Abstract
Bivalve shellfish play an important role in top-down control of primary symptoms of eutrophication. This short-circuits the process of organic decomposition and promotes an enhancement of underwater light climate, improved oxygenation of bottom water, and restoration of submerged aquatic vegetation. This review analyses this ecosystem service as a potential actor in watershed-level nutrient credit trading programmes and explores the possibilities of implementation of such programmes in Europe. We examine the different components of the issue, including the eutrophication status of European coastal waters, legal and management instruments, and the use of mathematical models at both the ecosystem and farm scales to evaluate the potential removal of nitrogen by cultivated shellfish such as oysters, mussels, and clams. The annual European bivalve shellfish production of over 700,000 metric tons is estimated to generate a nitrogen removal of 46,800 t year−1, equivalent to 14 × 106 population equivalent, and a minimum value of 507 × 106 €. We discuss future directions for this topic in Europe, drawing from ongoing research in the USA and elsewhere, in the light of the twin challenges of European aquaculture expansion and implementation of EU directives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and scope
The role of bivalve shellfish in improving water quality has not been widely recognized until the last decades (e.g. Higgins et al. 2011), although the filtration of water column particulates by different bivalve species has been studied for the best part of a century (Orton 1928; Loosanoff and Tommers 1948; Dral 1967; Loveland and Chu 1969; Tenore and Dunstan 1973; Bayne and Newell 1983; Shumway and Cucci 1987; Jørgensen et al. 1990; Bayne et al. 1993; Clausen and Riisgård 1996).
This lack of recognition may be due to historical reasons, since shellfish filtration was traditionally associated with disease outbreaks (e.g. Eyre 1925), and those public health issues in turn drove society to focus on land-based control of emissions. The development of industrial agriculture and the widespread use of fertilizers further sharpened this focus, resulting in a body of legislation aimed at source control, both for microbial vectors and for nutrient waste.
In the European Union, this is exemplified by first-generation directives such as the Urban Waste-Water Treatment Directive (UWWTD, 91/271/EEC) and its sister directive on agricultural waste (Nitrates, 91/676/EEC), and more recently by the Water Framework Directive (WFD, 2000/60/EC)—top-down control of eutrophication by bivalves merits no consideration in any of these legislative instruments.
By contrast, water management practices in Southeast Asia and China, particularly in integrated multi-trophic aquaculture (IMTA), have long recognized the importance of bivalves such as oysters, mussels, or razor clams in mitigating organic and inorganic loading to coastal systems.
An improved understanding of bivalve physiology and the environmental factors that determine key rates for processes such as feeding, pre-ingestive selection, and metabolism has led to the development of mathematical models for growth (e.g. Grant and Bacher 1998; Hawkins et al. 2002), which were subsequently extended to focus on production (Gerritsen et al. 1994; Bacher et al. 1998; Dame and Prins 1998; Gangnery et al. 2004; Grant et al. 2007).
More recently, as coastal aquaculture expansion has become a part of ecosystem-based management, and issues such as bioextraction have gained renewed traction (Lindahl et al. 2005); models combining production and environmental interactions have became available at both ecosystem and local scales (Nobre et al. 2010; Nunes et al. 2011; Ferreira et al. 2012, 2014a; Filgueira et al. 2014; Saurel et al. 2014).
Such tools are a powerful means to analyse the role of bivalve shellfish in reducing the primary symptoms of eutrophication and to quantify the potential of organically extractive aquaculture to participate in watershed-scale nutrient credit trading programmes as a full partner in integrated catchment management.
The main objectives of this chapter are therefore (1) to review nutrient-related ecosystem services provided by shellfish aquaculture; (2) to provide specific examples of application of models to this issue, including simulations of the economic value of top-down eutrophication control; and (3) to discuss practical aspects of implementation, including the appropriate valuation of these services.
Shellfish production in Europe
In order to evaluate the potential role of bivalve shellfish in nutrient credit trading at the European scale, it is important to understand: (1) which species are most important in terms of production, because of variations in feeding behaviour; (2) where that production is located, both with respect to latitude, because water temperature is a key driver of growth, and to proximity to the coastline—since we are addressing the substitution cost of land-based nutrient removal, offshore aquaculture typically does not meet the connectivity requirement with land-based sources.
In 2012, European aquaculture produced 2.88 × 106 t, representing 4.3 % of world supply, down from 12.2 % in 1990 (FAO 2014). For the European Union, those numbers are substantially worse: a 2012 production of 1.26 × 106 t, and a decrease from 7.9 % in 1990 to 1.9 % in 2012. The difference between Europe and the EU is largely explained by a significant increase in Norwegian salmon production.
The European shellfish harvest for 2011 (Table 1) was 713,000 tonnes, with Spain and France as the two major producers, accounting for 53 % of the total. They were followed by the UK with 13 %, although over half of that was due to the wild scallop fishery. Other countries with significant production were the Netherlands, Italy, Denmark, and Ireland. Although there is some interannual fluctuation (e.g. the Dutch mussel harvest ranges between 30 and 90 kt year−1 for the period 2000–2007), these data are considered as a best estimate for the analysis herein.
FAO (2014) estimates that bivalves constitute 20.1 % of world aquaculture production—the data in Table 1 (Eurostat 2014; DFPB 2008) yield a similar number of 24.7 % in Europe. However, this rises to 56.6 % for the European Union alone.
The country and species distributions shown in this table allow a better understanding of the potential in Europe for nutrient credit trading as a result of bivalve shellfish aquaculture.
Although Europe-wide mapping of shellfish areas is to our knowledge unavailable, country-level maps (Fig. 1; DEFRA 2014) show bivalve cultivation and harvesting is a near-shore activity that occurs within semi-enclosed systems or in relative proximity to the coastline. This is well established for all major European sites, such as the Rias Bajas in Galicia (Spain), where the majority of Mytilus galloprovincialis is grown, the Marennes-Oléron oyster-producing region in France, and for areas such as the Oosterschelde, Netherlands (Wong 2014), Sacca di Goro, Italy (Vincenzi et al. 2006), and the principal Northern Irish loughs (Ferreira et al. 2008).
The two overarching conclusions are therefore that (a) the volume of current shellfish production in Europe justifies consideration of bivalves as a part of integrated nutrient management and (b) the proximity of cultivation sites to the coast further support this option.
In order to analyse whether this is a relevant management tool in Europe, or merely a solution looking for a problem, the next section reviews the eutrophication status of European coastal waters.
Eutrophication in European coastal waters
Eutrophication is defined in the Marine Strategy Framework Directive (MSFD, 2008/56/EC) as a process driven by enrichment of water by nutrients, especially compounds of nitrogen and/or phosphorus, leading to increased growth, primary production, and biomass of algae; changes in the balance of organisms; and water quality degradation. The consequences of eutrophication are undesirable if they appreciably degrade ecosystem health and/or the sustainable provision of goods and services (Ferreira et al. 2011).
