end of the wintering phase, 29 % of early vitellogenic, 50 % of mid-vitellogenic, and 21 % of late vitellogenic follicles (LV) could be observed (Fig. 4). Thereafter, follicle growth (Fig. 3) and vitellogenesis (Fig. 4) progressed in all PTT groups, but with only minor differences between treatments: Generally, with increasing day length, higher ratios of late vitellogenic follicles were observed at an earlier time point. For example at 12:12 and 14:10, a maximum of 92 and 86 % of LV were recorded after 4 months, whereas at 08:16 and 10:14 the highest LV (85 and 93 %) was noticed one month later. Similarly, atretic follicles appeared first in the 12:12 and 14:10 treatments (except for one female at 08:16 after 4 months), again at a higher ratio than at shorter day lengths. Concomitant to the ongoing maturation during the PTT, the follicle diameter increased differentially, though not significant between groups (Fig. 3). Peak diameter was observed after 7 months in females of 12:12 (1113 ± 110 µm) and 14:10 (1142 ± 86 µm) treatments. Females of the short-day treatments developed only slightly smaller follicles with 1087 ± 64 (8:16) and 1067 ± 250 µm (10:14) also after 7 months.
Follicle diameter (mean ± SD) of female pikeperch after 0, I, II, III, IV, V, VI, and VII months of photo-thermal treatment (PTT) compared to control females kept at 12:12 and 23 °C. The dashed line indicates the status at day zero, the dotted line the end of wintering. The number of fish per treatment and sampling point is given in Table 1. To provide an optimal overview, data of the experimental groups at a respective sampling point are presented apart from one another, as identified on the x-axis. Significant differences between groups at a sampling point are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test). Significant differences within an experimental group over the experimental period are displayed in the supplemental material part (Tukey–Kramer/Dunn’s multiple comparison test)
Relative abundance of follicle stages after 0, I, II, III, VI, V, VI and VII months of photo-thermal treatment (PTT) compared to control females kept at 12:12 and 23 °C. The number of fishes per treatment and sampling point is given in Table 1
Gene expression analysis
In the control fish, basic expression levels of LHβ and FSHβ in the pituitary were comparable between males and females (Fig. 5). In all PTT groups, mRNA expression of the gonadotropins LHβ and FSHβ was significantly higher than in the control. However, there were no significant differences among the PTT groups, neither in males nor in females.
Pituitary mRNA expression (mean ± SD) of follicle-stimulating hormone β-subunit (FSHβ) and luteinizing hormone β-subunit (LHβ) in female (a) and male (b) pikeperch after 4 months of photo-thermal treatment (PTT) compared to the control fish kept at 12:12 and 23 °C. The number of samples is given in Table 1 (females) and Table 2 (males). Significant differences between groups are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test)
After the 4 months of PTT, FSHβ exhibited a fivefold (08:16) to sevenfold (14:10) increase in females compared to control females. In males, slightly lower expression levels of FSHβ were recorded, between three- (10:14 and 14:10) and fourfold (08:16 and 12:12). The expression of the LHβ subunit was even more prominently elevated after PTT. In males, expression was between 15 (12:12) and 20 times (08:16) higher than the control. In females, the upregulation was even more pronounced with expression level between 32 (08:16) and 38 times (12:12) higher than the control females.
Sex steroids
Maturation in both sexes was correlated to the sex-specific steroids: E2 in females (Fig. 6), and 11-KT and T in males (Fig. 7) were observed in high concentrations in the PTT groups. In the control, females exhibited baseline concentrations of 220 ± 50 up to 550 ± 250 pg/mL E2, 0.1 ± 0.1 up to 5.9 ± 10.7 ng/mL T, and 110 ± 40 up to 1200 ± 1500 pg/mL 11-KT (Fig. 6). Males of the control also showed continuously low steroid concentrations between 100 ± 40 and 750 ± 300 pg/mL E2, 0.23 ± 0.13 up to 1.8 ± 1.5 ng/mL of T, and between 0.3 ± 0.2 and 0.9 ± 0.5 ng/mL of 11-KT (Fig. 7).
