Abstract
This study shows the effects of dietary activated charcoal (AC) on health status, intestinal morphology and fillet geosmin content of Nile tilapia prior to harvesting (2 and 4 weeks). Four dietary treatments (each diet in six replicates) were formulated to incorporate AC at levels of 0, 10, 20 and 30 g kg−1 of the dry diet. Fish were reared in hapas, which were located in earthen ponds. There were not significant differences in growth performances among experimental treatments. The moisture and protein content in the fillet decreased and increased, respectively, as the incorporation level of AC increased. The hematological indices and several immune parameters did not differ significantly among treatment groups. Among the fifteen blood chemicals parameters examined, the significant reductions in protein and cholesterol and the changes in blood minerals were observed in fish fed dietary AC ≥20 g kg−1. Dietary AC tended to increase the height of intestinal villi and goblet cell number. Dietary AC also influenced the reduction in geosmin in the fish fillet. Taken together, these findings indicate that AC (at 10 g kg−1 diet) could be used as feed supplement for Nile tilapia prior harvesting to reduce geosmin without negative effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Activated charcoal (AC), which has non-nutritive and non-digestible components, has been used for medical and veterinary purposes as a universal poison antidote. AC is used as a detoxifying substance due to the influence of both physical and chemical characteristics, including surface area and pore size, on its adsorption capacity (McFarland and Chyka 1993; American Academy of Clinical Toxicology 1999; Bacaoui et al. 2001). AC is effective in the elimination of the mycotoxins, such as aflatoxins (Galvano et al. 1996), as well as pesticide residues (Wilson et al. 1971) that occasionally contaminate the feed’s ingredients. The adsorption effect of AC with other detrimental organic compounds would positively influence fish growth, immunity and flesh quality; therefore, AC could be used as dietary health supplement for Nile tilapia aquaculture. Recently, there has been only limited scientific research regarding the use of AC as a feed additive in livestock and aquatic animals (Villalba et al. 2002; Ruttanavut et al. 2009; Majewska et al. 2009; Thu et al. 2010).
The worldwide production of tilapia has been increased intensely to provide a staple source of protein for human consumption. Although Nile tilapia are hardy, easy to farm and fast growing, commercial intensive tilapia production has still been categorized as a type of business risk due to disease outbreaks, fluctuations in the water quality and/or contamination from any agricultural chemical pollutant during the culture period. Tilapia can be commercially raised in many culture systems, including ponds, cages, tanks and raceways. Pond culture is the most cost-effective grow-out system for growing tilapia because the fish can use natural food in addition to complete feed. A musty-earthy flavor is a critical problem of pond-raised tilapia. Geosmin and 2-methylisoborneol (MIB) are secondary metabolic products of some blue-green algae and bacteria (for a review, see Tucker 2000). Fish adsorb geosmin from the surrounding water primarily through their gills (From and Hǿrlyck 1984). Geosmin is also taken up intestinally, when fish ingest algae. In Nile tilapia, the highest amount of geosmin was detected in the intestine compared with the abdomen, skin and muscle (Yamprayoon and Noomhorm 2000). Several types of AC have been reported to have adsorption effects on geosmin in potable water resources (Ridal et al. 2001; Matsui et al. 2013). Therefore, an investigation of the effect of dietary AC on production performance, health status and fillet composition-odor of Nile tilapia would promote sustainable production of tilapia.
This study aims to investigate the potential use of dietary AC as a feed supplementation for Nile tilapia cultures during the period prior to harvesting. Hematological, blood chemical and immune parameters were evaluated to determine whether AC has detrimental effects on fish health. The intestinal morphology was also investigated. Furthermore, to determine whether AC could produce a reduction in the off-flavor, we analyzed the geosmin content in fillets.
Materials and methods
Experimental fish, design, fish culture and fish performance evaluation
The Nile tilapia (Oreochromis niloticus) used in this study were reared at the Suranaree University of Technology Farm (SUT Farm; Nakhon Ratchasima, Thailand). The experimental Nile tilapia were male Nile tilapia that had been produced by feeding the swim-up fry with a 50 mg kg−1 17α-methyltestosterone-treated diet for 4 weeks and then with a diet consisting of 350 g kg−1 crude protein until the start of the experiment. The experimental design was completely randomized into four treatment diets. To test the validity of the conclusions, each treatment was replicated six times with twenty fish per replicate. Twenty-four hapas (2 × 2 × 2.5 m3) were maintained in the earth pond of the SUT Farm, and the bottoms of the hapas were embedded 10 cm underneath the bottom of the pond. Prior to the start of the experiment, fish (380–460 g) were randomly distributed in the experimental hapas. The fish were fed Diet AC-0 to adapt to the experimental environment for 2 weeks. During the experiment, the fish were hand-fed twice daily for 4 weeks. Diets were fed ad libitum. Any dead fish was recorded and removed daily. The growth performance and feed utilization were evaluated at the end of weeks 2 and 4. During the experimental period, in order to investigate the effects of dietary AC on health status and fillet composition-odor, stable green water system was implemented to resemble semi-intensive tilapia farming. The daily fluctuation in water temperature was measured daily (at 6:00 and 18:00), and the dissolved oxygen and pH were determined weekly (at 6:00 and 18:00). The water temperatures (mean ± SD) at 6:00 and 18:00 were 28.3 ± 1.4 and 30.4 ± 2.6 °C, respectively. The pH and dissolved oxygen were within acceptable ranges, i.e., a pH of 8.20 ± 0.28 (6:00) and 8.76 ± 0.51 (18:00) and dissolved oxygen of 3.58 ± 0.45 mg L−1 [41.1–55.2 % of saturation] (6:00) and 12.25 ± 2.05 mg L−1 [135.72–205.58 % of saturation] (18:00). Total ammonia content was measured weekly using Tetratest NH3/NH4 + (Tetra GmbH, Melle, Germany). During the experimental period, total ammonia was maintained at <0.25 mg L−1.