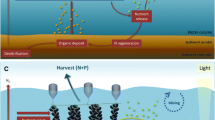
Delivery of land-based nutrients to coastal waterbodies has been greatly accelerated by human activities and may promote a complex array of undesirable symptoms, beginning with excessive growth of pelagic and opportunistic benthic algae which may lead to other, more serious water quality problems such as hypoxia, losses of seagrasses and occurrences of nuisance and toxic algal blooms (Fig. 2). Slow flushing coastal waters are more sensitive to inputs and may become impaired even with relatively small increases to nutrient loads. Eutrophic symptoms impair aesthetics and coastal water quality, which, in turn, have social and economic impacts on commercial and recreational fishing, tourism, and property value.
Due to evidence of nutrient-related degradation in EU coastal waters as early as the 1970s, several EU and international legislative mandates were passed and commissions formed to protect waters from further degradation and to improve conditions in impaired waters. Under EU legislation, stricter requirements apply to agriculture and urban wastewater treatment plants discharging into areas designated as sensitive or vulnerable to nutrient inputs. Combined areas under jurisdiction of the EU and the OSPAR and HELCOM Commissions cover most of EU coastal waters and mutually support objectives to combat eutrophication. These are similar to legislative mandates in the USA, China, Australia and elsewhere (Borja et al. 2008, 2012), evidencing that eutrophication is a global issue.
A range of tools exists to assess the degree and spatial extent of impairment and to track trends through time, measuring progress towards fulfilment of mandates (see review in Ferreira et al. 2011). State-of-the-art eutrophication assessment tools use key indicators including chlorophyll-a, dissolved oxygen, occurrence of harmful and toxic algal blooms, and loss of seagrasses, which allow an integrated evaluation of the overall status of eutrophication in coastal and marine waters, and enable managers and policy-makers to take decisions about the remediation of nutrient-related problems. The Assessment of Estuarine Trophic Status (ASSETS; Bricker et al. 2007) has been used most broadly and is one of the few methods that link nutrient loads to observed impairment, important to helping guide appropriate management. The OSPAR COMPP and HELCOM HEAT tools, while not as widely applied as ASSETS, are similar and also make a linkage between loads and water quality.
The most recent OSPAR COMPP assessment showed that eutrophication is still a problem—the assessments relating to the period 1990–2000 (OSPAR, 2008), in OSPAR regions II (Greater No. Sea), III (Celtic Sea), and IV (Bay of Biscay and Iberian Coast), report that the objective of no eutrophication by 2010 has only been partly achieved. The most recent application of the HELCOM HEAT assessment (HELCOM 2010) to 17 open areas and 172 coastal areas shows that the open waters in the Bothnian Bay and in the Swedish parts of the north-eastern Kattegat are not affected by eutrophication. The open parts of the Bothnian Bay are close to pristine, and the north-eastern Kattegat is influenced by Atlantic waters but open waters of all other basins are affected by eutrophication. Eleven coastal waters have been classified as not affected by eutrophication, while 161 areas are affected by eutrophication (HELCOM 2010).
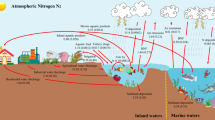
Nutrient loads to these coastal areas include point sources, such as wastewater treatment plants (WWTPs), and diffuse sources, such as agricultural and urban run-off, and atmospheric deposition from fossil fuel combustion in power plants and vehicles. Point sources generally dominate in urban areas, while diffuse sources dominate in agricultural areas.
Load estimates show that the main source of nutrients in both the OSPAR and HELCOM regions is diffuse, representing about 70 % of total nitrogen inputs in both cases (Table 2). Reductions in phosphorus discharges since 1985 exceed the OSPAR target of 50 %, but nitrogen discharges are still the main problem, especially those from agriculture. There were decreases in riverine and direct discharges of both nitrogen and phosphorus to the HELCOM area compared with the period 1995–2000, but these decreases are not yet reflected in reduced nutrient concentrations in the Baltic Sea—it can take decades for reduction measures to have positive effects since the rate of turnover of nutrients in soils and sediments means that they can be released to the marine environment for decades after loads from primary sources have been reduced.
Baseline and regime shifts (Duarte et al. 2008) further complicate these issues and can place management targets at risk.
The reductions in both the OSPAR and HELCOM regions confirm the effectiveness of measures taken to reduce nutrient loads; however, these have been largely due to point-source limitations. As point-source nutrient emissions become increasingly resolved through land-based treatment, the bulk of nutrient loading to coastal systems is due to diffuse sources (60–70 %; Table 2), which are both more difficult and more expensive to reduce. Management measures to support further reductions in nutrient loading must focus on diffuse sources, especially those from agriculture and atmospheric deposition. Furthermore, if the burden of reduction is placed on farmers, farming activity will be reduced due to costs, and the supply of agricultural produce will shift to other parts of the world where negative externalities of the activity are not penalized.
Water quality, shellfish, and European legislation
Bivalve aquaculture is organically extractive, which in practice means that the placement of shellfish such as mussels, oysters, and clams in the water inherently reduces the concentration of particulate organic matter (POM).
Figure 3 illustrates this for the Manila clam Venerupis philippinarum by means of an individual growth model applied in Samish Island, USA (Saurel et al. 2014).
For one individual, the equivalent annualized mass balance expressed in nitrogen gives a gross removal of 0.22 g N year−1 from phytoplankton and 0.89 g N year−1 from detritus. A proportion of that nitrogen (0.65 g N year−1) is returned as biodeposits from faeces and pseudofaeces, and dissolved nitrogen is also lost through excretion (0.09 g N year−1): this results in a net removal of 0.28 g N year−1 per animal.
A key aspect of the role of shellfish in nutrient control is the form in which bivalves remove nitrogenFootnote 1 and phosphorus from the water column. Nitrogen is defined as a causative factor of eutrophication rather than a symptom itself (Bricker et al. 2003), i.e. it is the phase shift from dissolved to particulate N that is responsible for decreased light availability and increased organic decomposition, and subsequent secondary symptoms (Fig. 2).
The direct introduction of detrital organics into a water body boosts the non-phytoplankton pool of organic matter available to bivalves. Although this fraction is not considered in the standard analysis of eutrophication, it effectively enters the eutrophication ‘chain’ (Fig. 2) at the point when primary symptoms develop into secondary symptoms—or, to use the OSPAR COMPP terminology, where direct effects lead to indirect effects.
Filter-feeding shellfish therefore act as a circuit-breaker between the two stages and curtail the development of hypoxia and the alterations in underwater light climate, which lead to losses in submerged aquatic vegetation such as eelgrass (Sagert et al. 2005; Dolch et al. 2013).
As point-source discharges are reduced, the increasing fraction of nutrient loading from diffuse sources (Table 2) makes source control more difficult and expensive (see Table 4) and supports the use of complementary strategies for top-down control of chlorophyll concentrations by shellfish—this provides both a source of income through bivalve harvest and an important ecosystem service.
In parallel, it has long been recognized (Eisma 1986) that the fraction rejected in bivalve feeding and digestion is consolidated into larger particles with higher settling velocities than the material filtered. The diameter of pseudofaecal and faecal aggregates may be 1–2 orders of magnitude greater than the ingested material (Giles and Pilditch 2004).