Plasma concentration of sex steroids (mean ± SD) in female pikeperch after 0, I, II, III, VI, V, VI and VII months of photo-thermal treatment (PTT) compared to control females kept at 12:12 and 23 °C. The dashed line indicates the status at day zero, the dotted line the end of wintering. The number of fish per treatment and sampling point is given in Table 1. To provide an optimal overview, data of the experimental groups at a respective sampling point are presented apart from one another, as identified on the x-axis. Significant differences between groups at a sampling point are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test). Significant differences within an experimental group over the experimental period are displayed in the supplemental material part (Tukey–Kramer/Dunn’s multiple comparison test). E2 17ß-estradiol, T testosterone, 11-KT 11-ketotestosterone, 17,20-P 17,20β-progesterone
Plasma concentration of sex steroids (mean ± SD) in male pikeperch after 0, I, II, III, VI, V, VI and VII months of photo-thermal treatment (PTT) compared to control males kept at 12:12 and 23 °C. The dashed line indicates the status at day zero, the dotted line the end of wintering. The number of fish per treatment and sampling point is given in Table 2. To provide an optimal overview, data of the experimental groups at a respective sampling point are presented apart from one another, as identified on the x-axis. Significant differences between groups at a sampling point are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test). Significant differences within an experimental group over the experimental period are displayed in the supplemental material part (Tukey–Kramer/Dunn’s multiple comparison test). E2 17β-estradiol, T testosterone, 11-KT 11-ketotestosterone
In females (Fig. 6), estradiol concentration already increased significantly during the wintering phase from 380 ± 230 (day zero) up to 2600 ± 1500 pg/mL (end of wintering after 3 months) and remained elevated in all PTT. Still, depending on the photoperiod, different peak values were observed over time. E2 decreased slightly after wintering during the first month (month four of the complete experiment, month one of the PTT) when the designated photoperiod of the experimental groups was established. Only the fish that remained at 12:12 revealed a constant increase in E2, peaking after 4 months at 4200 ± 3300 pg/mL. Higher peak levels of E2 were observed with increasing day lengths: Whereas females at the two shortest day lengths peaked at 2600 ± 1400 pg/mL (08:16) and at 2900 ± 2500 pg/mL (10:14), females exposed to the longest illumination time 14:10 reached a maximum concentration of 6800 ± 4200 pg/mL. During wintering, T increased only up to 7.9 ± 12 ng/mL. Thereafter, T increased further, but peak levels were quite different among the treatment groups: 140 ± 310 (08:16, sixth month), 17 ± 18 (10:14, seventh month), 410 ± 780 (12:12, fifth month), and 64 ± 110 ng/mL (14:10, seventh month). Interestingly, 11-KT dropped from day zero onwards (1200 ± 1500 pg/mL) and only increased thereafter, correlated to the appearance of late vitellogenic follicles in the respective PTT groups. Again, the highest level of 11-KT (1500 ± 2700 pg/mL) was observed in the group that remained at 12:12 after wintering, which was also observed earliest after a period of 5 months (2 month of PTT). In the following month, remaining treatments reached peak levels with 510 ± 930 (08:16), 1000 ± 1400 (10:14), and 1000 ± 1000 pg/mL (14:10). Similarly, in females of the control group, 11-KT concentrations decreased, and in contrast to the PTT, groups remained low, fluctuating around 110 ± 40 pg/mL. In the control females, 17,20-P constantly increased up to 150 ± 30 pg/mL in the fourth month, revealing the highest concentration of all experimental groups. After peaking, 17,20-P dropped and then increased again, irrespective of the maturation of the gonad in all PTT groups as well as in the control.
In males, T peaked at 21 ± 12 ng/mL during wintering, but returned to a baseline level of 1.5 ± 0.5 ng/mL after 3 months (Fig. 7). Subsequently, plasma T concentrations increased again. Here, respective peak concentrations increased with increasing day lengths from 9.6 ± 10.3 ng/mL in the 08:16 group to 27 ± 21 ng/mL in the 14:10 males. 11-KT concentrations increased rather constantly. Again, highest concentrations were recorded at the end of the experiment with increasing day lengths, revealing 3.1 ± 2.8 ng/mL at 08:16, 8.6 ± 21.7 ng/mL at 10:14, 21 ± 45 ng/mL at 12:12, and 39 ± 91 ng/mL at 14:10. E2 increased in all treatment groups until the end of the experiment. In contrast to the androgens, highest concentrations of E2 were recorded under short-day conditions, 1100 ± 230 pg/mL at 08:16 (month seven), 860 ± 150 pg/mL (month seven) at 10:14, 400 ± 80 pg/mL (month six) at 12:12, and 530 ± 120 pg/mL at 14:10 (month six).
