Abstract
This study investigated the effect of continuous temperature decrease on hemocyte apoptosis of the white shrimp Litopenaeus vannamei. In the stress group, water temperature decreased from 26 to 17 °C at a rate of 1 °C/h. Shrimp kept at 26 ± 0.5 °C were used as control group. Total hemocyte count (THC), reactive oxygen species (ROS) production, cytoplasmic free-Ca2+ (CF-Ca2+) concentration, mitochondrial membrane potential (MMP), apoptotic cell ratio, and caspase-3 activity of L. vannamei hemocytes were determined when water temperature decreased to 23, 20, and 17 °C, respectively. Increased ROS production in hemocytes was observed when water temperature decreased to 20 and 17 °C. Decreased THC and cellular MMP, increased CF-Ca2+ concentration, apoptotic cell ratio, and caspase-3 activity were shown when water temperature decreased to 17 °C. These results indicate that water temperature decrease can induce oxidative stress on shrimp hemocytes and then cause mitochondria and caspase-3 mediated hemocyte apoptosis and THC reduction, when water temperature decreased to an unconformable level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water temperature is one of the most important environmental factors which directly affect the survival, growth, and metabolism of shrimp (Diaz et al. 2004; Spanopoulos-Hernandez et al. 2005; Kumlu et al. 2010; Zhou et al. 2011). Shrimp farming in southern China has been adversely affected by winter mortality for several decades, especially in 2008. Recent studies of shrimp have shown that acute low temperature stress would induce reactive oxygen species (ROS) production and hemocyte apoptosis, increase caspase-3 transcription and activity levels, cause DNA damage and lipid peroxidation, reduce the immune functions and resistance against pathogen infection (Cheng et al. 2005; Wang et al. 2009; Chang et al. 2009; Qiu et al. 2011). These studies conducted acute low temperature stress experiment by transferring rapidly from a suitable temperature to a low temperature. However, in the natural environment, water temperature fluctuation is more complicated, and shrimp may suffer in the process of water temperature decrease. So far, only few study focus on the effects of temperature decrease on shrimp (Zhou et al. 2011).
Hemocytes play an essential role in physiology and immune defense of shrimp (Johansson et al. 2000). Loss and damage of circulating hemocytes would depress the immune ability, increase the susceptibility against pathogens, and even endanger the survival (Lorenzon et al. 1999; Cheng et al. 2005; Rodríguez-Ramos et al. 2008). Rapid low temperature transfer has been reported to cause total hemocyte count (THC) reduction which resulted from increased apoptotic hemocytes (Chang et al. 2009).
In crustacean, it had been reported that ROS overproduction could be induced by various stress factors, such as Cu2+ (Xian et al. 2010), pH (Wang et al. 2009), nitrite (Xian et al. 2011), and bacterial lipopolysaccharide (Xian et al. 2012). Oxidative stress was also found to be a damage mechanism of rapid low temperature transfer on shrimp (Qiu et al. 2011).
Oxidative stress is known to be an important induction factor for cell apoptosis (Ermak and Davies 2001). ROS overproduction paralleled hemocyte apoptosis was observed in shrimp exposed to some environmental stress factors (Xian et al. 2010, 2011). Mitochondria play a major role in apoptosis occurrence. Disruptions of mitochondrial functions are important events in cell apoptosis, such as loss of mitochondrial membrane potential (MMP), mitochondrial permeability transition, and release of cytochrome c (Green and Reed 1998; Orrenius 2007). Furthermore, mitochondrion is one of the main sites of ROS generation (Orrenius 2007). Cytoplasmic free-Ca2+ (CF-Ca2+) concentration elevation and caspase-3 activation are also important events of early apoptosis (Porter and Janicke 1999; Ermak and Davies 2001). Previous research has demonstrated that rapid low temperature transfer induced apoptosis of Litopenaeus vannamei hemocytes via caspase-3 pathway (Chang et al. 2009).
We speculate that similar to the rapid low temperature transfer, water temperature decrease would also induce hemocyte apoptosis. The present study is aimed to find out the temperature point of apoptosis occurrence and to confirm which apoptotic events happen. THC, ROS production, CF-Ca2+ concentration, MMP, apoptotic cell ratio, and caspase-3 activity of L. vannamei hemocytes were measured when the water temperature decreased from 26 to 17 °C at a rate of 1 °C/h.