Although this is not factored into nutrient credit trading at present, it provides an ecosystem service through the increase in underwater light availability, which may promote benthic primary production. This must be offset against the potential negative effect of increased organic deposition below shellfish cultivation areas. Models can play an important management role in analysing these trade-offs by optimizing site selection and stocking density, both with respect to current velocity and water column depth.
Both detrital POM and dissolved nutrients are released in fed aquaculture of species such as salmon, bream, and bass, and here too shellfish can play an important mitigation role. For the most part, this is not a direct linkage; in cage aquaculture of finfish, a substantial proportion of the solid waste from uneaten and undigested feed settles rapidly to the bottom based on sulphide levels found in close proximity to the fish cages (e.g. Chang et al. 2014).
The suspended fraction, at the culture densities typical of European, US, or Canadian aquaculture, disperses very rapidly (Reid et al. 2009; Liutkus et al. 2012; Cranford et al. 2013; Lander et al. 2013). It is often difficult to detect changes in both suspended detrital POM and shellfish yield whether bivalves are grown in monoculture or IMTA, even when shellfish are cultivated very close to fish cages (e.g. Peharda et al. 2007; Sara et al. 2009; Lander et al. 2012, 2013), but also see Cheshuk et al. (2003), Navarrete-Mier et al. (2010), Parsons et al. (2002), and Taylor et al. (1992). All of this suggest that the nutrient flow dynamics are location specific.
In Western (or Watershed Scale) IMTA (WIMTA)Footnote 2, the extractive species contribute on a larger scale to ecosystem balance, exactly as we propose bivalve shellfish should with respect to land-based discharge. This requires additional processing of deposited organic matter, i.e. mineralization in the benthos and stimulation of pelagic primary production.
Bivalves may be more physically connected to the benthos, as is the case with natural or restored reefs, and subtidal bottom culture, or less so in the case of raft or longline culture.
In any of these situations, as long as bay-scale (or even waterbody scale, sensu WFD) connectivity can be established, it is legitimate to include managed shellfish growing areas as part of a nutrient credit trading scheme, promoting market mechanisms for agriculture and fed aquaculture to offset emissions.
There is an interesting difference in public perception with respect to reef restoration and shellfish aquaculture. Particularly in the USA and in Northern Europe, social opposition to aquaculture contrasts sharply with support for restoration, although from the point of view of direct nutrient removal, both perform an identical service.
Moreover, oyster reefs in the Chesapeake Bay have been shown to enhance denitrification (Kellogg et al. 2013), and a comparison across a number of aquaculture and reef sites suggests that reefs display a greater potential than aquaculture for denitrification (Kellogg et al. 2014).
Despite the evidence that organically extractive aquaculture can provide an important ecosystem service, both the WFD and the MSFD are woefully short on the subject—when mentioned at all, aquaculture is seen only as a pressure—the implication is that only finfish culture is considered.
Since the MSFD includes all marine waters in the European exclusive economic zone (EEZ) and has a specific descriptor entitled ‘commercial fish and shellfish’, it is astonishing that the relevant guidance document (Piet et al. 2010) does not once mention the word ‘aquaculture’. This is in sharp contrast to European policy for aquaculture growth: EU regulation 508/2014 (EU 2014) of the European Maritime and Fisheries Fund (EMFF) refers to aquaculture over one hundred times; Article 49 states:
Aquaculture contributes to growth and jobs in coastal and rural regions. Therefore, it is crucial that the EMFF is accessible to aquaculture enterprises, in particular small and medium-sized enterprises (SMEs), and that it contributes to bringing new aquaculture farmers into the business. In order to increase the competitiveness and economic performance of aquaculture activities, it is vital to stimulate innovation and entrepreneurship. Therefore, it should be possible for the EMFF to support innovative operations, the business development of aquaculture enterprises in general, including non–food and off–shore aquaculture, and complementary activities such as angling-tourism, environmental services related to aquaculture or educational activities.
The phrase ‘environmental services’ clearly provides a framework for inclusion of bivalve aquaculture in coastal nutrient management and stimulates a discussion of its role—this can only be achieved if directives such as the WFD and MSFD evolve appropriately.
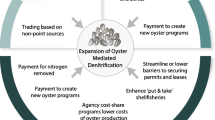
Credit trading for watershed-scale nitrogen control
In many parts of Europe and North America, implementation of point-source controls such as WWTP has reached the limits of removal technologies, with reductions in return on investment of continued treatment improvements. In addition, the majority of discharges to EU coastal waters are now from non-point sources (Table 2).
The concept of a nutrient credit trading program is to establish a market-based approach to help control nutrient discharges by providing economic incentives for achieving continued nutrient load reductions to meet water quality goals (Lindahl et al. 2005; Jones et al. 2010; Lal 2010). In a nutrient trading market, dischargers who reduce their nutrient discharges below allocated target levels can sell their surplus reductions or ‘credits’ to other dischargers in the same watershed. This approach allows dischargers who can reduce nutrients at low cost to sell credits to those facing higher-cost nutrient reduction options. Nutrient trading, therefore, could allow nutrient pollution sources such as WWTP and municipal stormwater programs to meet their pollution targets in a cost-effective manner and could create new revenue opportunities for farmers, entrepreneurs, and others who implement low-cost pollution reduction practices.
Nutrient credit trading at the watershed scale is already a reality in parts of the USA (Lal 2010; Branosky et al. 2011; Ferreira et al. 2011; STAC 2013). For example, the Connecticut Nitrogen Credit Exchange was created in 2002 to improve nutrient-related hypoxia conditions in Long Island Sound bottom waters. The Exchange provides an alternative compliance mechanism for 79 WWTPs throughout the state. During 2002–2009, the total value of credits bought and sold was $45.9 million, representing 15.5 million nitrogen credits exchanged.
The cost savings due to trading through the Exchange are estimated to be $300–$400 million over the alternative of implementing nitrogen removal projects at all 79 facilities (CT DEP 2010). At present, the Connecticut Exchange includes only point sources. There are four states in the Chesapeake Bay watershed that have also introduced nutrient trading programs to provide WWTP with flexible options for meeting and maintaining permitted nutrient load limits. In Virginia, the Nutrient Credit Exchange, established in 2005, is a voluntary association of 73 owners of 105 wastewater treatment facilities in the Chesapeake Bay watershed (http://www.theexchangeassociation.org/) that trade only within the state as is the case for the other three states that have trading programs (Maryland, Pennsylvania, West Virginia; Branosky et al. 2011). While there has been active and successful trade among point-source credits, inclusion of non-point sources in trading has been slow.
The U.S. Environmental Protection Agency’s (EPA) 2008 national water quality trading policy supports creation of non-point-source water quality trading credits through agricultural best management practices (BMPs), creation and restoration of wetlands, floodplains and wildlife and/or waterfowl habitat, and nutrient assimilation offsets that remove nutrients directly from the water, such as shellfish aquaculture (EPA 2008).