Growth
After wintering, correlated to the maturation of gonads, growth almost stagnated in females and males. No significant differences between the PTT groups were observed. In maturing females, weight and total length fluctuated around 52 ± 6 and 56 ± 4 cm, and wet weights ranged from 1300 ± 440 to 1730 ± 370 g (Fig. 8). Maturing males reached comparable values with weight and length between 1100 ± 260 and 1540 ± 410 g and 49 ± 4 and 54 ± 7 cm over the experimental period (Fig. 9). In contrast, control females and males grew moderately to maximum sizes around 63 ± 3 (females) and 62 ± 4 cm (males), increasing their peak body weight to 2300 ± 400 (females) and 2200 ± 400 g (males), respectively.
Growth (mean ± SD) in female pikeperch after 0, I, II, III, VI, V, VI and VII months of photo-thermal treatment (PTT) compared to control females kept at 12:12 and 23 °C. The dashed line indicates the status at day zero, the dotted line the end of wintering. The number of fish per treatment and sampling point is given in Table 1. To provide an optimal overview, data of the experimental groups at a respective sampling point are presented apart from one another, as identified on the x-axis. Significant differences between groups at a sampling point are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test). Significant differences within an experimental group over the experimental period are displayed in the supplemental material part (Tukey–Kramer/Dunn’s multiple comparison test)
Growth (mean ± SD) in male pikeperch after 0, I, II, III, VI, V, VI and VII months of photo-thermal treatment (PTT) compared to control males kept at 12:12 and 23 °C. The dashed line indicates the status at day zero, the dotted line the end of wintering. The number of fish per treatment and sampling point is given in Table 2. To provide an optimal overview, data of the experimental groups at a respective sampling point are presented apart from one another, as identified on the x-axis. Significant differences between groups at a sampling point are indicated by capital letters (Tukey–Kramer/Dunn’s multiple comparison test). Significant differences within an experimental group over the experimental period are displayed in the supplemental material part (Tukey–Kramer/Dunn’s multiple comparison test)
Discussion
As demonstrated by Hermelink et al. (2011, 2013), the onset of gonad maturation (puberty) in addition to subsequent maturation is temperature-controlled in the pikeperch (S. lucioperca). Hence, fine-tuning of the rearing protocol will substantially improve reproduction to overcome the shortage of stocking material for this emerging aquaculture species. In the Eurasian perch (P. fluviatilis), a closely related species, photoperiod plays a key role in the regulation of the reproductive cycle (Taranger et al. 2010; Wang et al. 2010).
The influence of the photoperiod on gonad maturation has not yet been experimentally studied in the pikeperch, although photoperiod has been examined in several studies on related species and is recently implemented by some commercial hatcheries during reproduction (Table 4). Thus, the focus of this study was to examine the influence of photoperiod on maturation in pikeperch and explore its potential for the reproductive management of pikeperch. The key hormones involved in the regulation of gonad development—E2, T and 11-KT—were assessed as well as both gonadotropins FSH and LH. This enabled a comprehensive description of the maturational progress and allowed the comparison between developmental stages. Firstly, we induced puberty as previously described (Hermelink et al. 2011) and then evaluated the influence of selected photoperiods at a temperature previously found to support optimal maturation of the gonads (Hermelink et al. 2013).
During the 3 months of wintering, vitellogenesis was successfully induced in all females. This was clearly demonstrated, firstly, by the constant follicle growth from 200 µm to more than 1100 µm, and secondly, by the transition to vitellogenesis and subsequent maturation. Additionally, the increase in E2 and T levels in the plasma indicated the activation of the HPG axis at the transition from previtellogenesis to vitellogenesis. Similarly in males, wintering-induced spermatogenesis correlated to increasing 11-KT blood concentrations.
Thus, at the end of the wintering period, stagnation of the gonad was overcome. In contrast, fish constantly kept at 23 °C and 12:12 confirmed complete stagnation of maturation. Only these fish exhibited substantial weight and length gain.
