Abstract
A feeding trial was conducted to investigate the effects of the dietary incorporation of autoclaved and raw (non-autoclaved) Ulva meal on juvenile rainbow trout (Oncorhynchus mykiss) growth, nutrient utilization, body composition, diet digestibility and fatty acid composition. An algae-free control diet and four experimental diets with either 5 or 10 % inclusion levels of raw Ulva meal (5 % = RU5; 10 % = RU10) or autoclaved Ulva meal (5 % = AU5; 10 % = AU10) were formulated. Fish were fed three times daily to apparent satiation for 12 weeks. The growth of fish fed RU10 (final weight = 76.7 ± 3.31 g; SGR = 2.8 ± 0.05 %) diet was significantly higher than that in fish fed the AU10 diet (67.5 ± 1.61 g; SGR = 2.6 ± 0.03 %). The feed conversion ratio (FCR) was significantly better in fish fed the RU10 diet (0.9 ± 0.06) compared with control diet (1.0 ± 0.06). Condition factor, viscerosomatic index and dress-out remained unaffected by dietary treatment. The hepatosomatic index (HSI) was significantly lower (P < 0.05) in fish fed the AU10 diet (1.2 ± 0.19) than fish fed the other diets (≥1.6). The level of eicosapentaenoic acid (20:5n-3—EPA) in muscle from fish fed the RU10, AU5 and AU10 diets was significantly higher than in fish fed control diet (P < 0.05). Dietary inclusion of Ulva meals resulted in a significant increase in muscle docosapentaenoic acid (22:5n-3—DPA) levels at the end of the feeding period. The results indicate that the dietary inclusion of raw Ulva meal at levels of up to 10 % can be used without significant negative effects on the growth performance, nutrient utilization, dietary digestibility and muscle fatty acid composition. In fact, the inclusion of dietary raw and autoclaved Ulva meal elevated n-3 fatty acids levels of rainbow trout muscle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fishmeal is traditionally the main protein source used in fish diets because it is a rich source of essential amino acids, essential fatty acids, energy and minerals (Hertrampf and Piedad-Pascual 2000). In the aquaculture industry, the increasing cost and inadequate supply of fishmeal limit sustainability. Therefore, reducing fishmeal inclusion levels and replacing fishmeal with cost-effective, widely available and sustainable feedstuffs are considered essential for the future development of the aquaculture industry (Tacon et al. 2006; Gatlin et al. 2007; Kaushik and Hemre 2008). Many alternative plant feedstuffs contain high levels of deleterious carbohydrate fractions, complex fiber-type components and anti-nutritional factors (ANF), which can limit their use in carnivorous fish diets (Drew et al. 2007; Krogdahl et al. 2010).
Macroalgae meals have emerged as interesting candidate ingredients for low inclusion level in aquafeeds (Valente et al. 2006; Dantagnan et al. 2009; Soler-Vila et al. 2009; Güroy et al. 2011). Enhancements of growth, feed utilization, lipid metabolism, physiological activity, stress response, disease resistance and carcass quality have been reported with low-level dietary algal inclusion (Mustafa et al. 1994, 1995, 1997; Mustafa and Nakagawa 1995; Nakagawa 1997; Nakagawa and Montgomery 2007). Of these algae, Ulva sp. are among the most commonly used as they are a good source of protein, vitamins, pigments and minerals (Garcia-Casal et al. 2007; Ortiz et al. 2006). The effects of dietary Ulva on fish growth performance, nutrient utilization and immune response have been investigated for several fish species, including rainbow trout (Oncorhynchus mykiss) (Yildirim et al. 2009; Güroy et al. 2011), tilapia (Oreochromis niloticus) (Güroy et al. 2007; Azaza et al. 2008; Ergun et al. 2009), common carp (Cyprinus carpio) (Diler et al. 2007) and the European sea bass (Dicentrarchus labrax) (Valente et al. 2006).
