Abstract
The objective of this study was to determine the effects of prawn juveniles and organic carbon source on the water quality and production performance of catfish. The experiment consisted of three treatments in triplicates, i.e. catfish monoculture as the control (treatment A); catfish–prawn co-culture at a prawn density of 20 prawn/m2 (treatment B) and catfish–prawn co-culture at a prawn density of 40 prawn/m2 (treatment C). Catfish was reared in a bamboo cage inside experimental concrete ponds at an initial density of 100/m2. Commercial feed pellets were only offered to the fish. In treatments B and C, organic carbon was added at an estimated C/N ratio of 10 to stimulate biofloc growth as the food source for the prawn juveniles. There was no significant difference in catfish growth among treatments. Dissolved inorganic nitrogen concentrations in B and C were generally lower than in the control. Although significantly affected by the initial stocking density, prawn growth was comparable to that fed with formulated feed. Catfish co-culture with prawn with C organic addition resulted in significantly higher feed nitrogen utilization. Overall results suggest that catfish–prawn co-culture with C organic addition may improve total food conversion ratio, reduce nitrogen waste and generate additional income for catfish farmers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In an intensive aquaculture system, fish stocking density is high and consequently, high levels of external feed are added to the system. As fish or shrimp only retain 16–41 % of feed nitrogen (Avmimelech 1999; Yi et al. 2003; Hari et al. 2004), and most of aquaculture feed contains more than 25 % protein, high fish stocking density and corresponding high levels of added feed result in high N loading into the water. Nitrogenous waste in an aquaculture system is mostly in the form of ammonia, which is the final result of protein metabolism and decomposition products of faecal matter and unconsumed feed (Crab et al. 2007). Because ammonia is toxic for most aquatic animals, the level of this compound in an aquaculture medium should be maintained at <0.01 mg/L (Wedemeyer 1996) by regular water exchange or by applying water treatment system.
Bioflocs technology is an aquaculture practice based on natural processes occurring in an aquatic ecosystem. In this system, the growth of heterotrophic microbial biomass that assimilates ammonia–nitrogen as their primary nutrient is facilitated by the addition of external organic carbon (Avnimelech 1999; De Schryver and Verstraete 2009; Azim and Little 2008). This microbial biomass may further aggregate with other microorganisms including phytoplankton and zooplankton forming what has been called bioflocs. Bioflocs can be consumed by the cultured organisms themselves or other detritus feeder aquatic organisms (Hari et al. 2004; Azim and Little 2008), or be harvested and processed as a feed ingredient (Kuhn et al. 2010; Bauer et al. 2012). The assimilation of ammonia by the bacteria and the consumption of this microbial biomass by the cultured organisms in this biofloc system may therefore result in higher nitrogen utilization efficiency and at the same time may also maintain the concentration of toxic ammonia at low level.
As most of aquaculture inputs become limited, aquaculture development has been directed towards a culture system that is efficient in nutrient use with less impact on environment (Diana et al. 2013). This includes developing aquaculture feeds with higher digestibility and nutrient-uptake efficiency, an integrated multitrophic aquaculture, as well as better water and effluent treatment technologies (Diana et al. 2013; Verdegem 2013). The application of various aquaculture species in an integrated multitrophic aquaculture and polyculture may improve the nutrient utilization as each species may play different roles in the food chain (Diana et al. 2013). Effluent water treatment technology has been developed not only to maintain good water quality to support the welfare of the cultured species but also to make efficient use of water, to reduce water pollution and thereby the environmental impact of aquaculture, as well as to recycle the wasted nutrient to improve overall feed nutrient utilization (Verdegem 2013).
The present study investigated the effect of catfish and prawn co-culture with the addition of an organic C source that stimulates the growth of microbial biomass as the food source for the prawn. Water quality, production performance of the system and feed nitrogen utilization efficiency were observed. Additionally, economic comparison between treatments was also performed to evaluate the economical viability of the system.
Materials and methods
Experimental setup
The experiment was performed in the Freshwater Aquaculture Research Station, Sukabumi, and in Bogor Agricultural University, West Java, Indonesia. Catfish Clarias batracus (75 days post-hatching) and prawn Macrobrachium rosenbergii (105 dph) at average initial body weight of 10.29 ± 0.29 and 1.07 ± 0.13 g, respectively, were used as the experimental animals. The study was conducted with three treatments in triplicates, i.e. catfish monoculture as the control (treatment A); catfish–prawn co-culture at a prawn density of 20 prawn/m2 (treatment B) and catfish–prawn co-culture at a prawn density of 40 prawn/m2 (treatment C), and lasted 49 days.