After the wintering phase, four different photo-thermal treatments tested whether day length influences maturation, as reported for other Perciformes such as the Eurasian perch (P. fluviatilis) where light has been suggested as the principal environmental cue triggering gametogenesis (Wang et al. 2006, 2010; Abdulfatah et al. 2011, 2013).
Remarkably, in female pikeperch reared at 14:10, the first appearance of atretic ovarian follicles, generally taken as evidence of spontaneous spawning (Sulistyo et al. 1998), was observed after 5 months, indicating an accelerated maturation induced by the longer day length.
Notably, even at the end of the experimental phase, the majority of females exposed to the shorter day lengths were still in a late vitellogenic stage with smaller follicles in average and therefore ready to undergo final maturation. Thus, an important technical note for commercial out-of-season reproduction is that shortened day length can be used to elongate the availability of potential spawners and therefore can be used to fine-tune reproduction. Furthermore, this study clearly suggests that photoperiod is only a minor factor modulating the progression of maturation, and finalization of maturation is equally achieved by temperature only.
The mRNA expression of the gonadotropin β-subunits was increased in both sexes and all treatment groups when compared to control fish, confirming the activation of the HPG axis and progression of gonad maturation. Nevertheless, there were no significant differences between the different day length treatments. Still, the expression levels of LHβ as well as FSHβ in females of the short-day treatments were slightly lower than those of the long-day treatments supporting the other findings here. As with our previous study, LHβ was more prominently increased compared to FSHβ. In this study, we can confirm the key role of LH toward the end of maturation as has been hypothesized in other fish species, e.g., Striped Bass (Morone saxatilis) (Hassin et al. 1999; Mylonas and Zohar 2001) and the European sea bass (Dicentrarchus labrax) (Rocha et al. 2009; Mazon et al. 2015). This suggests minor effects of the photoperiod on the expression of the gonadotropins during the mainly temperature-regulated maturation of the pikeperch.
Due to the experimental setup, the expression profiles of the gonadotropins exclusively reflect the situation at the end of the experiment. However, the repeated measurements of the sex steroids over the 4-month PTT provides a more precise insight into the influence of the photoperiod on maturation. Here, females reared under long-day conditions revealed the highest E2 concentrations, which is the crucial hormone of the female reproductive cycle. This was accompanied with a remarkable upregulation of 11-KT during the ongoing vitellogenesis (Hermelink et al. 2013). Interestingly, 11-KT is involved in the regulation of lipid (especially lipoprotein lipase) transportation during female maturation, which agrees with the results of the preceding study (Hermelink et al. 2013).
In male pikeperch, the HPG axis was activated during wintering, clearly indicated by the main androgens T as well as 11-KT. Similarly, these steroids were highest in the long-day treatments, as found with female specimens. It is well established that males of various percid fish such as the Eurasian perch (P. fluviatilis), yellow perch (Perca flavescens), white perch (Morone americana), and walleye (Stizostedion vitreum) show a clear, often biphasic, pattern of seasonal changes in the main androgens T and 11-KT, concomitant with the maturational stage (Malison et al. 1994; Jackson and Sullivan 1995; Dabrowski et al. 1996; Sulistyo et al. 2000). Less is known about the age-dependent production of the androgens. While Cierszko et al. (1998) detected similar levels of T and 11-KT in two- and three-year-old yellow perch (P. flavescens), the 11-KT concentrations in the three-year-old pikeperch observed during our experiment were up to three times higher at the end of the experiment than those found in two-year-old pikeperch previously reported (Hermelink et al. 2013). Yet, the highest androgen concentrations were observed in the long-day treatments and the lowest in the short-day treatments, indicating an effect of the photoperiod similarly found in females where the main estrogen E2 was highest under the long-day exposure.
In males, gonadotropin expression (in particular LH) did not reveal an effect of the photoperiod. Due to the presence of successive spermatogenic stages in the gonad, such effects are likely to have been further masked.