However, no information is available regarding the efficacy of using autoclaved Ulva meal in rainbow trout diets. The objective of the present study was to evaluate the effect of dietary raw and autoclaved Ulva meal on rainbow trout on the growth performance, nutrient utilization, body composition, digestibility and muscle fatty acid profile.
Materials and methods
Diet formulations
Ulva rigida was freshly collected from the near-shore waters of the Dardanelles, Canakkale, Turkey. Algal samples were thoroughly washed with sea water, dried at 40 °C for 48 h and fine-milled with a laboratory blender to produce raw Ulva meal. Fresh U. rigida was autoclaved (121 °C at 1.0 kg cm−2 for 30 min), dried at 40 °C for 48 h and ground to produce the autoclaved Ulva meal. An algae-free control diet and four experimental diets, with either 5 or 10 % inclusion levels of raw Ulva meal (5 % = RU5; 10 % = RU10) or autoclaved Ulva meal (5 % = AU5; 10 % = AU10), were formulated. The formulation and chemical composition of the experimental diets are displayed in Table 1. The dietary fatty acid composition is shown in Table 2. Feed ingredients were thoroughly mixed with the addition of oil and a vitamin/mineral premix using a laboratory food mixer. The moistened mixture was pelleted (2 mm pellet size) in a meat grinder and dried in a forced air oven (40 °C) until moisture content was reduced to approximately 10 %. The diets were stored in sealed plastic bags at −20 °C prior to use.
Experimental facility and fish
Juvenile rainbow trout, O. mykiss, were transported from a local fish farm (Keskinler Trout Farm, Bayramic, Canakkale, Turkey) and acclimatized to laboratory conditions at the Fish Nutrition Aquarium Unit of Canakkale Onsekiz Mart University, Faculty of Fisheries, for 4 weeks while being fed a commercial diet (Bagci Feed Company, crude protein: 50 %, crude lipid: 18 %). Three hundred fish (~7 g) were randomly allocated into 150-l circular tanks (20 fish per tank) within a recirculation freshwater system. Each treatment was conducted in triplicate tanks. Fish were fed to apparent satiation three times a day (08:00, 12:00 and 16:00) for 12 weeks. During the experimental period, temperature was maintained at 16 ± 1.5 °C and dissolved oxygen levels at >80 % saturation with a 12:12 h light:dark photoperiod. Water flow rate was approximately 12 l min−1 tank−1. Fish in each tank were individually weighed at the start and end of the feeding trial. Fish standard length (SL) was measured with fish measuring boards with 1-mm divisions at the start and end of the feeding trial. Daily feed intake was recorded, and each tank was weighed every 2 weeks to observe the growth and feed utilization parameters.
Sampling and proximate composition
Six fish were randomly taken from the initial pool of fish at the beginning of the experiment, and three fish from each tank (nine fish per treatment) were sampled at the end of the trial to determine whole body proximate analysis. At the end of the feeding trial, all fish were starved for 48 h to ensure that the digestive tract was devoid of feed. Analysis of crude protein, moisture, fiber and ash in the whole body of fish and the diets was performed according to standard AOAC (2000) procedures. Dietary and whole-body lipids were extracted according to the procedure of Folch et al. (1957) with chloroform/methanol (2:1 v/v). Nitrogen-free extract (NFE) was calculated by taking the sum values for crude protein, lipid, ash and crude fiber and then subtracting this value from 100. The gross energy content of the diets and fish was calculated using the conversion factors of 23.7 kJ g−1 for protein, 39.5 kJ g−1 for lipid and 17.2 kJ g−1 for carbohydrate (Brett and Groves 1979).
Samples of muscle from three fish per each tank were taken for fatty acid analyses. The fatty acids in the total lipid were esterified into methyl esters by saponification with 0.5 N methanolic NaOH and transesterified with 14 % boron trifluoride–methanol (AOAC 2000). Fatty acid methyl esters (FAME) were analyzed using a flame ionization gas chromatograph (Shimadzu GC-2014) equipped with an Omegawax 250 capillary column (30 m L X 0.25 mm internal diameter), a flame ionization detector (FID) and a split injection system with nitrogen carrier gas. The injector port and detector temperatures were maintained at 250 and 260 °C, respectively. The column temperature program was held at 140 °C for 5 min and then elevated at a rate of 3 °C/min to 200 °C. Total run time was 60 min per sample. Fatty acids were identified by comparing their retention times to that of fatty acid standards (Sigma-Aldrich Co, USA).