Feed formulation and pellet preparation
All test ingredients were obtained from animal feedstuff companies. Before formulating the feed, all feed ingredients were analyzed to determine gross composition (moisture, protein, lipid and ash) according to AOAC (1990). Four diets were formulated to incorporate activated charcoal (SKU V-1400, Charcoal House LLC, Crawford, NE, USA) at 0 (AC-0), 10 (AC-10), 20 (AC-20) or 30 (AC-30) g kg−1 (Table 1). All diets were formulated to contain same ingredients with little differences in proportions in order to produce isonitrogenous and isocaloric experimental diets. All diets were produced using a hammer grinder, mixer and extruder (Paktongchai Pasusat, Nakhon Ratchasima, Thailand). The dry ingredients were ground by a grinder and mixed by a ribbon screw mixer (22 rpm). The floating experimental diets were produced using a single screw extruder (an extruding temperature of 120–160 °C). All experimental diets were analyzed to determine their chemical compositions according to AOAC (1990) and stored at room temperature until use.
Fish sampling and blood collection
Hematological, blood chemical and immune parameters were evaluated to determine the effects of the activated charcoal on the health status of the Nile tilapia. At the end of weeks 2 and 4, the fish were not fed for 18 h prior to blood sampling. Four representative fish from each diet replicate were randomly selected and anesthetized with 2-phenoxyethanol (0.35 mL L−1). Blood sampling was conducted as described in Pitaksong et al. (2013). Blood was collected by a hypodermic syringe from the caudal vein. The collected blood sample was divided into two sets. One set was added to a tube containing K2EDTA as an anticoagulant. The second blood sample set was left to clot at 4 °C for at least 3 h and centrifuged at 5,000 rpm for 5 min at room temperature. Plasma was collected by the centrifugation of the K2EDTA-blood at 5,000 rpm for 10 min at 4 °C. The serum collected was stored at −80 °C for further analysis. After blood sampling, samples of intestine were collected for histological analysis. Then, the fillet were dissected and frozen for proximal analysis according to AOAC (1990) with slight modifications, such as sample weights (moisture; 2 g, protein; 5 g, fat and ash; 10 g), the fish fillet was minced for geosmin analysis.
Hematological assays
Immediately after blood sampling, the K2EDTA-blood was used to examine the hematological parameters. The red blood cell count (RBC) was analyzed in duplicate for each sample using a Neubauer hemocytometer after dilution with Grower’s solution (Voigt 2000). Hematocrit values (Ht) were measured in duplicate by placing K2EDTA blood into glass capillary tubes and centrifuging them for 5 min by microhematocrit centrifugation. The hemoglobin (Hb) content was measured using the photometrical cyanohemoglobin method. The white blood cell count (WBC) was determined in duplicate for each sample using a Neubauer hemocytometer after dilution with Dacie’s solution (Dacie and Lewis 2001).
Blood chemistry analysis
To investigate the effects of dietary activated carbon on fish health, several blood tests were performed such as glucose, triglyceride, cholesterol, total protein, albumin, blood urea nitrogen (BUN), total bilirubin (T-bilirubin), direct bilirubin (D-bilirubin), alkaline phosphatase (AP), amylase, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), calcium, chloride, and iron. Immediately after blood sampling, the K2EDTA treated blood was used to determine the blood glucose levels in whole blood drawn from the caudal vein using a hand-held glucometer (AccuTrend; Roche). Serum triglyceride was analyzed by the glycerol-3-phosphate oxidase-sodium N-ethyl-N-(3-sulfopropyl) m-anisidine (GPO-ESPAS) method described by Bucolo and David (1973). Serum was quantitatively determined using the cholesterol oxidase-phenol+aminophenazone (CHOD-PAP) technique described by Flegg (1973). Plasma proteins were estimated by the Biuret method (Gornall et al. 1949). Serum albumin was quantitatively evaluated using the bromocresol green method for albumin (Doumas et al. 1971). BUN was measured by a modified indophenol colorimetric method (Weatherburn 1967). Total and direct bilirubin (T- and D-bilirubin) were measured using the new diazo-DMSO method (Winsten and Cehelyk 1969). AP was examined by the phenolphthalein monophosphate (PMP) method (Babson et al. 1966). Amylase was quantitatively determined using the amyloclastic method utilizing decreases in starch-iodine reactions (Reif and Nabseth 1962). SGOT and SGPT were measured using Reitman and Frankel’s colorimetric method (Reitman and Frankel 1957). The calcium content in the serum was measured using the o-cresolphthalein direct method (Moorehead and Biggs 1974). Blood chloride was estimated using the thiocyanate method (Hamilton 1966). Iron ferene was examined using an iron quantitative determination in the serum or plasma (IDS, Liege, Belgium).