Conclusion
The investigation clearly highlighted temperature and not light as the main factor to control maturation in pikeperch (S. lucioperca). Therefore, a clear species-specific difference in the biology of pikeperch compared to the closely related Eurasian perch (P. fluviatilis) is described. Nevertheless, day length appears to “fine-tune” maturation. While “long days” with an illumination time of 12 and 14 h accelerated the maturation, a delay was observed under “short days” (8 or 10 h of light). Consequently, the manipulation of the photoperiod can be implemented as a simple and cost-effective measure to elongate the availability of pikeperch spawners under commercial conditions (e.g., to elongate the availability of spawnable fish), if the optimal temperature protocol is applied concurrently. This may be particularly useful for out-of-season reproduction in RAS-based aquaculture, also with regard to endogenous rhythms that may counteract pure thermal treatment. Although the data on females are more conclusive, males of the long-day treatments were characterized by the highest concentrations of both androgens T and 11-KT, indicating that similar effects are also modulating spermatogenesis. Thus, photoperiod should not be overlooked in the future development of pikeperch aquaculture and warrants consideration in the reproductive management of pikeperch.
Abbreviations
- 11-KT:
-
11-Ketotestosterone
- 17,20-P:
-
17,20β-Progesterone
- E2:
-
17β-Estradiol
- FSHβ:
-
Follicle-stimulating hormone β-subunit
- HPG axis:
-
Hypothalamus–pituitary–gonad axis
- LV:
-
Late vitellogenic follicles
- LHβ:
-
Luteinizing hormone β-subunit
- n. c.:
-
Natural conditions
- n. s.:
-
Not specified
- PTT:
-
Photo-thermal treatment
- RAS:
-
Recirculating aquaculture system
- T:
-
Testosterone
- us.:
-
Unspecified
References
Abdulfatah A, Fontaine P, Kestemont P, Gardeur JN, Marie M (2011) Effects of photothermal kinetics and amplitude of photoperiod decrease on the induction of the reproduction cycle in female Eurasian perch Perca fluviatilis. Aquaculture 322:169–176
Abdulfatah A, Fontaine P, Kestemont P, Milla S, Marie M (2013) Effects of the thermal threshold and the timing of temperature reduction on the initiation and course of oocyte development in cultured female of Eurasian perch Perca fluviatilis. Aquaculture 376:90–96
Barron JM, Jensen NR, Anders PJ, Egan JP, Ireland SC, Cain KD (2012) Effects of temperature on the intensive culture performance of larval and juvenile North American burbot (Lota lota maculosa). Aquaculture 364:67–73
Blecha M, Kristan J, Samarin AM, Rodina M, Policar T (2015) Quality and quantity of pikeperch (Sander lucioperca) spermatozoa after varying cold water treatments. J Appl Ichthyol 31:75–78
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Ciereszko RE, Dabrowski K, Ciereszko A, Ottobre JS (1998) Plasma concentrations of steroid hormones in male yellow perch, Perca flavescens: the effect of age and photothermal manipulation. Environ Biol Fish 51:97–105
Dabrowski K, Ciereszko RE, Ciereszko A, Toth GP, Christ SA, El-Saidy D, Ottobre JS (1996) Reproductive physiology of yellow perch (Perca flavescens): environmental and endocrinological cues. J Appl Ichthyol 12:139–148
Fleige S, Pfaffl MW (2006) RNA integrity and the effect on the real-time qRT-PCR performance. Mol Asp Med 27:126–139
Hassin S, Holland MCH, Zohar Y (1999) Ontogeny of follicle-stimulating hormone and luteinizing hormone gene expression during pubertal development in the female striped bass, Morone saxatilis (Teleostei). Biol Reprod 61:1608–1615
Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C (2011) Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol 172:282–292
Hermelink B, Wuertz S, Rennert B, Kloas W, Schulz C (2013) Temperature control of pikeperch (Sander lucioperca) maturation in recirculating aquaculture systems: induction of puberty and course of gametogenesis. Aquaculture 400:36–45