Materials and methods
Animals
The experimental shrimp L. vannamei were obtained from a commercial shrimp farm in Guangdong Province, China. They were maintained in the laboratory with diluted seawater at 5 ‰, pH 7.9–8.0 and controlled temperature (26 ± 0.5 °C), with continuous water circulation. Prior to experimental use, animals were acclimated to the laboratory conditions for 1 week and fed twice daily with shrimp feed (40 % protein, 5.0 % fat, 5.0 % fiber, and 16 % ash, supplied by a commercial diet, China). Only apparently healthy shrimp in the intermolt stage were used.
Temperature decrease trial
One hundred and twenty shrimp individuals with an average weight of 7.58 ± 0.21 g were randomly divided into six plastic tanks (50 cm × 35 cm × 30 cm), with 20 individuals per tank. Air pumps were turned on for continuous aeration. Two artificial climate incubators (temperature range 5–50 °C, RXZ-500D, Ningbo Dongnan Instrument Limited Company, China) were used for water temperature control. In one artificial climate incubator, water temperature of three tanks (stress group) was decreased from 26 to 17 °C at a rate of about 1 °C/h. In the other incubator, three tanks were maintained at 26 ± 0.5 °C as control group. Shrimps were not fed 24 h before trial to avoid interference of diet (González et al. 2010).
Preparation of hemocyte suspension and total hemocyte count (THC)
Hemolymph (300 μl) was withdrawn from the pericardial sinus of each shrimp with 25 gage needle and 1.5 ml syringe containing an equal volume of ice-cold anticoagulant solution (27 mM trisodium citrate, 385 mM sodium chloride, 115 mM glucose, pH 7.5) (Xian et al. 2012), when the water temperature in the stress group decreased to 26, 23, 20, and 17 °C. At each temperature, nine shrimps of each group from three tanks were analyzed.
A drop of hemolymph sample (10 μl) was placed on a hemocytometer to measure THC with light microscope (Olympus). One hundred microliters hemolymph was transferred to a new tube kept on ice for caspase-3 activity assay. The remaining diluted hemolymph was diluted with anticoagulant solution to obtain a final concentration of about 1 × 106 cells/ml and then used for the flow cytometric analysis of ROS production, CF-Ca2+ concentration, MMP, and apoptotic cell ratio.
Reactive oxygen species (ROS) production
The cell-permeant probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Sigma) was used to monitor the level of ROS as described previously (Xian et al. 2012). A volume of 200 μl hemocyte suspension was incubated with 10 μM DCFH-DA for 30 min in the dark at room temperature. Then, the DCF fluorescence (FL1-H) in hemocytes was recorded by flow cytometer (Becton–Dickinson FACSCalibur). ROS production was expressed as mean fluorescence of DCF.
Cytoplasmic free-Ca2+ (CF-Ca2+) concentration
The cell-permeant probe fluo-3/acetoxymethyl ester (fluo-3/AM, Sigma) was used to monitor the concentration of CF-Ca2+ as described previously (Xian et al. 2012). A volume of 200 μl hemocyte suspension was incubated with 10 μM fluo-3/AM for 30 min in the dark. Then, the fluo-3 fluorescence in hemocyte was analyzed by flow cytometer. CF-Ca2+ concentration was expressed as mean fluorescence of fluo-3.
Mitochondrial membrane potential (MMP)
Mitochondrial membrane potential change was examined using the MMP-sensitive fluorescent dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazoylcarbo-cyanine iodide (JC-1, Sigma). JC-1 emits light at red and green wavelengths according to its concentrations absorbed by the mitochondrion: JC-1 forms aggregate which emits orange–red fluorescence at a normal MMP, but MMP decrease would cause increase in JC-1 monomer which emits green fluorescence. Therefore, the red fluorescence and green fluorescence of the JC-1 reflect the change in MMP. A volume of 200 μl hemocyte suspension was incubated with 10 μM JC-1 at room temperature for 30 min in the dark. Then, the hemocytes were analyzed by flow cytometer. Results were expressed as JC-1 red-fluorescent/JC-1 green-fluorescent dot plot. The percentage of MMP decreased cells was analyzed.