Immune assay
Immune parameters, including total immunoglobulin, lysozyme activity and alternative complement activity (ACH50), were measured. Total immunoglobulin was measured according to Siwicki et al. (1994). Using experimental fish serum, lysozyme activity was analyzed as described by Pitaksong et al. (2013), and ACH50 were determined according to Sunyer and Tort (1995).
Histological analysis
To determine the effect of dietary activated charcoal on intestinal morphology, two fish from each replication of each treatment were sampled and prepared for histological study, as described previously (Phumyu et al. 2012). Portions of the anterior, middle and posterior of the intestines were dissected and preserved in 10 % phosphate-buffered formalin with a pH 7.2. After dehydration, the tissue was embedded in a paraffin wax, cut into slices 5 μm thick and mounted on glass slides. After deparaffinization, the slides were dehydrated and stained with Hematoxylin and Eosin (H&E). The height of the villus was measured on stained sections under a microscope using an ocular micrometer at 100× magnification. The ten longest intact villi in each intestinal position were selected for measurement in duplicate cross-sections for each sample.
Geosmin analysis
Extraction of the geosmin was carried out according to Yamprayoon and Noomhorm (2000), with modifications. Fish fillet samples of known weights (100 g) were minced and mixed with 50 mL of distillated water and 20 g of NaCl. Volatiles were distilled from the mixture of minced fillet for 3 h. The distillate was extracted with 2 mL hexane containing internal standard [isobornyl acetate (2.5 μg mL−1) and 12 g of NaCl for 15 min]. Anhydrous sodium sulfate was used to remove water from the hexane extract. Geosmin was then analyzed with gas chromatography–mass spectrometry (GC-CP3800, MS-1200, Varian Inc., Walnut Creek, CA, USA) equipped with a capillary column (Varian VF-5 ms, 30 m*0.25 mm ID*0.25 μm film thickness) and a quadrupole mass detector. The injector port temperature was 260 °C, and a 2 μL sample of the extract was used for injection. The flow rate of the helium carrier gas was 1.0 mL min−1, and splitless mode was applied. The transfer line temperature was set at 250 °C. The initial column temperature was maintained at 60 °C for 2 min before being increased to 240 °C at a rate of 8 °C min−1 and held for 5.5 min. The mass spectra were obtained by electron ionization (EI) at 70 eV. The scan range was 50–200 m/z at 0.5 s intervals. The identification of geosmin was performed by comparing retention times with geosmin standards [2.5, 5, 7.5, 10, 15, 20 ppb] and mass spectra with mass spectral libraries (National Institute of Standards, NIST). The amounts of geosmin were calculated from peak areas by comparing them with the geosmin standard curve; isobornyl acetate was used as an internal standard.
Data analysis
All data were analyzed by one-way analysis of variance (ANOVA) using SPSS for Windows (Release 10) (SPSS Inc., Chicago, IL, USA). When significant differences were found among the groups, Duncan’s multiple range tests were used to rank the groups. The statistical model utilized was y ij = μ + τ I + ε ij , where y ij was the response; μ, the general means; τ I, the dietary AC effects; and ε ij , the random error. In addition, regression analysis and goodness of fit (R 2) for the responses (Y) and for the level of incorporated AC parameters (x) were conducted. Throughout the experiment, effects and differences were declared to be significant when their values were less than 0.05 (P < 0.05).
Results
The growth performance of Nile tilapia fed different experimental feeds was determined at the end of 2 and 4 weeks (Table 2). Through the whole experimental period, the growth performances, including final weight, weight gain (WG), specific growth rate (SGR) and feed conversion ratio (FCR), of the fish fed all experimental diets showed no significant differences. Fish fed the AC-10 diet had the highest final weight, WG, SGR, feed intake (FI) and the lowest FCR, compared to the other experimental diets; however, due to the high standard deviations, differences were not significant. The condition factor (K) of Nile tilapia did not vary significantly among the groups. Until the end of the experimental period (4 weeks), the survival rates remained high in all experimental groups, ranging from 90 to 100 %.
The effects of supplemental AC on the chemical composition of the fillets are given in Table 3. During the experimental period, an increase in supplemental AC decreased the moisture of the fillets, and a significant reduction was observed at the end of 4 weeks. A significant linear relationship (y = −0.971x + 778.669, R 2 = 0.510) was found between AC supplementation levels and moisture. At the end of week 2, an increase in supplemental AC significantly increased protein contents of the fillets. By the end of week 4, a significant linear relationship (y = 0.609x + 140.849, R 2 = 0.446) was observed between AC and protein contents. No significant differences were observed on the fat or ash content in fillets among the experimental groups.