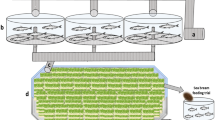
Nine units of concrete tanks with a dimension of 5 × 3 × 1.5 m3, each with a bamboo cage (1.5 × 1 × 1 m3), were used for the experiment (Fig. 1). Catfish juveniles were placed in the bamboo cages, whereas the prawns were distributed in the water outside the cages. The cages were placed 20 cm above the tank bottom and were supported by bamboo at each of their four sides. Prior to experimentation, the ponds were cleaned and filled with 12 m3 of water. The water was subsequently disinfected using calcium hypochlorite at a concentration of 30 mg/L followed by strong aeration for neutralization. Water addition was applied during the culture period only to replace losses due to evaporation.
Feed containing 32 % of crude protein was offered only to the catfish, twice a day, at a level range of 5–3 % of the fish biomass. The feeding level was regularly adjusted according to fish biomass estimations following weekly sampling. Molasses was used as the organic carbon source in the co-culture treatments (B and C) at a dose of 72.5 % of the total feed given to the catfish (De Schryver et al. 2008). To promote floc formation, 1 mg/L of sodium silicate was daily added to the tanks with biofloc during the first week of culture period.
Sampling was performed weekly to determine fish feeding level and growth, whereas survival was calculated based on daily monitoring of fish mortality. Total bacterial count was weekly measured in pond water samples using tryptic soy agar (TSA) medium with 24-h incubation at 37 °C. The water quality parameters, such as dissolved oxygen (DO), pH and temperature, were measured in situ using hand-held pH meter, and DO meter. Total ammonia nitrogen, nitrite-N, nitrate-N, alkalinity, carbon dioxide, biological oxygen demand (BOD), volatile suspended solids (VSS) and total suspended solids (TSS) concentrations were measured using procedures as described in APHA (2005). Protein content of fish and shrimp was measured according to Kjeldahl method (Takeuchi 1988).
Specific growth rate, survival and feed efficiency were calculated according to Huisman (1987). Food conversion ratio (FCR) was calculated according to Eq. (1):
Nitrogen retention was calculated according to Eq. (2):
where both N in fish and in feed are in grams. Gross and net productivity were calculated according to Huisman (1987).
Statistical analyses
The data were statistically analysed using one-way analyses of variance using SPSS statistics software version 13 (SPSS Inc., Chicago, USA) at 95 % confidence level. Post hoc analyses using Fisher’s least significant difference test were performed for any significant difference.
Results
Water quality
The water quality parameters observed in this experiment are presented in Table 1. There was no significant difference observed in most water quality parameters except in CO2, TSS and VSS concentrations. Carbon dioxide concentrations were significantly higher in co-culture treatments than those in the control. Similarly, total and volatile suspended solids levels in co-culture treatments were also higher than in the control; however, the differences were only significant in co-culture treatment with high prawn density.
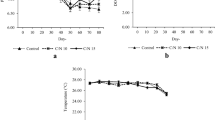
Figure 2 presents diurnal dissolved oxygen concentrations in the culture water on week 5. It can be seen that diurnal DO concentration and fluctuation in the control tend to be higher than those in the co-culture treatments. Diurnal oxygen concentration on the fifth week of culture showed strong reductions of dissolved oxygen in the treatments with organic C addition.
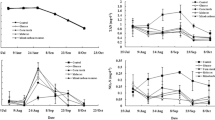
While there were no significant differences among treatments in TAN and NO2-N concentrations when considering the whole experimental period, higher values in the control were recorded by the end of the culture period (Fig. 3). A higher concentration of NO3-N was also noticed in the control on day 21.
Total heterotrophic bacteria in the water of treatments B and C were two log units higher (P < 0.05) than the treatment without any addition, with a tendency to increase during the culture period (Fig. 4).
Mean values of (n = 3) total bacteria count (log CFU/mL) in pond water of catfish monoculture (treatment A) and catfish–prawn co-culture at prawn densities of 20/m2 (treatment B) and 40/m2 (treatment C). Bars marked with a different superscript in each sampling point are significantly different (P < 0.05)
Production and economic performance
No significant differences among treatments were observed in growth, survival and FCR of catfish (Table 2). Prawn harvesting average body weight, growth and survival were significantly higher in the treatment with lower prawn density, respectively 43, 49 and 14 % higher. In terms of prawn biomass, no significant differences were observed in production and productivity. However, when calculated as the number of juveniles produced, the productivity of treatment C, 1.5 million juveniles/ha/year, was significantly higher than that of treatment B (900,000 juveniles/ha/year). Significantly lower FCR was observed in the co-culture treatments than in the control (Table 2).