Lindahl et al. (2005) examined the potential to use mussel aquaculture in an ‘agro–aqua’ recycling system that conceptualized a nutrient trading system that would monetize nutrient management by substituting the cost of treatment plant upgrades with mussel farming as a cost-effective way to assure water quality improvements and provide seafood product and animal feed. Their comparison of costs of wastewater upgrades of 8.17–10.43 € kg−1 of nitrogen removed compared favourably to 12.13 € kg−1 of nitrogen removed in small ponds. By comparison, the calculated cost of 4.43 € kg−1 of nitrogen removed via mussel farming was one of the first indications that shellfish farming might be used as an offset for improvements to wastewater treatment technologies in a nutrient trading scenario (Lindahl et al. 2005).
One noted drawback was the potential negative environmental effects of ammonia build-up and biodeposition under the mussel operation. On balance, given that these negative effects could be limited by careful monitoring of the sites and adjustment of mussel densities, nutrient trading of shellfish aquaculture credits seemed a promising and cost-effective solution. More recently, Petersen et al. (2014) showed that costs related to establishment, maintenance and harvest of mussel production optimized for mitigation can be carried out at a lower cost compared to mussel production for (human) consumption (at 14.8 € kg−1 N removed), confirming the potential for water quality improvement through inclusion of shellfish in a nutrient trading program.
In an attempt to address the lack of non-point-source trading success in the Chesapeake Bay Nutrient Exchange in Virginia, Stephenson et al. (2010) evaluated and compared the cost, feasibility, and administrative risk of non-point-source offset alternatives; we focus here on the cost and feasibility results. Agricultural non-point offset costs ranged from 15 to 926 € kg−1 N offset, much higher than the Lindahl et al. (2005) and Petersen et al. (2014) estimates. Additionally, the amount of land required to offset point-source expansion is impractical, with a range across all agricultural offset options of 610–26,000 ha. Urban non-point offsets were equally costly with all categories ranging from 56 to 4074 € kg−1 N removed.
The urban BMPs had equally challenging area requirements of 1700–4100 ha required to offset the point-source expansion. By comparison, nutrient assimilation offset options (shellfish aquaculture, algal turf scrubber, restored floodplain wetlands) ranged in cost from 0 to 396 € kg−1, with oyster aquaculture ranging from 0 to 278 € kg−1 N of nitrogen removed. Stephenson et al. (2010) estimated that this would correspond to 22–62 million oysters while in 2008 there was a total harvest of 16 million oysters and suggest a considerable expansion of shellfish aquaculture would be needed to produce the required offsets for the point-source expansion.
However, if the annualized ecosystem service of shellfish in removing nitrogen is evaluated based on a whole population approach, as described in the next section, then about 21 kg N t−1 FW are removed (Bricker et al. 2015), which means the 2008 production might be at the low end of the offset needs (11–31 × 106 oysters), considering a 3-year oyster growth cycle for Crassostrea virginica.
The results of Stephenson et al. (2010) are included in a compilation of costs and comparison of modelled nitrogen removal efficiencies by shellfish farms (Rose et al. 2015). Although these are farm-scale estimates and thus do not account for potential interactions among neighbouring farms (e.g. lower production and lower removal rates due to food depletion), they compare favourably to reported nitrogen removal effectiveness of agricultural best management practices and stormwater control measures (Table 3). The approved agricultural BMP removal rates are in general lower than removal via stormwater control measures, suggesting that both stormwater control measures and shellfish aquaculture would be more desirable for non-point-source credit trading than agricultural credits.
Rose et al. (2015) additionally compared costs for nitrogen removal strategies for comparison to the cost of shellfish aquaculture (Table 4). The production of non-point-source credits by shellfish compares favourably to agricultural non-point nutrient management strategies and is more cost-effective than urban stormwater strategies and wastewater treatment upgrades. Inclusion in a nutrient trading program seems desirable based on removal efficiencies and cost-effectiveness in comparison with these other strategies.
It should be noted (Rose et al. 2015) “that shellfish can provide a number of other ecosystem services in addition to nitrogen removal, and nutrient management plans that include shellfish aquaculture will likely receive many of these ancillary benefits. Oyster reefs or mussel ropes may increase provision of habitat for other macrobenthic species by an order of magnitude (Šegvić-Bubić et al. 2011; Kellogg et al. 2013). Shellfish may also increase photic depth (Pollack et al. 2013), potentially helping restore submerged aquatic vegetation (SAV), which in turn provides oxygenation of bottom water and habitat for juvenile fish.” These services, together with the supply of goods that generate additional local income and employment and reduce dependency on imported seafood, leverage the positive externalities of shellfish aquaculture as to other methods of nutrient abatement.
These results support the inclusion of shellfish aquaculture as a non-point-source offset credit in nutrient trading programs. However, there are other considerations that must be taken into account prior to full acceptance into a credit exchange such as the negative environmental concerns noted by Lindahl et al. (2005). Additionally, as shown by Rose et al. (2015) and noted by others (e.g. STAC 2013; Kellogg et al. 2014) removal efficiencies are highly variable from site to site and year to year; in one catastrophic event, it is possible to destroy removal capabilities at a site. The uncertainty in performance must be acknowledged and addressed before shellfish aquaculture can be fully integrated into a nutrient credit trading program.
Role of shellfish in eutrophication control
Mathematical models have made it possible to analyse the role of shellfish in reducing symptoms of eutrophication, not just by simulating the changes in concentration of key indicators such as chlorophyll or dissolved oxygen, but by evaluating mass balances for such indicators, and the corresponding balance for nitrogen and phosphorus (e.g. Nobre et al. 2010; Nunes et al. 2011).
It is common to determine the role (for instance in nitrogen removal) of shellfish, whether cultivated, or in restored reefs, by considering the harvested or collected biomass (STAC 2013) and applying a conversion factor (usually about 1 %; e.g. Higgins et al. 2011) for the nitrogen component—in other words, to consider only the fraction of shellfish physically taken out of the water. The difficulty in this approach is obvious when we consider an oyster or mussel reef, particularly in a pristine natural system with little or no human intervention, where the gathering of shellfish is either non-existent or insignificant at the scale of the reef.
Although the shellfish that are cultivated, or used in reef restoration, are in the water, the eutrophication symptoms are not or are greatly reduced. This reduction promotes increased water clarity, and the effect of shellfish, while in the water, is to short-circuit the eutrophication process, greatly reducing secondary symptoms of eutrophication such as hypoxia (through lack of detrital organic supply) and loss of submerged aquatic vegetation (through improved underwater light climate). In other words, nutrient bioextraction by means of shellfish aquaculture or reef restoration is not the physical removal of nutrients from an aquatic ecosystem, but the nutrient bioextraction from a particular component of that ecosystem, in order to modify the processes or rates within that ecosystem.
Whereas for the shellfish harvest approach there is no need to apply a complex ecosystem- or farm-scale model, evaluation of the true eutrophication-related ecosystem service provided by shellfish aquaculture or reefs requires a methodology that accounts for the full sources and sinks of carbon, nitrogen, and phosphorus, related to bivalve physiology.