The hematological indices of the Nile tilapia fed different experimental diets are shown in Table 4. Throughout the experimental period, the red blood cell count (RBC), hemoglobin and hematocrit did not vary significantly among diets. By the end of week 2, an increase in supplemental AC led to a significant increase in the white blood cell count. At week 4, white blood cell counts did not differ according to the level of supplemental AC. The effects of AC supplementation on several parameters of humoral, non-specific immunity, including lysozyme activity, total immunoglobulin and alternative complement (ACH-50), were assessed (Table 5). By the end of the experimental period, values for lysozyme activity, total immunoglobulin and ACH-50 did not vary among experimental diets.
Table 6 shows the blood chemical parameters of Nile tilapia fed different experimental diets. Several blood chemical analyses performed, such as glucose, triglyceride, albumin, BUN, T-bilirubin, D-bilirubin, AP, amylase, SGOT and SGPT, did not reveal any significant difference among different diets through the end of the experimental period. However, there were significant differences in cholesterol values which appeared to decrease with an increase in AC supplementation in the diets. By the end of week 4, a significant reduction was determined in Nile tilapia fed diets AC-20 and AC-30, comparing with Diet AC-0. At week 2, the total protein content of Nile tilapia that were fed diets AC-20 and AC-30 was decreased, comparing with those on Diet AC-0. However, at week 4, the total proteins appear to be similar among diets. AC supplementation had effects on the blood minerals of Nile tilapia, by the end of week 2, where a significant decrease in the calcium levels was evident, while had no significant effects on the chloride or iron levels. A significant linear relationship (y = −0.013x + 3.687, R 2 = 0.778) was observed between AC supplementation levels and the blood calcium content. At the end of week 4, the calcium tended to decrease with an increase in AC supplementation in the experimental diets, although the decrease was not significant. Additionally, significantly higher blood chloride and lower blood iron levels were observed in Nile tilapia fed Diet AC-30 compared to Diet AC-0 (P < 0.05).
The intestinal histomorphometry of the Nile tilapia that were fed various levels of AC-supplemented diets is given in Fig. 1 and Table 7. By the end of week 2, AC-supplemented diets led to significant increases in the height of villi in all parts of the intestine (P < 0.05). At week 4, fish fed Diet AC-10 diet had higher villi heights in the anterior and middle parts, whereas supplementation with AC at 20 and 30 g kg−1 did not generate any further increase in villi height. AC supplementation did not have any effect on the villi of the posterior intestine. At week 2, a significantly higher goblet cell count was observed in only the middle intestine of fish fed Diet AC-30. By the end of week 4, compared with fish on Diet AC-0, the number of goblet cells in all portions of the intestines of fish fed dietary AC was higher. Moreover, dietary AC seemed to increase the thickness of the intestinal muscle in posterior part (Fig. 1) which would indicate the effect of dietary AC on chronic constipation.
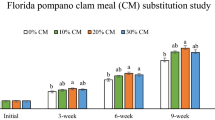
Fillet’s geosmin content of Nile tilapia fed the AC-supplemented diets are shown in Fig. 2. By the end of week 2, fish fed on AC-30 diet had significantly lower geosmin contents in their fillets (P < 0.05). At the end of week 4, significantly lower geosmin contents in the fillets were observed for fish fed the AC-10, AC-20 and AC-30 diets, compared with fish fed the control diet (AC-0) (P < 0.05). In addition, a significant nonlinear relationship (y = 0.016x 2 − 0.830x + 15.856, R 2 = 0.702) was observed between AC and the geosmin content in fillets.
Geosmin content in the fillets of Nile tilapia fed on activated charcoal (AC) at 2 weeks and 4 weeks. Different superscript letters in the bar graph indicate significant differences at P < 0.05. Note that a nonlinear relationship was predicted for the AC and geosmin contents at week 4 (y = 0.016x 2 − 0.830x + 15.856, R 2 = 0.702)
Discussion
AC, a non-nutritive substance, has the potential to be used as feed additive due to its detoxifying effects. In pond-reared fish, potential areas of concern for fish production include contamination with harmful substances, such as mycotoxins and/or pesticides in feed ingredients, and an off-flavor. To address the latter concern, AC has been widely used in medical, veterinary and wastewater treatments, as well as in water treatments for aquaculture. So far, studies in the utilization of AC in animal feed have been controversial. For example, while nonsignificant protective effects against fumonisin have been observed in pigs, dietary AC marginally alleviated the growth performance-inhibitory effects of aflatoxin in growing chickens and increased the FI of shrubs in small ruminants (Edrington et al. 1997; Piva et al. 2005; Rogosic et al. 2006). Because AC is a universal adsorbent, the administration of AC could have both positive and negative influences on the digestive physiology and metabolism. In addition, long-term consumption of AC would induce adverse effect on Nile tilapia due to its non-nutritive and non-digestible substance. In this study, we demonstrated both positive and negative effects of AC supplementation on the growth, health status, chemical composition and geosmin content in fillet and intestinal morphology of Nile tilapia during marketable size (an ideal market size for pond-reared Nile tilapia is generally greater than or equal to 400–500 g).