Fish performance and somatic indices
Fish growth performance and nutrient utilization were calculated according to the following formulae:
Six fish were randomly taken from the initial pool at the beginning of the experiment, and three fish from each tank (nine fish per treatment) were randomly sampled at the end of the trial to determine somatic indices.
The somatic indices were calculated according to the following formulae:
where FBW is the final body weight (g), IBW is the initial body weight (g), FI = feed intake (g), WG = weight gain (g), T = time (days), PI = dietary protein intake (g), PG = protein gain (g), EG = energy gain, EI = dietary energy intake, SL = standard length (cm), LW = liver weight (g) and VW = viscera weight (g).
Digestibility trial
The digestibility trial was immediately conducted at the end of the growth trial. Feces were collected from each fish within each tank using stripping techniques. Fish were fed in excess by hand 10 h before stripping. Manual stripping of fish was accomplished by netting and anesthetizing all fish in the tank. The feces were then removed from the distal intestine using gentle abdominal pressure. Care was taken to exclude urinary excretions or mucus from the collection. Wet fecal samples were then dried at 50 °C (48 h) before analysis. All diets contained Celite (1 g kg−1) as the indigestible marker. Analysis of acid-insoluble ash in diets and feces was performed according to standard AOAC methodology (2000).
Digestibility coefficients of nutrients were determined with the following formula:
Statistical analysis
All data were subjected to a one-way analysis of variance (ANOVA) and post hoc LSD using the statistical software package Statgraphics 7.0 (Manugistics Incorporated, Rockville, MD, USA) (Zar 2001). All percentage data and ratios were arcsine transformed before being subjected to the analysis. The results were treated statistically significant at the 5 % level.
Results
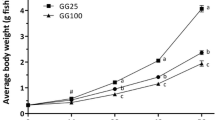
The overall growth performance and nutrient utilization data are displayed in Table 3. The mean weights of fish at the end of the 12-week feeding trial ranged from 76.7 g (RU10 diet) to 67.5 g (AU10 diet) (Fig. 1). The mean weight and specific growth rate (SGR) of fish fed diet RU10 were significantly higher than fish fed the AU10 diet. However, there were no significant differences between any of the remaining treatments (Table 3). The feed conversion ratio (FCR) of fish fed the RU10 diet was significantly lower than fish fed the control diet (Table 3). Although the highest protein efficiency ratio (PER) was recorded in fish fed the RU10 diet, no significant differences were observed among the groups (Table 3). Net protein utilization (NPU) of fish fed AU5 was significantly lower than fish fed the control, RU5 and RU10 diets (P > 0.05). Furthermore, feed intake (FI) was significantly lower for AU5, AU10 and RU10 fed fish than fish fed the control or RU5 diets.
The apparent digestibility coefficients (ADCs) of fish fed the experimental diets are displayed in Table 4. The ADCs of protein (ranging from 93.45 to 98.94 %) were significantly affected (P < 0.05) by the various dietary treatments. The protein ADCs of fish fed Ulva diets were significantly higher than fish fed the control diet. No significant differences were observed in the ADCs of lipid among dietary treatments (P > 0.05).
Data on biometric parameters of fish fed the different diets are displayed in Table 5. Aside from the hepatosomatic index (HSI), which was significantly lower (P < 0.05) in fish fed the AU10 diet than in fish fed the other diets, no differences were observed between the groups. The viscerosomatic index (VSI) of fish fed diets AU5 and AU10 was significantly higher than the fish fed the other diets. Inversely, this trend was observed with dress-out (DO).
The whole-body composition of fish fed the experimental diets is presented in Table 6. Fish fed AU5 contained a significantly lower (P < 0.05) protein content than fish fed the other diets. Furthermore, the body protein content of fish fed the control and RU5 diets was significantly higher than fish fed the AU5 and AU10 diets.