If a particular species of shellfish is cultivated for 3 years prior to harvest, as is the case, e.g. with mussels in Ireland (Nunes et al. 2011), oysters in France (Raillard and Ménesguen 1994), or clams in the NW USA (Saurel et al. 2014), the annualized eutrophication-related ecosystem service integrates: (1) the Year 3 cohort, much of which will be physically removed (harvested); (2) the Year 2 cohort, which will be harvestable only the following year; and (3) the Year 1 cohort, which will take a further 2 years to reach harvestable size.
An identical rationale is applicable to a reef: in the case where an adult animal takes 3 years to grow, bivalves of different sizes coexist—they all provide an ecosystem service of nutrient management through drawdown of phytoplankton and organic detritus, even though a significant proportion of bivalves are not of harvestable size, and those that are may not be harvested at all.
This holistic approach was applied in the REServ project for Long Island Sound, USA, (Fig. 4; Table 5), using an ecosystem model that includes two cultivated bivalve species: the Eastern oyster Crassostrea virginica and the quahog Mercenaria mercenaria (Bricker et al. 2015).
Figure 4 illustrates the chlorophyll drawdown provided by the shellfish under the standard model aquaculture scenario—a relatively low cultivation density; this top-down control results in an 8–12 % reduction in peak concentrations for the western part of the Sound. At the potential scenario of aquaculture development, the percentile 90 value for chlorophyll decreases by 16–21 % in the western Long Island Sound (Bricker et al. 2015).
Table 5 shows results only for the oysters, which for the standard aquaculture scenario provide a net nitrogen removal of 656 t year−1; using a standard population equivalent (PEQ) of 3.3 kg N PEQ−1 year−1 (Ferreira et al. 2007), this corresponds to about 199,000 PEQ year−1.
An equivalent calculation for quahogs provides an aggregate estimate for both species of over 600,000 PEQ year−1 for the standard model and over double that for the potential development scenario.
The financial value of this service can be calculated in several ways (Table 4 and explanatory text). For a trading price of $13 (or 10.8 €) kg−1, referred by Piehler and Smyth (2011) for the North Carolina nutrient offsets program, the aggregate ecosystem service for both shellfish species in Long Island Sound is 22.4 million €. This is about 7 % higher than the estimate based on unit costs reported by Lindahl et al. (2005) for land-based treatment, i.e. both methods provide very similar estimates.
Oysters and quahogs are relatively high value products: for the Eastern oyster (farmgate price of $0.40 per oyster), the services:goods ratio for nutrient removal is about 6 %.
However, two aspects need to be considered: (1) this ratio will be substantially higher for mussels or Manila clams, because product values are substantially lower; (2) the ratio will also increase (see Table 4) if the unit costs are estimated for diffuse sources.
The type of ecological model applied in REServ provides results on a system-wide scale, taking into account multiple culture cycles, spatial and temporal interactions, hydrodynamics, and the appropriate biogeochemical components. Simulations are run for periods of a decade or more, and the current state of the art integrates watershed nutrient loading models such as SWAT into a powerful multi-model analytical framework (Ferreira et al. 2014b), where the effects of changing nutrient drivers in the watershed (i.e. source control measures) can be tested.
Such models are valuable for management, but they are lengthy and costly to develop, calibrate, and validate. As a consequence, the estimation of regional potential for incorporation of bivalve shellfish for nutrient credit trading in Europe has been developed using the simpler methodology applied by Saurel et al. (2014) to Puget Sound, USA.
Rather than base our calculations on the harvested biomass for each major species reported in Table 1, which underestimates the ecosystem service provided, as discussed above, we opted to apply the FARM screening model (e.g. Silva et al. 2011 and references therein) to different species of bivalve shellfish.
A full set of example farms, representative of European shellfish culture, was used by Ferreira et al. (2009) to validate the FARM model for a range of bivalve species cultivated in Europe. The environmental driver and culture practice data collated for that work have been used to run FARM in order to obtain the results that make up the final part of this review, taking advantage of numerous improvements made to the model over the past 5 years. In some cases, new farms were used for calculations—this is particularly the case for Mediterranean mussel (Western Portugal) and Good Clam (south-eastern Portugal).
Model outputs were annualized both for production and for net nitrogen removal (Table 6). The ratio for each species reflects typical culture practice with respect to seeding, cultivation period, stocking density, harvest weight, and culture placement (e.g. rafts, longlines, and trestles).
The annual European bivalve shellfish production of over 700,000 metric tons is estimated to generate a nitrogen removal of 46,800 t year−1, equivalent to 14 × 106 PEQ, and a minimum value of 507 × 106 €, based on 10.8 € kg−1 N (Piehler and Smyth 2011). Table 6 provides a breakdown by country and by species and suggests that Spain is the largest potential market for credit trading, with a market value of 170 million euros, about one-third of the overall European market.
Care must be taken in using these figures, not least because the origin of the nutrients and algae is germane to this analysis. For instance, Spanish production of M. galloprovincialis takes place in the Galician Rias, where the food sources are primarily phytoplankton advected from offshore, and autochthonous primary production driven by nutrient-rich upwelled water.
For shellfish growers to participate in watershed-level nitrogen trading schemes, it is important that the service provided by the filter-feeders is offsetting land-based inputs, rather than natural contributions from offshore.
Future directions
The growth of the aquaculture sector in Europe is currently a hotly debated theme, as it is in the USA and elsewhere. An increase in finfish culture is important for food security, employment, and trade balance; shellfish aquaculture also contributes to all of these, but supplies a number of additional services, including eutrophication management.
We certainly do not advocate shellfish as a replacement for land-based nutrient control, mainly because of moral hazard, and but also because of the areal coverage and stocking densities that would be required. Furthermore, the financial viability of shellfish culture from a product perspective is severely constrained in various parts of Europe.
In some Northern European countries, it is difficult to make a business case for cultivation of lower value shellfish species due to labour costs.
In Southern Europe, Mediterranean countries are limited in their possibilities for bivalve-related credit trading for two reasons: (1) oligotrophic waters are a severe limitation to expansion of shellfish aquaculture, except close to nutrient-enriched coastal areas, where competition for space with, e.g. the tourism industry, is a major challenge; (2) since food supply is limited for marine trophic webs, the partition of this resource with cultivated organic extractive species is an important issue for ecosystem-based management.
Despite these various caveats, there is certainly room for market-based policies to stimulate shellfish aquaculture in Europe, taking advantage of watershed-scale nutrient management services.
Shellfish growers should be considered as stakeholders in such integrated catchment management plans, with a particular focus on offsetting emissions from non-point agricultural sources, and point sources from finfish aquaculture.
In both cases, since the top-down control of shellfish is exerted not on the emissions themselves, but indirectly on the transformative effect of those emissions on production of organic matter, site selection can be based on appropriate criteria for production, ecological, and social carrying capacity (Inglis et al. 2000), as long as connectivity exists at the waterbody scale.