In comparison with catfish monoculture, catfish co-culture with prawn juvenile resulted in higher profit (Table 3). Furthermore, when the prawn density increased from 20 to 40/m2, the total net return increased 37 % and 2.3 times higher than that of catfish monoculture.
Feed nitrogen utilization
There was no significant difference in feed N recovery by catfish among treatments (Table 4). However, as prawn recovered 3.25–3.81 % of the total feed N, co-culture of catfish with prawn resulted in significantly higher total feed N recovery (P < 0.05).
Discussion
The ranges of water temperature and pH were similar between treatments and within acceptable ranges. Diurnal oxygen concentration on the fifth week of culture showed that there were significant reductions of dissolved oxygen observed in the treatments with organic C addition. The reduction of DO towards the end of the culture period and the increase of CO2 concentrations in treatments B and C indicate that the addition of prawn biomass and the stimulation of heterotrophic bacteria growth by C organic addition resulted in higher respiration activities in the prawn–catfish culture system, mainly under higher prawn density. The high nitrogenous waste loaded into the water and the addition of organic C stimulated heterotrophic microbial growth in treatment B and especially so in treatment C, as shown by the levels of total heterotrophic bacteria, TSS and VSS in the water of the respective treatments. The high total bacterial count in treatment C confirms previous studies in the application of biofloc technology that showed that the addition of an external organic carbon source resulted in an increase in total heterotrophic bacteria (Hari et al. 2004; Azim and Little 2008). Total and volatile suspended solids are two physical characteristics that are frequently used as the main parameters for the characterization of floc formation in biofloc-based aquaculture systems (De Schryver et al. 2008). Highly suspended solids were particularly noticed in treatment C that was typical for a biofloc system where suspended solids may reach a value of more than 200 mg/L (Azim and Little 2008; De Schryver et al. 2008).
Dissolved inorganic nitrogen including TAN, NO2-N and NO3-N fluctuated over the culture period. There was a tendency towards the end of the culture period that the concentrations of dissolved inorganic nitrogen in the control without external C addition were higher than in the co-culture treatments with C addition. The concentrations of TAN, NO2-N and NO3-N that represent nitrification processes in the systems showed that during the first 3 weeks of culture, nitrification occurred in all treatments. After day 21, on the other hand, nitrification in the control was not completely working as indicated by the accumulation of TAN and NO2-N but not of NO3-N. Nevertheless, the range of inorganic N was appropriate for normal growth of both catfish and prawn (Peteri et al. 1992; New 2002).
As it was expected, the growth of prawn juvenile stocked at lower density (20/m2) in treatment B was significantly higher (P < 0.05) than that of treatment C (40/m2). The growth performance of prawn juvenile in the present study was comparable to that of a still unpublished stocking density experiment performed in the Freshwater Aquaculture Research Station, Sukabumi. In that experiment, prawn juveniles fed with 28 % crude protein feed at a stocking density of 20/m2 resulted in a specific growth rate of 3 %/day, survival of 91 % and FCR of 1.4. At a stocking density of 40/m2, on the other hand, slower growth rate (2.58 %/day) and higher FCR (1.9) were observed. In this regard, the growth and survival of prawn juveniles in the present experiment, where external feeding for prawn was not provided, was supported by the availability of bioflocs as the food source in the system. This result supports a previous study by Crab et al. (2010), who demonstrated that prawn post-larvae could utilize bioflocs grown with different carbon sources as their food. The similar growth of prawn juveniles in the present experiment and in that of prawn fed with artificial feed also indicates that the nutritional quality of the bioflocs available continuously in the system was comparable to that of artificial feed. Previous studies showed that bioflocs contain considerably high protein and lipid levels as well as other essential nutrients, such as essential amino acids and fatty acids, carotenoids, exogenous digestive enzymes and other bioactive compounds (Ju et al. 2008; Ekasari et al. 2010; Crab et al. 2010; Xu et al. 2012; Xu and Pan 2012).
Although survival was higher in treatment B, prawn juvenile productivity (prawn/ha/year) was noticeably higher in treatment C. This may indicate that even though higher mortality occurred at higher prawn density, the total surviving prawn juveniles was still higher than that at the lower prawn density. As prawn nursery productivity is determined by the number of juvenile produced, treatment C resulted in higher economical viability than treatment B (Table 3). Overall FCR of catfish–prawn co-culture regardless of the prawn density was significantly lower than that of catfish monoculture, which can be attributed to the utilization of bioflocs as food by the prawn. Furthermore, co-culture and C source addition in treatments B and C also contribute to the increase in total feed N utilization. The present study showed that the addition of prawn and organic C might recover approximately 10 % of the unutilized N from the feed provided to the catfish or 3 % of the total feed N input.