The muscle fatty acid composition of rainbow trout is shown in Table 7. The level of eicosapentaenoic acid (20:5n-3—EPA) of fish fed RU10, AU5 and AU10 diets was significantly higher when compared to fish fed the control diet (P < 0.05). Dietary inclusion of Ulva meals resulted in a significant increase of docosahexaenoic acid (22:6n-3—DHA) in the muscle at the end of the feeding period. The n-3 fatty acids level of the groups fed dietary Ulva meal was significantly higher (P < 0.05) than the control group.
Discussion
The results from the present trial indicate that the inclusion of raw Ulva meal at levels of up to 10 % in practical diets did not cause adverse effects on the growth performance, nutrient utilization, digestibility and muscle fatty acid composition of rainbow trout. Similarly, Valente et al. (2006) observed that 10 % dietary raw Ulva meal had no significant effect on growth performance of European sea bass and Güroy et al. (2007) reported that dietary Ulva rigida meal inclusion at 10 % had no negative effects on the growth of tilapia. Other studies have shown that a 5 % inclusion of dietary Ulva can have a positive influence on the growth performance of black sea bream Acanthopagrus schlegeli (Nakagawa et al. 1993) and snakehead Channa striatus (Hassan and Hashim 1995). In the present study, the inclusion of autoclaved Ulva meal, at both inclusion levels, led to significant reductions in body protein levels. This was not the case when using raw Ulva meal. No other body composition parameters (i.e., moisture, ash and lipid) were affected by the dietary treatments.
Many studies have sought to improve the digestibility and nutritive value of plant feedstuffs by thermal treatment methods (Barrows et al. 2007), and thus in the present study, autoclaved Ulva meal was studied in comparison with raw Ulva meal. The growth performance of fish fed diets containing 10 % autoclaved Ulva meal (group AU10) was significantly lower than that of fish fed 10 % raw Ulva meal (RU10); however, the performance was not significantly different to the control. To the authors’ knowledge, there is no information regarding the effects of autoclaved Ulva meal with respect to growth performance of fish. However, many studies have assessed the effects of thermal processing on plant ingredients incorporated into aquafeeds (Satoh et al. 1998; Mwachireya et al. 1999; Allan and Booth 2004; Davies and Gouveia 2010). Thermal treatment applied throughout the commercial processing of plant products inactivates many of the anti-nutritional factors. The duration of the heat treatment should be kept to a minimum in order to minimize the possibility of destroying indispensable amino acids and vitamins, and reducing the availability of other nutrients (Peres et al. 2003).
The linolenic acid (18:3n-3) content of fish fed the AU5 and AU10 diets was significantly higher than control group. The sums of polyunsaturated fatty acid in the muscle of trout fed the Ulva diets (raw and autoclaved) were significantly superior when compared to control diet. Little information is available regarding muscle fatty acid composition of fish fed dietary algae. However, Dantagnan et al. (2009) reported that the inclusion of 3 and 6 % macroalgae meal resulted in a significant increase in polyunsaturated fatty acids (PUFAs), especially EPA (20:5n-3), DHA (22:6n-3) and linolenic acid, in rainbow trout muscle, and Walker et al. (2009) observed that the arachidonic acid (20:4n-6) levels were elevated in Atlantic cod Gadus morhua juveniles fed Porphyra (30 % fishmeal replace).
The ADCs of dietary lipid were high, with no significant differences being observed between treatment groups. The ADCs for protein of all Ulva groups was significantly higher than the control group, and no differences between the Ulva treatment groups were observed. In contrast to these findings, previous studies have reported reduced ADCs for protein (Appler 1985) and lipid (Valente et al. 2006) in fish fed algal meals.