Other issues that must be considered in designing a successful trading program that includes shellfish aquaculture are listed in Table 7 (adapted from STAC 2013; Walker and Selman 2014). These are mainly policy related and include governance aspects and costs. Models such as FARM can be certified after appropriate validation to help reduce the cost burden on the shellfish industry with respect to certification of removal efficiencies and to increase trust within the sector by illustrating and quantifying the positive externalities of the activity.
The focus on diffuse sources from different types of farming (agri/aqua) presents three main advantages: (1) point-source discharges from land are associated with microbial risks. This occurs also with agricultural emissions, but to a much lesser extent, which increases social acceptance of waterbody offsets; (2) the valuation of credits is much greater, due to the economic and social costs of control measures—whereas point-source control is widely supported, reductions in fertilizer application, and instruments such as the Netherlands nitrogen and phosphorus levy (MINAS, RIVM 2002) have potential consequences for the competitiveness and risk of agricultural activities, with repercussions on productivity and employment—these aspects lead to substantial controversy in the application of land-based controls and to the economic migration of farmers to parts of Europe where such instruments do not exist; (3) social and cultural values may be better preserved in remote areas, where farming is under pressure due to economic and social (generational) challenges; further emission-based controls may be the tipping point for the collapse of such areas—an agri–aqua economy may be a solution that will allow economic growth and avoid a desertification of European remote rural areas.
We face a dual challenge in the Europe of the twenty-first century: (1) to preserve and enhance the quality and sustainability of food production, which builds on two centuries of successive improvement; (2) to remain competitive in the context of world markets, reducing the trade deficit and affording generations to come the perspective of stable employment.
Integrated nutrient management can help address both challenges, and shellfish aquaculture should be a full partner in that process.
Notes
This review focuses on nitrogen since it is generally the limiting nutrient for primary production in estuarine and coastal systems.
Defined as cultivation of different trophic levels at the system scale (e.g. embayment, fjord, estuary), promoting ecosystem services at that scale, rather than direct benefits from cultivation at close proximity (such as seen in ponds in Asia).
References
Bacher C, Duarte P, Ferreira JG, Heral M, Raillard O (1998) Assessment and comparison of the Marennes-Oleron Bay (France) and Carlingford Lough (Ireland) carrying capacity with ecosystem models. Aquat Ecol 31(4):379–394
Bayne BL, Newell RC (1983) Physiological energetics of marine molluscs. Academic Press, Mollusca, pp 407–515
Bayne BL, Iglesias JIP, Hawkins AJS, Navarro E, Heral M, Deslous-Paoli JM (1993) Feeding behaviour of the mussel Mytilus edulis. Responses to variations in quantity and organic content of the seston. J Mar Biol Ass UK 73:813–829
Borja A, Basset A, Bricker S, Dauvin J-C, Elliott M, Harrison T, Marques JC, Weisberg S, West R (2012) Classifying ecological quality and integrity of estuaries. Chapter 1.9 within the ‘Treatise on Estuarine and Coastal Science’ (Editors-in-Chief, Eric Wolanski & Donald McLusky), Elsevier. doi: 00109 is 10.1016/B978-0-12-374711-2.00109-1
Borja A, Bricker SB, Dauer DM, Demetriades NT, Ferreira João G, Forbes AT, Hutchings P, Jia i X, Kenchington R, Marques JC, Zhu C (2008) Overview of integrative tools and methods in assessing ecological integrity in estuarine and coastal systems worldwide. Mar Pollut Bull 56:1519–1537
Branosky E, Jones C, Selman M (2011) Comparison tables of state nutrient trading programs in the Chesapeake Bay Watershed. Washington, DC: World Resources Institute. http://www.wri.org/publication/comparison-tables-of-state-chesapeake-baynutrient-trading-programs
Breetz HL, Fisher-Vanden K, Jacobs H, Schary C (2005) Trust and communication: mechanisms for increasing farmers' participation in water quality trading. Land Econ 81(2):170–190
Bricker, SB, Ferreira JG, Zhu CB, Rose J, Galimany E, Wikfors G, Saurel C, Landeck-Miller R, Wands J, Trowbridge P, Grizzle R, Wellman K, Rheault R, Steinberg J, Jacob A, Davenport E, Tedesco M, Ayvazian S (2015) REServ: an ecosystem services assessment using bioextraction technologies for removal of nitrogen and other substances in long Island Sound and the Great Bay/Piscataqua Region. NOAA/EPA, (in press)
Bricker SB, Ferreira JG, Simas T (2003) An integrated methodology for assessment of estuarine trophic status. Ecol Model 169(1):39–60
Bricker S, Longstaff B, Dennison W, Jones A, Boicourt K, Wicks C, Woerner J (2007). Effects of nutrient enrichment in the nation’s estuaries: a decade of change, national estuarine eutrophication assessment update. NOAA coastal ocean program decision analysis series No. 26. National Centers for Coastal Ocean Science, Silver Spring, MD. 322 pp http://ccma.nos.noaa.gov/publications/eutroupdate/
Chang B, Page F, Losier R, McCurdy E (2014) Organic enrichment at salmon farms in the Bay of Fundy, Canada: DEPOMOD predictions versus observed sediment sulfide concentrations. Aquac Environ Interact 5:185–208
Cheshuk BW, Purser GJ, Quintana R (2003) Integrated open-water mussel (Mytilus planulatus) and Atlantic salmon (Salmo salar) culture in Tasmania, Australia. Aquaculture 218:357–378
Clausen IB, Riisgård HU (1996) Growth, filtration and respiration in the mussel Mytilus edulis: no regulation of the filter-pump to nutritional needs. Mar Ecol Prog Ser 141:37–45
Connecticut Department of Environmental Protection (CT DEP) (2010) An incentive-based water quality trading program. The connecticut Department of Environmental Protection, Bureau of water protection and land reuse, Hartford, CT. 10 pp
Cranford PJ, Reid GK, Robinson SMC (2013) Open water integrated multi-trophic aquaculture: constraints on the effectiveness of mussels as an organic extractive component. Aquac Environ Interact 4:163–173
Dame RF, Prins TC (1998) Bivalve carrying capacity in coastal ecosystems. Aquat Ecol 31:409–421
DEFRA (2014) MAGIC GIS. http://magic.defra.gov.uk/MagicMap.aspx
Dolch T, Buschbaum C, Reise K (2013) Persisting intertidal seagrass beds in the northern Wadden Sea since the 1930s. J Sea Res 82:134–141