Apoptotic cell ratio
The apoptotic hemocytes were examined using Vybrant® Apoptosis Assay Kit #2 (Invitrogen) following the manufacturer’s instructions. Annexin V-Alexa Fluor® 488 is used to quantitatively determine the percentage of cells that are actively undergoing apoptosis. Propidium iodide (PI) is a viability probe and used to distinguish viable cells from nonviable cells. A volume of 300 μl hemocyte suspension was centrifuged (800×g for 10 min at 4 °C) and then resuspended at about 3 × 106 cells/ml in 1× Annexin V binding buffer. One hundred microliters of hemocyte sample was stained with 5 μl of Annexin V-Alexa Fluor® 488 and 1 μl of 100 μg/ml PI working solution for 15 min in the dark. Then, 400 μl 1× Annexin V binding buffer was added to each tube, and the cells were immediately analyzed by flow cytometer. Typically, 10,000 events were collected using excitation/emission wavelengths of 488/525 and 488/675 nm for Alexa Fluor® 488 and PI, respectively. Results were expressed as Annexin V-Alexa Fluor® 488/PI dot plot (Fig. 6). Cells stained negative with both probes represent live cells (quadrant a). Cells stained positive with Annexin V-Alexa Fluor® 488 and negative with PI are early apoptotic cells (quadrant b). Cells stained positive with both Annexin V-Alexa Fluor® 488 and PI are in the end stage of apoptosis, undergoing necrosis, or already dead (quadrant c). Apoptotic cell ratio was expressed as the percentage of cells in quadrant b and c.
Caspase-3 activity
Caspase-3 activity was measured spectrophotometrically using a caspase-3 activity assay kit (Beyotime, China) following the manufacturer’s instructions with a slight modification. Optical density of chromophore p-nitroaniline (pNA) produced from Ac-DEVD-pNA cleaved by caspase-3 was quantified at 405 nm. Hemolymph (100 μl) was centrifuged at 800×g, 4 °C for 10 min. The supernatant was discarded, and the pellet was resuspended in 100 μl cell lysis buffer on ice for 15 min and then centrifuged at 20,000×g, 4 °C for 15 min. The supernatant (60 μl) was incubated for 12 h at room temperature with 120 μl caspase assay buffer and 20 μl Ac-DEVD-pNA (10 mM). The optical density at 405 nm was measured using a microplate reader (iMark™, BIO-RAD) and was estimated from the absorbance readings after 0 and 12 h incubation with Ac-DEVD-pNA.
Statistical analyses
Cell Quest® software (Becton–Dickinson Immunocytometry Systems, San Jose, CA, USA) was used to create logical regions and color gating analyses of fluorescence data. All data are presented as mean ± standard deviation for three replications. A multiple comparison (Tukey) test was conducted to compare the significant differences of the data using SPSS 13.0 program (SPSS Inc., Chicago, IL, USA). A p value <0.05 was considered significant.
Results
THC
The mean of THC ranged from 137.3 ± 15.37 × 105 to 97.0 ± 6.2 × 105 cells/ml as temperature decreased from 26 to 17 °C. There was no significant difference in THC when water temperature decreased to 23 and 20 °C. When water temperature decreased to 17 °C, THC decreased significantly by 29.4 % compared to the initial level (Fig. 1).
ROS production
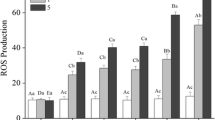
The ROS content of hemocytes increased from 63.9 ± 12.5 to 200.3 ± 38.5 as temperature decreased from 26 to 17 °C. ROS production increased significantly by 119.3 and 213.4 % compared to the initial level, when water temperature decreased to 20 and 17 °C, respectively, while no significant difference in ROS production of hemocytes was observed among shrimp kept at 26 °C at the different sampling times (Figs. 2, 3).
CF-Ca2+ concentration
Significant increase in CF-Ca2+ concentration was observed when the water temperature declined to 17 °C, compared to the level at the initial temperature, while no significant difference in CF-Ca2+ concentration of hemocytes was observed among shrimp kept at 26 °C at the different sampling times (Figs. 4, 5).
MMP
The initial percentage of MMP decreased cells (R2) was 2.7 ± 0.2 %. There was no significant change in MMP while water temperature was decreased from 26 to 20 °C. When the water temperature dropped to 17 °C, MMP of hemocytes significantly decreased, and the percentage of MMP decreased hemocytes increased to 11.9 ± 1.6 % (Figs. 6, 7).