Jackson LF, Sullivan CV (1995) Reproduction of white perch: the annual gametogenic cycle. Trans Am Fish Soc 124:563–577
Kristan J, Alavi SMH, Stejskal V, Policar T (2013) Hormonal induction of ovulation in pikeperch (Sander lucioperca L.) using human chorionic gonadotropin (hCG) and mammalian GnRH analogue. Aquac Int 21:811–818
Ljubobratovic U, Kucska B, Feledi T, Poleksic V, Markovic Z, Lenhadt M, Ronyai A (2013) Combined methods for artificial reproduction of pikeperch, Sander lucioperca. In: Conference proceedings VI international conference “Water and Fish”, Faculty of Agriculture, University of Belgrade – Serbia, Belgrade, pp 261–266. 12–14 June 2011
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Malison JA, Procarione LS, Barry TP, Kapuscinski AR, Kayes TB (1994) Endocrine and gonadal changes during the annual reproductive-cycle of the fresh-water teleost, Stizostedion vitreum. Fish Physiol Biochem 13:473–484
Mazon MJ, Moles G, Rocha A, Crespo B, Lan-Chow-Wing O, Espigares F, Munoz I, Felip A, Carrillo M, Zanuy S, Gomez A (2015) Gonadotropins in European sea bass: endocrine roles and biotechnological applications. Gen Comp Endocrinol 221:31–41
Migaud H, Fontaine P, Sulistyo I, Kestemont P, Gardeur JN (2002) Induction of out-of-season spawning in Eurasian perch Perca fluviatilis: effects of rates of cooling and cooling durations on female gametogenesis and spawning. Aquaculture 205:253–267
Migaud H, Gardeur JN, Kestemont P, Fontaine P (2004a) Off-season spawning of Eurasian perch Perca fluviatilis. Aquac Int 12:87–102
Migaud H, Fontaine P, Kestemont P, Wang N, Brun-Bellut J (2004b) Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture 241:561–574
Migaud H, Wang N, Gardeur JN, Fontaine P (2006) Influence of photoperiod on reproductive performances in Eurasian perch Perca fluviatilis. Aquaculture 252:385–393
Migaud H, Davie A, Taylor JF (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol 76:27–68
Mueller-Belecke A, Zienert S (2008) Out-of-season spawning of pike perch (Sander lucicoperca L.) without the need for hormonal treatments. Aquac Res 39:1279–1285
Mylonas CC, Zohar Y (2001) Use of GnRHa-delivery systems for the control of reproduction in fish. Rev Fish Biol Fish 10:463–491
Okuzawa K (2002) Puberty in teleosts. Fish Physiol Biochem 26:31–41
Palinska-Zarska K, Zarski D, Krejszeff S, Nowosad J, Bilas M, Trejchel K, Brylewski A, Targonska K, Kucharczyk D (2014) The effect of age, size and digestive tract development on burbot, Lota lota (L.) larvae weaning effectiveness. Aquac Nutr 20:281–290
Palinska-Zarska K, Zarski D, Krejszeff S, Kupren K, Laczynska B, Kucharczyk D (2015) Optimal feeding level of burbot larvae fed Artemia spp. and reared under controlled conditions. N Am J Aquac 77:295–301
Pankhurst NW, Porter MJR (2003) Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish Physiol Biochem 28:385–389
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acid Res 29:e45
Pimakhin A, Kouril J, Stejskal V, Zak J (2015) The effect of geographical origin of perch (Perca fluviatilis L. 1758) populations on growth rates under natural and aquaculture conditions: a review. J Appl Ichthyol 31:56–63
Rocha A, Zanuy S, Carrillo M, Gomez A (2009) Seasonal changes in gonadal expression of gonadotropin receptors, steroidogenic acute regulatory protein and steroidogenic enzymes in the European sea bass. Gen Comp Endocrinol 162:265–275
Ronyai A (2007) Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquac Res 38:1144–1151
Schaefer F, Overton J, Wuertz S (2016) Pikeperch Sander lucioperca egg quality cannot be predicted by total antioxidant capacity and mtDNA fragmentation. Anim Reprod Sci. doi:10.1016/j.anireprosci.2016.02.016
Selman K, Wallace RA, Sarka A, Qi XP (1993) Stages of oocyte development in the Zebrafish, Brachydanio rerio. J Morphol 218:203–224
Stacey NE (1984) Control of the timing of ovulation by exogenous and endogenous factors. In: Potts GW, Wootton RJ (eds) Fish reproduction: strategies and tactics. Academic Press, London, pp 207–222