Dral ADG (1967) The movements of the latero-frontal cilia and the mechanism of particle retention in the mussel. Neth J Sea Res 3(3):391–422
Duarte CM, Conley DJ, Carstensen J, Sánchez-Camacho M (2008) Return to Neverland: shifting baselines affect eutrophication restoration targets. Estuar Coasts 32(1):29–36
Dutch Fish Product Board (DFPB) (2008) Fish facts mussels (Sept. 2008). http://www.pvis.nl/fileadmin/user_upload/pvis/Documenten/Verantwoorde_vis/Fish_facts_mussels.pdf
Eisma D (1986) Flocculation and de-flocculation of suspended matter in estuaries. Neth J Sea Res 20(2–3):183–199
European Union (2014) Regulation (EU) No 508/2014 of the European Parliament on the European maritime and fisheries fund. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2014.149.01.0001.01.ENG
Eurostat (2014) Total fishery production—total all fishing areas. http://ec.europa.eu/eurostat
Eyre JWH (1925) The Oyster and the public health. Public Health 38:6–27
FAO (1999) Regional review on trends in aquaculture development—Europe. Food and Agricultural Organization of the United Nations, Fish Culture Research Institute, Szarvas, Hungary
FAO (2014) The State of world fisheries and aquaculture (SOFIA). FAO, Rome
Ferreira JG, Hawkins AJS, Monteiro P, Moore H, Service M, Pascoe PL, Ramos A, Sequeira A (2008) Integrated assessment of ecosystem-scale carrying capacity in shellfish growing areas. Aquaculture 275:138–151
Ferreira JG, Sequeira A, Hawkins AJS, Newton A, Nickell T, Pastres R, Forte J, Bodoy A, Bricker SB (2009) Analysis of coastal and offshore aquaculture: application of the FARM model to multiple systems and shellfish species. Aquaculture 292:129–138
Ferreira JG, Andersen JH, Borja A, Bricker SB, Camp J, Cardoso da Silva M, Garcés E, Heiskanen A-S, Christoph Humborg L, Ignatiades C, Lancelot A, Menesguen P, Tett N, Hoepffner U Claussen (2011) Indicators of human-induced eutrophication to assess the environmental status within the European marine strategy framework directive. Estuar Coast Shelf Sci 93:117–131
Ferreira JG, Saurel C, Ferreira JM (2012) Cultivation of gilthead bream in monoculture and integrated multi-trophic aquaculture. Analysis of production and environmental effects by means of the FARM model. Aquaculture 358–359(2012):23–34
Ferreira JG, Falconer L, Kittiwanich J, Ross L, Saurel C, Wellman K, Zhu CB, Suvanachai P (2014a) Analysis of production and environmental effects of Nile tilapia and white shrimp culture in Thailand. Aquaculture. doi:10.1016/j.aquaculture.2014.08.042
Ferreira JG, Saurel C, Lencarte Silva JD, Nunes JP, Vazquez F (2014b) Modelling of interactions between inshore and offshore aquaculture. Aquaculture 426:154–164
Filgueira R, Guyondet T, Comeau LA, Grant J (2014) A fully-spatial ecosystem-DEB model of oyster (Crassostrea virginica) carrying capacity in the Richibucto Estuary, Eastern Canada. J Mar Sys 136:42–54
Gangnery A, Bacher C, Buestel D (2004) Modelling oyster population dynamics in a Mediterranean coastal lagoon (Thau, France): sensitivity of marketable production to environmental conditions. Aquaculture 230:323–347
Gerritsen J, Holland AF, Irvine DE (1994) Suspension-feeding bivalves and the fate of primary production: an estuarine model applied to Chesapeake Bay. Estuaries 17(2):403–416
Giles H, Pilditch CA (2004) Effects of diet on sinking rates and erosion thresholds of mussel Perna canaliculus biodeposits. Mar Ecol Prog Ser 282:205–219
Grant J, Bacher C (1998) Comparative models of mussel bioenergetics and their validation at field culture sites. J Exp Mar Biol Ecol 219(1–2):21–44
Grant J, Curran KJ, Guyondet TL, Tita G, Bacher C, Koutitonsky V, Dowd M (2007) A box model of carrying capacity for suspended mussel aquaculture in Lagune de la Grande-Entrée, Iles-de-la-Madeleine. Québec Ecol Mod 200:193–206
Hawkins AJS, Duarte P, Fang JG, Pascoe PL, Zhang JH, Zhang XL, Zhu MY (2002) A functional model of responsive suspension-feeding and growth in bivalve shellfish, configured and validated for the scallop Chlamys farreri during culture in China. J Exp Mar Biol Ecol 281(1–2):13–40
Higgins CB, Stephenson K, Brown BL (2011) Nutrient bioassimilation capacity of aquacultured Oysters: quantification of an ecosystem service. J Environ Qual 40(1):271–277
Houle J, Roseen R, Ballestero T, Puls T, Sherrard J (2013) A comparison of maintenance cost, labor demands, and system performance for LID and conventional stormwater management. J Environ Eng 139:932–938
Inglis GJ, Hayden BJ, Ross AH (2000) An overview of factors affecting the carrying capacity of coastal embayments for mussel culture. National Institute of Water & Atmospheric Research (NIWA) Client Report CHC00/69, Christchurch
Jones C, Branosky E, Selman M, Perez M (2010) How nutrient trading could help restore the Chesapeake Bay. Washington, DC: World Resources Institute. http://www.wri.org/publication/how-nutrient-trading-could-help-restore-chesapeake-bay
Jørgensen CB, Larsen PS, Riisgård HU (1990) Effects of temperature on the mussel pump. Mar Ecol Prog Ser 64:89–97
Kellogg ML, Cornwell JC, Owens MS, Paynter KT (2013) Denitrification and nutrient assimilation on a restored oyster reef. Mar Ecol Prog Ser 480:1–19
Kellogg ML, Smyth AR, Luckenbach MW, Carmichael RH, Brown BL, Cornwell JC, Piehler MF, Owens MS, Dalrymple DJ, Higgins CB (2014) Use of oysters to mitigate eutrophication in coastal waters. Estuar Coast Shelf Sci 151:156–168
Lal H (2010) Nutrient credit trading- a market-based approach for improving water quality. In: Delgado JA, Follett RF (eds) Advances in nitrogen management for water quality. Soil and Water Conservation Society, Ankeny, IA, pp 344–361
Lander TR, Robinson SMC, MacDonald BA, Martini JD (2012) Enhanced growth rates and condition index of blue mussels (Mytilus edulis) held at Integrated Multitrophic aquaculture sites in the Bay of Fundy. J Shellfish Res 31:997–1007
Lander TR, Robinson SMC, MacDonald BA, Martin JD (2013) Characterization of the suspended organic particles released from salmon farms and their potential as a food supply for the suspension feeder, Mytilus edulis in integrated multi-trophic aquaculture (IMTA) systems. Aquaculture 406:160–171
Lindahl O, Hart R, Hernroth B, Kollberg S, Loo L, Olrog L, Rehnstam-Holm A (2005) Improving marine water quality by mussel farming: a profitable solution for Swedish Society. Ambio 34(2):131–138
Liutkus M, Robinson S, MacDonald B, Reid G (2012) Quantifying the effects of diet and mussel size on the biophysical properties of the blue mussel, Mytilus Spp., feces egested under simulated imta conditions. J Shellfish Res 31:69–77