Mitochondrial membrane potential of hemocytes from L. vannamei of L. vannamei in control group (kept at 26 ± 0.5 °C) and stress group (water temperature decreased from 26 to 17 °C at a rate of 1 °C/h). Dates in the same group with different letters are significantly different (p < 0.05). N = 9 shrimps
Apoptotic cell ratio
The initial ratio of apoptotic hemocytes was 2.7 ± 0.6 %. The percentage of apoptotic hemocytes was not affected by temperature decrease from 26 to 23 and 20 °C. Apoptotic cell ratio of hemocytes significantly increased to 10.8 ± 1.0 % when water temperature decreased to 17 °C ( Figs. 8, 9).
Caspase-3 activity
The caspase-3 activity in hemocytes of shrimp in stress groups significantly increased by 182.3 % when water temperature decreased to 17 °C, while no significant difference was observed among shrimp kept at 26 °C at the different sampling times (Fig. 10).
Discussion
Hemocytes play vital role in immunity and physiology of crustaceans. THC is known to be an important indicator for immune function. Decrease in hemocyte number in crustaceans might lead to impaired immune capability, decreased resistance to bacterial infection and ultimately death (Cheng et al. 2005; Xian et al. 2010). The number of hemocytes could be modulated by various factors, such as salinity (Wang and Chen 2006a), ammonia (Rodríguez-Ramos et al. 2008), nitrite (Xian et al. 2011), Cu2+ (Xian et al. 2010), and pathogens (Li et al. 2008). Rapid low temperature transfer has also been reported to result in decrease in hemocyte count in white shrimp L. vannamei (Cheng et al. 2005; Chang et al. 2009; Qiu et al. 2011), tiger shrimp Penaeus monodon (Wang and Chen 2006b), giant freshwater prawn Macrobrachium rosenbergii (Cheng and Chen 2000), and lobster Panulirus interruptus (Gomez-Jimenez et al. 2000). For the white shrimp L. vannamei transferred from 28 to 24 and 20 °C, the THC decreased significantly after 24 h (Cheng et al. 2005). Another study reported that THC significantly reduced when L. vannamei were rapidly transferred from 28 to 22 °C for 2 days (Chang et al. 2009). Effect of temperature decrease on THC was analyzed in the present study. THC did not change when temperature decreased from 26 to 20 °C at a rate of 1 °C/h. Significant decrease in THC only could be observed when water temperature reduced to 17 °C (Fig. 1). This result suggests that the process of temperature decrease has similar effect with rapid low temperature transfer on the hemocyte count of shrimp. There is no doubt that the rapid transfer is a more acute stress than temperature decrease. Suppression in THC reduction did not occur until temperature decreased to 17 °C in the present study, but could be observed when shrimp transferred from 28 to 24 °C (Cheng et al. 2005).
It has previously been reported that a small increase in ROS is considered to be beneficial with respect to increased immunity (Raman et al. 2008). In contrast, high ROS generation may be cytotoxic and lead to oxidative damage to tissue macromolecules including DNA, proteins, and lipids (Orrenius 2007). Previous studies had showed that oxidative stress was one of the damage mechanisms for acute low temperature stress (Qiu et al. 2011). The ROS level in L. vannamei hemocyte increased at 3 h after transfer from 25 to 12 °C (Qiu et al. 2011). Inversely, another study showed that content of superoxide anion (O2 −) in shrimp hemocyte reduced at 24 h after transfer from 28 to 20 °C (Cheng et al. 2005). However, this study did not measure the change of other ROS, such as H2O2 and OH. In the present study, significant increases of ROS content occurred when water temperature decreased to 20 and 17 °C. The results may suggest that temperature decrease also induce oxidative stress on shrimp hemocytes.
Many studies have demonstrated that intracellular ROS generation is intimately associated with apoptotic cell death (Ermak and Davies 2001; Oh and Lim 2006; Orrenius 2007; Xian et al. 2010, 2012). We measured the hemocyte apoptosis by the detection of phosphatidylserine (PS) externalization. Results of apoptosis indicators showed the damage of temperature decrease on hemocytes (Fig. 8). Increase in apoptotic cell ratio was observed when water temperature decreased to 17 °C (Fig. 9). Pervious study of acute low temperature stress showed that percentage of apoptotic hemocytes increased by 88.7 and 200.1 %, after shrimps L. vannamei were transferred from 28 to 22 °C for 2 and 7 days, respectively (Chang et al. 2009). These results indicate that both temperature decrease and temperature transfer would cause hemocyte apoptosis in shrimp. ROS has also been demonstrated to induce cell apoptosis.