The present experiment indicates that raw Ulva meal has potential as a novel feed additive for inclusion in diets for rainbow trout juveniles. Dietary inclusion at levels up to 10 % can be used without detrimental effects on the growth performance, nutrient utilization, digestibility or muscle fatty acid composition. In fact, the inclusion of raw and autoclaved Ulva meal in rainbow trout diets improved the n-3 fatty acids content in rainbow trout muscle. Further studies are required to examine the effects on health and lipid metabolism, but it should be noted that high levels of autoclaved Ulva meal lead to poorer performance than raw Ulva meal.
References
Allan GL, Booth MA (2004) Effects of extrusion processing on digestibility of peas, lupins, canola meal and soybean meal in silver perch Bidyanus bidyanus (Mitchell) diets. Aquac Res 35:981–991
AOAC (Association of Official Analytical Chemists) (2000) Official methods of analysis, 19th edn. AOAC, Arlington
Appler HN (1985) Evaluation of hydrodictyon-reticulatum as protein source in feeds for Oreochromis (Tilapia) niloticus and Tilapia zilli. J Fish Biol 27:327–334
Azaza MS, Mensi F, Ksouri J, Dhraief MN, Brini B, Abdelmouleh A, Kraiem MM (2008) Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae ulva meal (Ulva rigida) reared in geothermal waters of southern Tunisia. J Appl Ichthyol 24:202–207
Barrows FT, Stone DAJ, Hardy RW (2007) The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 265:244–252
Brett J, Groves TDD (1979) Physiological energetics. In: Hoar S, Randall DJ, Brett JR (eds) Fish physiology bioenergetics and growth, vol 8. Academic Press, New York, pp 279–352
Dantagnan P, Hernandez A, Borquez A, Mansilla A (2009) Inclusion of macroalgae meal (Macrocystis pyrifera) as feed ingredient for rainbow trout (Oncorhynchus mykiss): effect on flesh fatty acid composition. Aquac Res 41:87–94
Davies SJ, Gouveia A (2010) Response of common carp fry fed diets containing a pea seed meal (Pisum sativum) subjected to different thermal processing methods. Aquaculture 305:117–123
Diler İ, Tekinay A, Güroy D, Güroy B, Soyuturk M (2007) Effects of Ulva rigida on the growth, feed intake and body composition of common carp, Cyprinus carpio. J Biol Sci 7:305–308
Drew MD, Borgeson TL, Thiessen DL (2007) A review of processing of feed ingredients to enhance diet digestibility in finfish. Anim Feed Sci Technol 138:118–136
Ergun S, Soyuturk M, Guroy B, Guroy D, Merrifield D (2009) Influence of Ulva meal on growth, feed utilization, and body composition of juvenile Nile tilapia (Oreochromis niloticus) at two levels of dietary lipid. Aquac Int 17:355–361
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Garcia-Casal MN, Pereira AC, Leets I, Ramirez J, Quiroga ME (2007) High iron content and bioavailability in humans from four species of marine algae. J Nutr 137:2691–2695
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu GS, Krogdahl A, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Güroy BK, Cirik S, Güroy D, Sanver F, Tekinay AA (2007) Effects of Ulva rigida and Cystoseira barbata meals as a feed additive on growth performance, feed utilization, and body composition of Nile tilapia, Oreochromis niloticus. Turk J Vet Anim Sci 31:91–97
Güroy D, Güroy B, Merrifield DL, Ergun S, Tekinay AA, Yigit M (2011) Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. J Anim Physiol Anim Nutr 95:320–327
Hassan N, Hashim R (1995) The use of varying levels of Ulva spp. meal as binders for practical diets and their effect on growth of snakehead (Channa striatus) fry. J Biosci 6:123–131
Hertrampf J, Piedad-Pascual F (2000) Handbook on ingredients for aquaculture feeds. Kluwer, The Netherlands
Kaushik S, Hemre G (2008) Plant proteins as alternative sources for fish feed and farmed fish quality. In: Lie Ø (ed) Improving farmed fish quality and safety. Woodhead Publishing, Cambridge, pp 300–319