Loosanoff VL, Tommers FD (1948) Effect of suspended silt and other substances on rate of feeding of oysters. Science 107:69–70
Loveland RE, Chu DSK (1969) Oxygen consumption and water movement in Mercenaria mercenaria. Comp Biochem Physiol 29(1):173–184
Mariola MJ (2012) Farmers, trust, and the market solution to water pollution: the role of social embeddedness in water quality trading. J Rural Stud 28:577–589
Navarrete-Mier F, Sanz-Lazaro C, Marin A (2010) Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 306:101–107
Nobre AM, Ferreira JG, Nunes JP, Yan X, Bricker S, Corner R, Groom S, Gu H, Hawkins A, Hutson R, Lan D, Lencart e Silva JD, Pascoe P, Telfer T, Zhang X, Zhu M (2010) Assessment of coastal management options by means of multilayered ecosystem models. Estuar Coast Shelf Sci 87:43–62
Nunes JP, Ferreira JG, Bricker SB, O’Loan B, Dabrowski T, Dallaghan B, Hawkins AJS, O’Connor B, O’Carroll T (2011) Towards an ecosystem approach to aquaculture: assessment of sustainable shellfish cultivation at different scales of space, time and complexity. Aquaculture 315:369–383
Orton JH (1928) On rhythmic periods in shell-growth in O. edulis with a note on fattening. J Mar Biol Assoc 15:365–427
OSPAR, 2010. C.Moffat, R. Emmerson, A. Weiss, C. Symon, L. Dicks (Eds.) Quality status report 2010. OSPAR Commission, London
OSPAR Commission (2000) Quality status report 2000. OSPAR Commission, London. 108 + vii pp
Parsons GJ, Shumway SE, Kuenstner S, Gryska A (2002) Polyculture of sea scallops (Placopecten magellanicus) suspended from salmon cages. Aquacult Int 10:65–77
Peharda M, Zupan I, Bavcevic L, Frankic A, Klanjscek T (2007) Growth and condition index of mussel Mytilus galloprovincialis in experimental integrated aquaculture. Aquac Res 38:1714–1720
Petersen JK, Hasler B, Timmermann K, Nielsen P, Tørring DB, Larsen MM, Holmer M (2014) Mussels as a tool for mitigation of nutrients in the marine environment. Mar Pollut Bull 82:137–143
Piehler MF, Smyth AR (2011) Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2(1):1–16
Piet G, Albella AJ, Aro E, Farrugio H, Lleonart J, Lordan C, Mesnil B, Petrakis G, Pusch C, Radu G, Rätz HJ (2010) Marine strategy framework directive—task group 3 report—commercially exploited fish and shellfish. JRC57750
Pollack JB, Yoskowitz D, Kim H-C, Montagna PA (2013) Role and value of nitrogen regulation provided by oysters (Crassostrea virginica) in the Mission-Aransas Estuary, Texas, USA. PLoS One 8:e65314
Raillard O, Ménesguen A (1994) An ecosystem box model for estimating the carrying capacity of a macrotidal shellfish system. Mar Ecol Prog Ser 115:117–130
Reid GK, Liutkus M, Robinson SMC, Chopin TR, Blair T et al (2009) A review of the biophysical properties of salmonid faeces: implications for aquaculture waste dispersal models and integrated multi-trophic aquaculture. Aquac Res 40:257–273
RIVM (2002) MINAS and Environment. Balance and Outlook, RIVM, Bilthoven, The Netherlands
Rose JM, Bricker SB, Ferreira JG (2015) Comparative analysis of modeled nitrogen removal by shellfish farms. Mar Pollut Bull 91(1):185–190
Sagert S, Krause Jensen D, Henriksen P, Rieling T, Schubert H (2005) Integrated ecological assessment of Danish Baltic Sea coastal areas by means of phytoplankton and macrophytobenthos. Estuar Coast Shelf Sci 63(1–2):109–118
Sara G, Zenone A, Tomasello A (2009) Growth of Mytilus galloprovincialis (mollusca, bivalvia) close to fish farms: a case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 636:129–136
Saurel C, Ferreira JG, Cheney D, Suhrbier A, Dewey B, Davis J, Cordell J (2014) Ecosystem goods and services from Manila clam culture in puget sound: a modelling analysis. Aquac Environ Interac 5:255–270
Šegvić-Bubić T, Grubišić L, Karaman N, Ticina V, Mišlov JK, Katavić I (2011) Damages on mussel farms potentially caused by fish predation—self service on the ropes? Aquaculture 319:497–504
Shumway SE, Cucci TL (1987) The effects of the toxic dinoflagellate Protogonyaulax tamarensis on the feeding and behaviour of bivalve molluscs. Aquat Toxicol 10(1):9–27
Silva C, Ferreira JG, Bricker SB, DelValls TA, Martín-Díaz ML, Yañez E (2011) Site selection for shellfish aquaculture by means of GIS and farm-scale models, with an emphasis on data-poor environments. Aquaculture 318:444–457
STAC (Chesapeake Bay Program Scientific and Technical Advisory Committee) (2013) Evaluation of the use of shellfish as a method of nutrient reduction in the Chesapeake Bay. STAC Publ. #13-005, Edgewater, MD. 65 pp
Stephenson K, Aultman S, Metcalfe T, Miller A (2010) An evaluation of nutrient nonpoint offset trading in Virginia: a role for agricultural nonpoint sources? Water Resour Res 46:W04519. doi:10.1029/2009WR008228
Taylor BE, Jamieson G, Carefoot TH (1992) Mussel culture in British Columbia: the influence of salmon farms on growth of Mytilus edulis. Aquaculture 108:51–66
Tenore KR, Dunstan WM (1973) Comparison of feeding and biodeposition of three bivalves at different food levels. Mar Biol 21:190–195
U.S. Environmental Protection Agency (2008) EPA water quality trading evaluation, final report. Washington, D.C
Vincenzi S, Caramori G, Rossi R, De Leo GA (2006) Estimating clam yield potential in the Sacca di Goro lagoon (Italy) by using a two-part conditional model. Aquaculture 261:1281–1291
Walker S, Selman M (2014) Addressing risk and uncertainty in water quality trading markets. Washington, DC: World Resources Institute. http://www.wri.org/sites/default/files/wri_issuebrief_uncertainty_3-9_final.pdf
Wong I (2014) GIS mapping on mussel culture. http://hz.nl/Documents/Delta%20Academy%20Applied%20Research%20Centre/IW_FinalReport.pdf
Acknowledgments
The authors acknowledge support from the EU IDREEM (FP7) and AQUASPACE (H2020) projects and from the EPA/NOAA RESERV project. We are additionally grateful to Shawn Robinson and Peter Cranford, DFO Canada, for text suggestions, and to all our colleagues on the various project teams mentioned above.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Elena Mente & Aad Smaal / European Aquaculture Development since 1993: The benefits of aquaculture to Europe and the perspectives of European aquaculture production.
Rights and permissions
About this article
Cite this article
Ferreira, J.G., Bricker, S.B. Goods and services of extensive aquaculture: shellfish culture and nutrient trading. Aquacult Int 24, 803–825 (2016). https://doi.org/10.1007/s10499-015-9949-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-015-9949-9