The ability of AC to absorb a wide range of noxious substances and gases (McFarland and Chyka 1993; American Academy of Clinical Toxicology 1999; Bacaoui et al. 2001) would most likely increase feed efficiency, thereby improving the growth performances of the animals. The present study showed that the growth performance (WG, SGR, FCR) of Nile tilapia at the grow-out stage appeared to be similar in all diets. However, an improvement was observed in fish fed diet at 10 g kg−1 AC supplementation level, but differences were not significant because of the high standard deviations observed. In our experience, although the experimental diets were formulated to contain similar levels of nutritive chemical composition, the incorporation of AC at high levels affected the physical characteristics of the extruded diets, including palatability, color and smell. These qualities could influence the fish’s appetites. The variable effects of charcoal-supplemented diets on growth performance have been demonstrated in chicken and fish. For example, growth performances (FI, WG, FCR) were linearly improved as the supplementation level of wood charcoal increased from 0 to 100 g kg−1 in broiler chicken at 28 days of age; however, the positive effects were limited at the older ages (Kutlu et al. 2001). Charcoal supplementation up to 6 g kg−1 could improve growth performances in broilers during the 21st to 49th days of age (Kana et al. 2011). Supplementation with bamboo charcoal at 5 g kg−1 significantly improved the growth performances (FE, WG, SGR) of flounder, Paralichythys olivaceus, at juvenile stages (Thu et al. 2010). In addition, a mixture of dietary charcoal and wood vinegar at 5 and 10 g kg−1 for 8 weeks was shown to increase feed efficiency and WG, respectively, of flounder at later stages (Yoo et al. 2005). Combining these data, it seems that whether charcoal influences growth performance depends on the supplemental dosage and growth stages of the animals.
The approximate composition of fish body and fish fillet has generally been determined to assess the nutritional status of the fish. Supplementation of AC at the level of 20 and 30 g kg−1 effected changes in some proximal composition parameters of Nile tilapia fillet. AC supplementation linearly increased the protein and decreased the water contents in the flesh of Nile tilapia. This finding was similar to the results of Thu et al. (2010) who reported a positive effect of bamboo charcoal supplementation on digestibility, leading to an increase in the protein content of the fish bodies. However, this positive result did not significantly affect moisture, lipid or ash levels in the carcasses of the flounder. In contrast, the mixture of dietary charcoal and wood vinegar did not influence the approximate composition in flounder (Yoo et al. 2005). The reduction in the proportion of moisture in the fillets was most likely caused by an increase in other contents such as protein, fat and ash. Because AC adsorb significant amount of water (Deng 2006), it remains to be further investigated whether dietary AC, particularly at high levels and/or over long periods, may affect the fluid homeostasis processes in fish.
Hematological parameters could be used as indices of the health status of the fish. The present study demonstrated that dietary AC did not significantly affect such hematological parameters as RBC, hemoglobin or hematocrit throughout the experimental period (Table 4). These findings were similar to the results performed in turkeys (Majewska et al. 2009). Because AC is considered a non-specific detoxifier, its use should contribute to improve health conditions. Certain immune parameters, including the white blood cell counts, lysozymes, total immunoglobulin and ACH50, were determined in order to evaluate the effects of supplementation of charcoal on immune status. White blood cell count of Nile tilapia in the present study was lower when compared to the values reported by Phumyu et al. (2012) and Li et al. (2011). The different number of white blood cell number may be due to age- or size-related changes. It seems that white blood cell decreases with fish size increases. It was reported that tilapia exposed to pesticide and mycotoxin led to decrease in white blood cell count (Hart et al. 1997; Kumar et al. 2011; Selim et al. 2013). AC supplementation at high levels (for a short-term feeding period) had positive effects on increasing the white blood cell number. An increment in white blood cell number by dietary AC could be explained by the acute effect of AC as non-specific detoxifier when the diet contained a high level of AC. In addition, in Nile tilapia, diet contaminated with aflatoxin led to decrease in white blood cell number, and mycotoxin adsorbent showed to increase number of white blood cell (Selim et al. 2013). Dietary AC did not significantly influence the tested humoral immune parameters. In general, fish immune system comprises of complex network of circulating cells, organs and physiological systems. Therefore, further investigation is required to determine whether dietary AC has any effects on other immune parameters in order to clearly understand the effect of dietary AC on fish health.
Blood chemicals parameters measured as health indices of Nile tilapia were presented in Table 6. Supplementation with bamboo charcoal was reported to increase protein digestibility (Thu et al. 2010). Because AC is considered a universal adsorbent, it also has the potential to adsorb many nutrients. Plasma protein was consequently manifested in the effect of AC supplementation on nutrition status, immune and liver function in fish. The present result showed that although supplementation with AC ≥20 g kg−1 (2 weeks) had significantly negative effects on plasma proteins, its values are in the reference interval for tilapia (Hrubec et al. 2000). Additionally, the plasma protein levels after 4 weeks of experimentation appeared to be similar among the experimental groups. These evidences suggested that dietary AC had no obviously adverse effects on protein assimilation. The present results showed that supplementation of AC ≥20 g kg−1 influenced blood cholesterol levels. Supplementation with a mixture of charcoal and wood vinegar was demonstrated to alter fatty acid profiles and to decrease saturated fatty acids in flounder (Yoo et al. 2005). Moreover, AC interfered with the enterohepatic circulation of bile acids and cholesterol; therefore, ingestion of AC was demonstrated to reduce serum cholesterol in patients with hypercholesterolemia (Neuvonen et al. 1989). The present result demonstrated that dietary AC had effects on the mineral content of blood, including calcium, chloride and iron. The absorptive properties of AC with mineral (Olsen 2010) and the release mineral content would affect plasma electrolyte in fish bodies.