Calcium acts as a secondary messenger in a variety of cellular processes. Marked elevations of CF-Ca2+ activate hydrolytic enzymes and then lead to cell apoptosis (Nicotera and Orrenius 1998). Our results showed that significant increase in CF-Ca2+ concentration was observed when water temperature decreased to 17 °C. This result correlated well with the increased apoptotic ratio at the same temperature, suggesting that Ca2+ might play a major role in apoptosis induced by temperature decrease. The increased CF-Ca2+ concentration also correlated well with the elevated production of ROS, indicating that elevated Ca2+ content in the cytoplasm resulted from oxidative stress, as reported in diverse cell types (Ermak and Davies 2001; Mehta and Shaha 2006).
Mitochondrial membrane potential is maintained in a definite degree to sustain the physiological activity in normal cells (Orrenius 2007). Excessive ROS seems to be lethal by inducing the opening of mitochondrial permeability transition pore and leading to the disruption of MMP (Green and Reed 1998; Orrenius 2007). MMP collapse is considered to be an early apoptotic event (Green and Reed 1998). In the present study, loss of MMP occurred when water temperature decreased to 17 °C, presenting a positive correlation with the elevation of ROS production. These results suggest that MMP collapse caused by temperature decrease may be modulated by ROS-mediated signaling pathways.
Caspase activation plays a central role in the execution of programmed cell death (Shi 2004). Caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins (Porter and Janicke 1999). Knocking down caspase-3 by RNAi reduced mortality in L. vannamei challenged with a low dose of white-spot syndrome virus (Rijiravanich et al. 2008). Caspase-3 in L. vannamei hemocytes could be induced by acute low temperature transfer (Chang et al. 2009). In the present study, caspase-3 activation occurred when water temperature decreased to 17 °C, indicating that the increased caspase-3 activity contributed to the occurrence of hemocyte apoptosis induced by temperature decrease.
The present study clearly demonstrated the occurrence of apoptosis in shrimp hemocyte when water temperature decreased to 17 °C. Apoptotic events characterized by CF-Ca2+ increase, MMP loss, PS externalization, and caspase-3 activation paralleled ROS content elevation in terms of temperature response, suggesting that hemocyte apoptosis induced by temperature decrease was mediated by superfluous ROS. Under stress of temperature decrease, ROS overproduction triggers hemocyte apoptosis via the caspase-3 dependent pathway and the mitochondrial pathway. On the other hand, our findings showed that at a decrease rate of 1 °C/h, 17 °C is the unconformable point of water temperature. This fact suggests that shrimp farmers should do defensive measures before the water temperature decreases to 17 °C at this situation. However, the decreasing rate of water temperature may also affect the unconformable point. More studies should be done to confirm the influence of decreasing rate.
References
Chang CC, Yeh MS, Cheng W (2009) Cold shock-induced norepinephrine triggers apoptosis of haemocytes via caspase-3 in the white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 27:695–700
Cheng W, Chen JC (2000) Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 10:387–391
Cheng W, Wang LU, Chen JC (2005) Effect of water temperature on the immune response of white shrimp Litopenaeus vannamei to Vibrio alginolyticus. Aquaculture 250:592–601
Diaz F, Re AD, Sierra E, Diaz-Iglesias E (2004) Effects of temperature and salinity fluctuation on the oxygen consumption, ammonium excretion and osmoregulation of the blue shrimp Litopenaeus stylirostris (Stimpson). J Shellfish Res 23:903–910
Ermak G, Davies KJA (2001) Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol 38:713–721
González RA, Díaz F, Licea A, Re AD, Sanchez LN, Garcia-Esquivel Z (2010) Thermal preference, tolerance and oxygen consumption of adult white shrimp Litopenaeus vannamei (Boone) exposed to different acclimation temperatures. J Therm Biol 35:218–224
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Johansson MW, Keyser P, Sritunyalucksana K, Soderhall K (2000) Crustacean haemocytes and haematopoiesis. Aquaculture 191:45–52