Krogdahl A, Penn M, Thorsen J, Refstie S, Bakke AM (2010) Important antinutrients in plant feedstuffs for aquaculture: an update on recent findings regarding responses in salmonids. Aquac Res 41:333–344
Mustafa MG, Nakagawa H (1995) A review: dietary benefits of algae as an additive in fish feed. Isr J Aquac Bamid 47:155–162
Mustafa MG, Umino T, Nakagawa H (1994) The effect of Spirulina feeding on muscle protein deposition in red sea bream, Pagrus major. J Appl Ichthyol 10:141–145
Mustafa MG, Wakamatsu S, Takeda TA, Umino T, Nakagawa H (1995) Effects of algae meal as feed additive on growth, feed efficiency, and body composition in red sea bream. Fish Sci 61:25–28
Mustafa MG, Umino T, Nakagawa H (1997) Limited synergistic effect of dietary Spirulina on vitamin C nutrition of red sea bream Pagrus major. J Mar Biotechnol 5:129–132
Mwachireya SA, Beames RM, Higgs DA, Dosanjh BS (1999) Digestibility of canola protein products derived from the physical, enzymatic and chemical processing of commercial canola meal in rainbow trout Oncorhynchus mykiss (Walbaum) held in fresh water. Aquac Nut 5:73–82
Nakagawa H (1997) Effect of dietary algae on improvement of lipid metabolism in fish. Biomed Pharmacother 51:345–348
Nakagawa H, Montgomery W (2007) Algae. In: Nakagawa H, Sato M, Gatlin D III (eds) Dietary supplements for the health and quality of cultured fish. CABI Publishing, Cambridge, pp 133–167
Nakagawa H, Nematipour G, Yamamoto M, Sugiyama T, Kusaka K (1993) Optimum level of Ulva meal diet supplement to minimize weight loss during wintering in Black Sea Bream Acanthopagrus schlegeli (Bleeker). Asian Fish Sci 6:139–148
Ortiz J, Romero N, Robert P, Araya J, Lopez-Hernandez J, Bozzo C, Navarrete E, Osorio A, Rios A (2006) Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem 99:98–104
Peres H, Lim C, Klesius PH (2003) Nutritional value of heat-treated soybean meal for channel catfish (Ictalurus punctatus). Aquaculture 225:67–82
Satoh S, Higgs D, Dosanjh B, Hardy R, Eales J, Deacon G (1998) Effect of extrusion processing on the nutritive value of canola meal for chinook salmon (Oncorhynchus tshawytscha) in seawater. Aquac Nut 4:115–122
Soler-Vila A, Coughlan S, Guiry MD, Kraan S (2009) The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss): effects on growth, feed efficiency, and carcass composition. J Appl Phycol 21:617–624
Tacon A, Hasan M, Subasinghe R (2006) Use of fishery resources as feed inputs for aquaculture development: trends and policy implications. FAO Fisheries Circular No. 1018. 99
Valente LMP, Gouveia A, Rema P, Matos J, Gomes EF, Pinto IS (2006) Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252:85–91
Walker AB, Fournier HR, Neffus CD, Nardi GC, Berlinsky DL (2009) Partial replacement of fish meal with laver porphyra spp. in diets for Atlantic cod. N Am J Aquac 71:39–45
Yildirim O, Ergun S, Yaman S, Turker A (2009) Effects of two seaweeds (Ulva lactuca and Enteromorpha linza) as a feed additive in diets on growth performance, feed utilization, and body composition of rainbow trout (Oncorhynchus mykiss). Kafkas Univ Vet Fak Derg 15:455–460
Zar JH (2001) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River
Acknowledgments
The authors kindly thank Zeki Sönmez, İzzet Şahin, Volkan Tepe and Serhan Mantoğlu for their technical assistance. We would also like to thank Agromey Feed Mill Company and Kartal Chemical Incorporated for providing feed ingredients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güroy, B., Ergün, S., Merrifield, D.L. et al. Effect of autoclaved Ulva meal on growth performance, nutrient utilization and fatty acid profile of rainbow trout, Oncorhynchus mykiss . Aquacult Int 21, 605–615 (2013). https://doi.org/10.1007/s10499-012-9592-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-012-9592-7