Mycotoxins were demonstrated to affect the morphology of intestine by a decrease in villi height in pig and chicken (Awad et al. 2006; Kolf-Clauw et al. 2009). Intestinal integrity affects the physiological metabolism of nutrient absorption. Dietary AC contributes to the excretion and elimination of pathogens, toxins and noxious gases, and thereby, it is considered a gastrointestinal decontamination agent. Therefore, AC would have positive effect on intestinal morphology. Nevertheless, medical case studies have reported adverse effects from the administration of AC on gastrointestinal obstruction (Watson et al. 1986; Atkinson et al. 1992; Green and McCauley 2006). The present study demonstrated that AC supplementation significantly increases the villus height in all parts (for 2 weeks) and in the anterior and the middle parts (for 4 weeks) of intestines. These results were similar to the results of studies in piglets, which involved supplementation with charcoal powders and wood vinegar compound liquids (Mekbungwan et al. 2004). A positive correlation between villus height and growth performance was reported in Nile tilapia (Vechklang et al. 2011; Phumyu et al. 2012), and this positive correlation was not observed in the present study. Due to its non-specific adsorptive capacity, AC most likely binds to and excretes certain essential nutrients. Thus, this study suggests that AC has positive effects on the intestinal integrity and negative effects on metabolism of nutrient absorption, and thereby, dietary AC had nonsignificantly positive effects on growth performance. Goblet cells play an essential role in the synthesis and secretion of mucin into the mucus layer, destroying pathogens (Blomberg et al. 1993). Mycotoxins were also revealed to reduce number of goblet cells in pig (Bracarense et al. 2012). The present study demonstrated that AC supplementation led to increases in the goblet cell counts. The AC adsorption of pathogen and/or noxious substances in the intestine leads to increases in the number of goblet cells along the intestinal villi.
Musty-earthy odors, which are primarily caused by trans-1,10-dimethyl-trans-9-decalol (geosmin) and 2-methylisoborneol (MIB), have become a problem not only in potable water resources but also in pond-based farming systems. Tilapia collected from commercially freshwater and brackish ponds which were revealed to contain geosmin at 1.56–9.85 μg kg−1 (Yamprayoon and Noomhorm 2000). Compared to the previous report, lower geosmin concentration in Nile tilapia in the present study might be due to the different in pond condition and culture system. For instance, comparing to fish ingest algae, Nile tilapia in this study was fed practical diet ad libitum which resulted in low geosmin content in flesh. Several types of AC, including granular, powdered and superfine powdered AC, have been demonstrated to be suitable for use in the removal of geosmin and MIB from drinking water (Ridal et al. 2001; Cook et al. 2001; Matsui et al. 2013). It is impractical to remove both of these earthy-odor substances from the water in fish ponds. Fish primarily adsorb geosmin through their gills and intestines via the ingestion of algae and bacteria on the pond bottom, and the highest geosmin content was found in the intestine (Yamprayoon and Noomhorm 2000). Our results showed that AC supplementation for 4 weeks led to a significant decrease in the geosmin content of fish fillet, following a quadratic response, while MIB was not detected. It was revealed that reduction (96–98 %) in geosmin from Nile tilapia flesh took 16 days when fish were reared in aerated clean water without geosmin (Yamprayoon and Noomhorm 2000). The present study provides a method to reduce off-flavors in fish fillet using dietary AC at 30 mg kg−1 for 2 weeks or at 10 mg kg−1 for 4 weeks caused by geosmin.
In conclusion, this study demonstrated that AC supplementation at low levels (10 g kg−1) for 4 weeks prior to harvesting did not have any detrimental effects on the health status of Nile tilapia. Our findings provide evidence of the effect of the use of AC as feed additive and of the potential benefits of utilizing AC to reduce off-flavors in pond-based tilapia production.