Steffens W, Geldhauser F, Gerstner P, Hilge V (1996) German experiences in the propagation and rearing of fingerling pikeperch (Stizostedion lucioperca). Ann Zool Fenn 33:627–634
Sulistyo I, Rinchard J, Fontaine P, Gardeur JN, Capdeville B, Kestemont P (1998) Reproductive cycle and plasma levels of sex steroids in female Eurasian perch Perca fluviatilis. Aquat Living Resour 11:101–110
Sulistyo I, Fontaine P, Rinchard J, Gardeur JN, Migaud H, Capdeville B, Kestemont P (2000) Reproductive cycle and plasma levels of steroids in male Eurasian perch Perca fluviatilis. Aquat Living Resour 13:99–106
Szczepkowski M, Zakes Z, Szczepkowska B, Piotrowska I (2011) Effect of size sorting on the survival, growth and cannibalism in pikeperch (Sander lucioperca.) larvae during intensive culture in RAS. Czech J Anim Sci 56:483–489
Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien FA, Dufour S, Karlsen O, Norberg B, Andersson E, Hausen T (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515
Trubiroha A, Wuertz S, Frank SN, Sures B, Kloas W (2009) Expression of gonadotropin subunits in roach (Rutilus rutilus, Cyprinidae) infected with plerocercoids of the tapeworm Ligula intestinalis (Cestoda). Int J Parasitol 39:1465–1473
Wang N, Gardeur JN, Henrotte E, Marie M, Kestemont P, Fontaine P (2006) Determinism of the induction of the reproductive cycle in female Eurasian perch, Perca fluviatilis: identification of environmental cues and permissive factors. Aquaculture 261:706–714
Wang N, Teletchea F, Kestemont P, Milla S, Fontaine P (2010) Photothermal control of the reproductive cycle in temperate fishes. Rev Aquac 2:209–222
Wocher H, Harsanyi A, Schwarz FJ (2012) Larviculture of burbot (Lota lota L.): larval rearing using Artemia and weaning onto dry feed. Aquac Res 44:106–113
Wuertz S, Nitsche A, Jastroch M, Gessner J, Klingenspor M, Kirschbaum F, Kloas W (2007) The role of the IGF-I system for vitellogenesis in maturing female sterlet, Acipenser ruthenus Linnaeus, 1758. Gen Comp Endocrinol 150:140–150
Zakes Z (2007) Out-of-season spawning of cultured pikeperch [Sander lucioperca (L.)]. Aquac Res 38:1419–1427
Zakes Z, Demska-Zakes K (2009) Controlled reproduction of pikeperch Sander lucioperca (L.): a review. Arch Pol Fish 17:153–170
Zakes Z, Szczepkowski M, Partyka K, Wunderlich K (2013) Effect of gonadotropin hormonal stimulation on out-of-season propagation success of different year classes of indoor-reared pikeperch (Sander lucioperca (L.)). Aquac Int 21:801–810
Zarski D, Kucharczyk D, Targonska K, Palinska K, Kupren K, Fontaine P, Kestemont P (2012) A new classification of pre-ovulatory oocyte maturation stages in pikeperch, Sander lucioperca (L.), and its application during artificial reproduction. Aquac Res 43:713–721
Zarski D, Targonska K, Kaszubowski R, Kestemont P, Fontaine P, Krejszeff S, Kupren K, Kucharczyk D (2013) Effect of different commercial spawning agents and thermal regime on the effectiveness of pikeperch, Sander lucioperca (L.), reproduction under controlled conditions. Aquac Int 21:819–828
Acknowledgments
This study was supported by the German research funding DFG as part of the project “Influence of exogenous factors on the endocrine regulation of gonad maturation in pikeperch S. lucioperca” (SCHU 2308/1-1 AOBJ: 530719). The authors acknowledge AquaPri (Egved Denmark), Landesforschungsanstalt Landwirtschaft & Fischerei (Mecklenburg-West Pomerania, Germany), Institute of Inland Fisheries Potsdam-Sacrow (Brandenburg, Germany), Müritz AquaArt GmbH (Mecklenburg-West Pomerania, Germany), and the Fischzucht Rietschen (Saxonia, Germany) for the information on the reproductive management of pikeperch (Table 4).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hermelink, B., Kleiner, W., Schulz, C. et al. Photo-thermal manipulation for the reproductive management of pikeperch Sander lucioperca . Aquacult Int 25, 1–20 (2017). https://doi.org/10.1007/s10499-016-0009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0009-x