Kumlu M, Türkmen S, Kumlu M (2010) Thermal tolerance of Litopenaeus vannamei (Crustacea: Penaeidae) acclimated to four temperatures. J Therm Biol 35:305–308
Li CC, Yeh ST, Chen JC (2008) The immune response of white shrimp Litopenaeus vannamei following Vibrio alginolyticus injection. Fish Shellfish Immunol 25:853–860
Lorenzon S, De Guarrini S, Smith VJ, Ferrero EA (1999) Effects of LPS injection on circulating haemocytes in crustaceans in vivo. Fish Shellfish Immunol 9:31–50
Mehta A, Shaha C (2006) Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic Bio Med 40:1857–1868
Nicotera P, Orrenius S (1998) The role of calcium in apoptosis. Cell Calcium 23:173–180
Oh SH, Lim SC (2006) A rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulation. Toxicol Appl Pharm 212:212–223
Orrenius S (2007) Reactive oxygen species in mitochondria-mediated cell death. Drug Metab Rev 39:443–455
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Qiu J, Wang WN, Wang LJ, Liu YF, Wang AL (2011) Oxidative stress, DNA damage and osmolality in the Pacific white shrimp, Litopenaeus vannamei exposed to acute low temperature stress. Comp Biochem Physiol C 154:36–41
Raman T, Arumugam M, Mullainadhan P (2008) Agglutinin-mediated phagocytosis-associated generation of superoxide anion and nitric oxide by the hemocytes of the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 24:337–345
Rijiravanich A, Browdy CL, Withyachumnarnkul B (2008) Knocking down caspase-3 by RNAi reduces mortality in Pacific white shrimp Penaeus (Litopenaeus) vannamei challenged with a low dose of white-spot syndrome virus. Fish Shellfish Immunol 24:308–313
Rodríguez-Ramos T, Espinosa G, Hernández-López J, Gollas-Galván T, Marrero J, Borrell Y, Alonso ME, Bécquer U, Alonso M (2008) Effects of Echerichia coli lipopolysaccharides and dissolved ammonia on immune response in southern white shrimp Litopenaeus schmitti. Aquacluture 274:118–125
Shi YG (2004) Caspase activation: revisiting the induced proximity model. Cell 117:855–858
Spanopoulos-Hernandez M, Martinez-Palacios CA, Vanegas-Perez RC, Rosas C, Ross LG (2005) The combined effects of salinity and temperature on the oxygen consumption of juvenile shrimps Litopenaeus stylirostris (Stimpson, 1874). Aquaculture 244:341–348
Wang FI, Chen JC (2006a) Effect of salinity on the immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae. Fish Shellfish Immunol 20:671–681
Wang FI, Chen JC (2006b) The immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae under temperature stress. Aquaculture 258:34–41
Wang WN, Zhou J, Wang P, Tian TT, Zheng Y, Liu Y, Mai WJ, Wang AL (2009) Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp Biochem Physiol C 150:428–435
Xian JA, Wang AL, Ye CX, Chen XD, Wang WN (2010) Phagocytic activity, respiratory burst, cytoplasmic free-Ca2+ concentration and apoptotic cell ratio of haemocytes from the black tiger shrimp, Penaeus monodon under acute copper stress. Comp Biochem Physiol C 152:182–188
Xian JA, Wang AL, Chen XD, Gou NN, Miao YT, Liao SA, Ye CX (2011) Cytotoxicity of nitrite on haemocytes of the tiger shrimp, Penaeus monodon, using flow cytometric analysis. Aquaculture 317:240–244
Xian JA, Miao YT, Li B, Guo H, Wang AL (2012) Apoptosis of tiger shrimp (Penaeus monodon) haemocytes induced by Escherichia coli lipopolysaccharide. Comp Biochem Physiol A 164:301–306
Zhou M, Wang AL, Xian JA (2011) Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: effects of continuous cold stress. Aquaculture 317:182–186
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31302164 and 30671628), China Postdoctoral Science Foundation (2012M511829), Guangdong Provincial Natural Science Foundation (S2011020003256 and S2012040008093), Scientific and Technological Planning Project of Guangdong Province (2011B020307010 and 2012B020307004), Project of Guangdong Provincial Oceanic and Fishery Administration (A200901B06), and the Scientific and Technological Planning Project of Guangzhou City (11A82090870).
Author information
Authors and Affiliations
Corresponding author
Additional information
Bin Li and Jian-An Xian contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, B., Xian, JA., Guo, H. et al. Effect of temperature decrease on hemocyte apoptosis of the white shrimp Litopenaeus vannamei . Aquacult Int 22, 761–774 (2014). https://doi.org/10.1007/s10499-013-9704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9704-z