References
American Academy of Clinical toxicology (1999) Position statement and practical guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol 37:731–751
AOAC (1990) Association of Official Analytical Chemists. Official methods of analysis of the Association of Official Analytical Chemists, vol 1, 14th edn. AOAC, Arlington, VA
Atkinson SW, Young Y, Trotter GA (1992) Treatment with activated charcoal complicated by gastrointestinal obstruction requiring surgery. BMJ 305:563
Awad WA, Bohm J, Razzazi-Fazeli E, Zentek J (2006) Effects of feeding deoxynivalenol contaminated wheat on growth performance, organ weights and histological parameters of the intestine of broiler chickens. J Anim Physiol Anim Nutr 90:32–37
Babson AL, Greeley SJ, Coleman CM, Philips GE (1966) Phenolphthalein monophosphate as a substrate for serum alkaline phosphatase. Clin Chem 12:482–490
Bacaoui A, Yaacoubi A, Dahbi A, Bennouna C, Phan Tan Luu R, Maldonado-Hodar FJ, Rivera-Utrilla J, Moreno-Castilla C (2001) Optimization of conditions for the preparation of activate carbons from olive-waste cakes. Carbon 39:425–432
Blomberg L, Krivan HC, Cohen PS, Conway PL (1993) Piglet ileal mucus protein and glycolipid (galactosylceramide) receptors specific for Escherichia coli K88 fimbriae. Infect Immun 61:2526–2531
Bracarense A-PFL, Lucioli J, Grenier B, Pacheco GD, Moll W-D, Schatzmayr G, Oswald IP (2012) Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br J Nutr 107:1776–1786
Bucolo G, David H (1973) Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem 19:476–482
Cook D, Newcombe G, Sztajnbok P (2001) The application of powdered activated carbon for MIB and geosmin removal: predicting PAC doses in four raw waters. Water Res 35:1325–1333
Dacie JV, Lewis SM (2001) Practical haematology, 9th edn. Churchill Livingstone, London, p 633
Deng S (2006) Sorbent technology. In: Lee S (ed) Encyclopedia of chemical processing. Taylor & Francis Group, New York, pp 2825–2845
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31:87–96
Edrington TS, Kubena LF, Harvey RB, Rottinghaus GE (1997) Influence of a superactivated charcoal on the toxic effects of aflatoxin or T-2 toxin growing broilers. Poult Sci 76:1205–1211
Flegg HM (1973) An investigation of determination of serum cholesterol by an enzymatic method. Ann Clin Biochem 10:79–84
From J, Hǿrlyck V (1984) Sites of uptake of geosmin, a cause of earthy-flavor, in rainbow trout (Salmo gairdneri). Can J Fish Aquat Sci 41:1224–1226
Galvano F, Pietri A, Fallico B, Bertuzzi T, Scire S, Galvano M, Maggione R (1996) Activate carbons: in vitro affinity for aflatoxin B1 and relation of adsorption ability to physicochemical parameters. J Food Prot 59:545–550
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Green JP, McCauley W (2006) Bowel perforation after single-dose activated charcoal. Can J Emerg Med 8:358–360
Hamilton RH (1966) A direct photometric method for chloride in biological fluids, employing mercuric thiocyanate and perchloric acid. Clin Chem 11:1–17
Hart LJ, Smith SA, Smith BJ, Robertson J, Holladay SD (1997) Exposure of tilapian fish to the pesticide lindane results in nypocellularity of the primary hematopoietic organ (pronephros) and the spleen without altering activity of phagocytic cells in these organs. Toxicol 118:211–221
Hrubec TC, Cardinale JL, Smith SA (2000) Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet Clin Pathol 29:7–12
Kana JR, Teguia A, Mungfu BM, Tchoumboue J (2011) Growth performance and carcass characteristic of broiler chickens fed diets supplemented with graded levels of charcoal from maize cob or seed of Canarium schweinfurthii Engl. Trop Anim Health Prod 43:51–56
Kolf-Clauw M, Castellote J, Joly B, Bourges-Abella N, Raymond-Letron I, Pinton O, Oswald IP (2009) Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol in vitro 23:1580–1584
Kumar N, Antony Jesu Prabhu P, Pal AK, Remya S, Aklakur Md, Rana RS, Gupta S, Raman RP, Jadhao SB (2011) Anti-oxidative and immune-hematological status of tilapia (Oreochromis mossambicus) during acute toxicity test of endosulfan. Pestic Biochem Phys 99:45–52
Kutlu HR, Ünsal I, Görgülü M (2001) Effects of providing dietary wood (oak) charcoal to broiler chicks and laying hens. Anim Feed Sci Technol 90:213–226
Li E, Lim C, Cai C, Klesius PH (2011) Growth response and resistance to Streptococcus iniae of Nile tilapia, Oreochromis nimoticus, fed diets containing different levels of wheat distiller’s dried grains with solubles with or without lysine supplementation. Anim Feed Sci Technol 170:246–255
Majewska T, Mikulski D, Siwik T (2009) Silica grit, charcoal and hardwood ash in turkey nutrition. J Elem 14:489–500
Matsui Y, Nakao S, Taniguchi T, Matsushita T (2013) Geosmin and 2-methylisoborneol removal using superfine powdered activate carbon: shell adsorption and branched pore kinetic model analysis and optimal particle size. Water Res 47:2873–2880
Mauel MJ, Miller DL, Merrill AL (2007) Hematologic and plasma biochemical values of healthy hybrid tilapia (Oreochromis aureus × Oreochromis nilotica) maintained in a recirculating system. J Zoo Wildl Med 38:420–424
McFarland AF, Chyka PA (1993) Selection of activated charcoal products for the treatment of poisonings. Ann Pharmacother 27:358–361