Conclusion
The water quality of catfish–prawn co-culture with organic C addition was generally better than the catfish monoculture. Co-culture with prawn with C source addition in catfish culture also resulted in significantly lower total FCR and significantly higher feed nitrogen utilization. Overall results suggest that catfish co-culture with prawn nursery with C organic addition would reduce nitrogen waste and also generate additional income for the catfish farmer.
References
APHA (2005) Standard methods for the examination of the water and wastewater, American Public Health Association, Washington, DC
Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 176:227–235
Azim ME, Little DC (2008) The biofloc technology (BFT) in indoor tanks: water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 283:29–35
Bauer W, Prentice-Hernandez C, Tesser MB et al (2012) Substitution of fishmeal with microbial floc meal and soy protein concentrate in diets for the pacific white shrimp Litopenaeus vannamei. Aquaculture 342–343:112–116
Crab R, Avnimelech Y, Defoirdt T et al (2007) Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 270:1–14
Crab R, Chielens B, Wille M et al (2010) The effect of different carbon sources on the nutritional value of bioflocs a feed for Macrobrachium rosenbergii postlarvae. Aquac Res 41:559–567
De Schryver P, Verstraete W (2009) Nitrogen removal from aquaculture pond water by heterotrophic nitrogen assimilation in lab-scale sequencing batch reactors. Bioresour Technol 100:1162–1167
De Schryver P, Crab R, Defoirdt T et al (2008) The basics of bio-flocs technology: the added value for aquaculture. Aquaculture 277:125–137
Diana JS, Egna HS, Chopin T et al (2013) Responsible aquaculture in 2050: valuing local conditions and human innovations will be key to success. Bioscience 63:255–262
Ekasari J, Crab R, Verstraete W (2010) Primary nutritional content of bio-flocs cultured with different organic carbon sources and salinity. Hayati J Biosci 17:125–130
Hari B, Kurup BM, Varghese JT et al (2004) Effect of carbohydrate addition on production in extensive shrimp culture systems. Aquaculture 241:179–194
Huisman EA (1987) The principles of fish culture production. Department of Aquaculture, Wageningen University, Netherland, p 100
Ju ZY, Forster I, Conquest L, Dominy W (2008) Enhanced growth effects on shrimp (Litopenaeus vannamei) from inclusion of whole shrimp floc or floc fractions to a formulated diet. Aquac Nutr 14:533–543
Kuhn DD, Lawrence AL, Boardman GD et al (2010) Evaluation of two types of bioflocs derived from biological treatment of fish effluent as feed ingredients for Pacific white shrimp, Litopenaeus vannamei. Aquaculture 303:28–33
New MB (2002) Farming freshwater prawns: a manual for culture of the gaint river prawn (Macrobrachium rosenbergii). Roma: Food and Agriculture Organization of the United Nations
Peteri A, Nandi S, Chowdhury SN (1992) Manual on seed production of African catfish (Clarias gariepinus). Rome: Food and Agriculture Organization of the United Nations
Takeuchi T (1988) Laboratory work-chemical evaluation of dietary nutrients. In: Watanabe T (ed) Fish nutrition and mariculture. Kanagawa International Fisheries Training Center, Japan International Cooperation Agency, Kanagawa, pp 179–233
Verdegem MCJ (2013) Nutrient discharge from aquaculture operations in function of system design and production environment. Rev Aquac 4:1–14
Wedemeyer GA (1996) Physiology of fish in intensive culture systems. Chapman and Hall, New York
Xu WJ, Pan LQ (2012) Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 356–357:147–152
Xu WJ, Pan LQ, Zhao DH et al (2012) Preliminary investigation into the contribution of bioflocs on protein nutrition of Litopenaeus vannamei fed with different dietary protein levels in zero-water exchange culture tanks. Aquaculture 350–353:147–153
Yi Y, Lin CK, Diana S (2003) Hybrid catfish (Clarias macrocephalus x C. gariepinus) and Nile tilapia (Oreochromis niloticus) in an integrated pen-cum pond system: growth performance and nutrient budgets. Aquaculture 217:395–408
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohmana, D., Surawidjaja, E.H., Sukenda, S. et al. Water quality and production performance of catfish–prawn co-culture with organic carbon source addition. Aquacult Int 23, 267–276 (2015). https://doi.org/10.1007/s10499-014-9814-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9814-2