Mekbungwan A, Yamauchi K, Sakaida T (2004) Intestinal villus histological alterations in piglets fed dietary charcoal powder including wood vinegar compound liquid. Anat Histol Embryol 33:11–16
Moorehead WR, Biggs HG (1974) 2-Amino-2-methyl-1-propanol as the alkalizing agent in an improved continuous-flow cresolphthalein complexone procedure for calcium in serum. Clin Chem 20:1458–1460
Neuvonen PJ, Kuusisto P, Vapaatalo H, Manninen V (1989) Activate charcoal in the treatment of hypercholesterolaemia: dose-response relationships and comparison with cholestyramine. Eur J Clin Pharmacol 37:225–230
Olsen KR (2010) Activated charcoal for acute poisoning: One toxicologist's journey. J Med Toxicol 6:190–198
Phumyu N, Booanuntanasarn S, Jangprai A, Yoshizaki G, Na-Nakorn U (2012) Pubertal effects of 17α-methyltestosterone on GH-IGF genes of the hypothalamic-pituitary-liver-goandal axis and other biological parameters in male, female and sex-reversed Nile tilapia. Gen Comp Endocrinol 177:278–292
Pitaksong T, Kupittayanant P, Boonanuntanasarn S (2013) The effects of vitamins C and E on the growth, tissue accumulation and prophylactic response to thermal and acidic stress of hybrid catfish. Aquac Nutr 19:148–162
Piva A, Casadei G, Pagliuca G, Cabassi E, Galvano F, Solfrizzo M, Riley RT, Diza DE (2005) Activated carbon does not prevent the toxicity of culture material containing fumonisin B1 when fed to weanling piglets. J Anim Sci 83:1939–1947
Reif AE, Nabseth DC (1962) Serum amylase determination by Somogyi’s amyloclastic method with use of a photometric end-point. Clin Chem 8:113–129
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol 28:56–63
Ridal J, Brownlee B, McKenna G, Levac N (2001) Removal of taste and odour compounds by conventional granular activate carbon filtration. Water Qual Res J Can 36:43–54
Rogosic J, Pfister JA, Provenza FD, Grbesa D (2006) The effect of activate charcoal and number of species offered on intake of Mediterranean shrubs by sheep and goats. Appl Anim Behav Sci 101:305–317
Ruttanavut J, Yamauchi K, Goto H, Erikawa T (2009) Effects of dietary bamboo charcoal powder including vinegar liquid on growth performance and histological intestinal change in aigamo ducks. Int J Poult Sci 8:229–236
Selim KM, El-hofy H, Khalil RH (2013) The efficacy of three mycotoxin adsorbents to alleviate aflatoxin B1-induced toxicity in Oreochromis niloticus. Aquac Int. doi:10.1007/s10499-013-9661-6
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affect non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41:125–139
Sunyer JO, Tort L (1995) Natural hemolytic and bactericidal activities of seabream, Sparus aurata serum are affected by the alternative complement pathway. Vet Immunol Immunopathol 45:333–345
Thu M, Koshio S, Ishikawa M, Yokoyama S (2010) Effects of supplementation of dietary bamboo charcoal on growth performance and body composition of juvenile Japanese flounder, Paralichthys olivaceus. J World Aquac Soc 41:255–262
Tucker CS (2000) Off-flavor problems in aquaculture. Rev Fish Sci 8:45–88
Vechklang K, Boonanuntanasarn S, Ponchunchoovong S, Ponchunchoovong S, Pirarat N, Wanapu C (2011) The potential for rice wine residual as an alternative protein source in a practical diet for Nile tilapia (Oreochromis niloticus) at the juvenile stage. Aquac Nutr 17:685–694
Villalba JJ, Provenza FD, Banner RE (2002) Influence of macronutrients and activated charcoal on intake of sagebrush by sheep and goats. J Anim Sci 80:2099–2109
Voigt GJ (2000) Hematology techniques and concepts for veterinary technicians. Iowa State University Press, Ames, p 139
Watson WA, Cremer KF, Chapman JA (1986) Gastrointestinal obstruction associated with multiple-dose activated charcoal. J Emerg Med 4:401–407
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971
Wilson LL, Kurtz DA, Rugh MC, Chase LE, Zieglek JH, Varela-Alvarez H, Borger ML (1971) Effects of feeding activate carbon on growth rate and pesticide concentrations in adipose tissues of steers fed apple waste. J Anim Sci 33:1361–1364
Winsten S, Cehelyk B (1969) A rapid micro diazo technique for measuring total bilirubin. Clin Chim Acta 25:441–446
Yamprayoon J, Noomhorm A (2000) Geosmin and off-flavor in Nile tilapia (Oreochromis niloticus). J Aquat Food Prod Technol 9:29–41
Yoo JH, Ji SC, Jeong GS (2005) Effect of dietary charcoal and wood vinegar mixture (CV82) on body composition of olive flounder Paralichthys olivaceus. J World Aquac Soc 36:203–208
Acknowledgments
This study was supported by grants from the Suranaree University of Technology, the Higher Education Research Promotion and the National Research University Project of Thailand, Office of the Higher Education Commission, and the National Research Council of Thailand (SUT3-303-52-24-23). We acknowledge Mr. Sunai Plymee (SUT Farm) for maintaining the fish throughout this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonanuntanasarn, S., Khaomek, P., Pitaksong, T. et al. The effects of the supplementation of activated charcoal on the growth, health status and fillet composition-odor of Nile tilapia (Oreochromis niloticus) before harvesting. Aquacult Int 22, 1417–1436 (2014). https://doi.org/10.1007/s10499-014-9756-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9756-8