Abstract
The findings of this study provide useful insights into the key aspects of fish cage farming to be considered during setting and overall management of multi-purpose water resources so as to maximise their aquacultural potential. The study was conducted in Machakos District, Kenya, to characterise limnochemistry and algal composition of three small man-made reservoirs Ngeki, Ngei and Kavovi, used for cage culture of Nile tilapia (Oreochromis niloticus). In Ngeki, the open water was compared with areas in between the cages and significant differences in dissolved oxygen were found. For N, P and chlorophyll-a, no differences were noticed. In all the reservoirs, temporal variations were noticed for all parameters except NO3N and NH4N. Peak concentrations of nutrients occurred during the rainy season with lower values in the dry season (below detectable limits for SRP). All reservoirs were classified as hyper-eutrophic with a strong positive correlation between chlorophyll-a and TP. Algal abundances changed with season. Chlorophyta dominated at the start of the rainy season, while cyanobacteria prevailed at the onset of thermal stratification. Diatoms prevailed in the dry season but were notably absent in the rainy season. It was concluded that all the reservoirs are suitable for cage culture of Nile tilapia, but this should be undertaken with caution considering critical periods when anoxic conditions may occur.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The government of Kenya development policy has always focused on alleviating poverty through increased food production and minimisation of environmental degradation. Consequently, a major priority development need of the government has been low-cost aquaculture, which will increase available protein to local communities (Ngugi and Manyala 2004). Aquaculture in Kenya occurs mainly in semi-intensive ponds, where cheap feed supplements and fertilisers are applied to produce low-cost yields (Waidbacher et al. 2006). A more recent management strategy for optimisation of tilapia production, which is still under development, is based on the concept of cage-pond integration (Liti et al. 2005). This technique has been successfully introduced to several East African water bodies under a unifying fish cage culture project known as BOMOSA with the main focus being on small public reservoirs. In Kenya, reservoirs are scattered in arid and semi-arid lands. The majority of the reservoirs are for multi-purpose use, including irrigation, domestic water supply to the rural communities in addition to serving as water points for domestic animals. As the main source of water in these reservoirs is rainfall, they are subject to rapid hydrological fluctuations, as well as changes in water quality.
Cage culture is well established in developed countries as indicated from various studies (McGhie et al. 2000). In these countries, it is mostly practiced in marine coastal waters and reservoirs and here, its impact on water quality is fairly well understood (Alongi et al. 2003). In cage culture, a large amount of wastes (mainly faeces and unconsumed feed) that are rich in nitrogen (N) and phosphorus (P) compounds are released directly into the water (Pawar et al. 2001; Tomaszek and Koszelnik 2003). This results in anoxic conditions, which may create extensive negative ecological impacts, such as internal P loading or accumulation of reduced toxic compounds, leading to heavy mortalities of aquatic life (Troell and Berg 1997; McGhie et al. 2000; Cho and Bureau 2001). The magnitude of these effects depends mainly on the intensity of fish production, waste dispersion by currents and the environmental carrying capacity to assimilate any organic loading (Aguardo-Giménz and García-García 2004). These effects are more apparent in small water bodies where water exchange and the consequent waste dilution through wind-generated currents are limited (Alongi et al. 2003; Guo and Li 2003).
The establishment of cage culture in developed countries has relied heavily on thorough understanding of the systems in which the cages are placed (DeSilva 1998). However, in the tropics where fish cage aquaculture is on the rise, and investigations into small water bodies that offer a huge resource for the development of such activities are still lacking. As the need for cage culture in tropical developing countries becomes more apparent due to the fisheries decline in wild stocks, such investigations are urgently needed, and this study was therefore designed to address some of these gaps.
The main objective of this study was to monitor temporal changes in key water-quality parameters that may be influenced by associated cage culture activities in one of the reservoirs and to characterise the limnochemistry and algal composition of three small reservoirs used for cage culture in Kenya. It is anticipated that the information obtained will be essential in achieving and maintaining sustainable multiple utilisation of these reservoirs. The findings from this research are also expected to be applicable for other tropical semi-arid areas.
Materials and methods
Study area and sites description
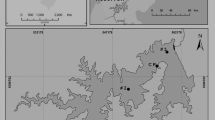
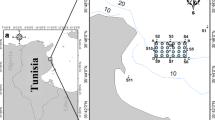
The study was conducted in the three reservoirs Ngeki, Ngei and Kavovi (Fig. 1, Table 1) located in the Machakos District. The district is situated on a predominantly semi-arid, eastward-facing slope and is part of Kenya’s eastern Foreland Plateau, an eroded basement complex broken by residual hill masses and occasionally over-lain by tertiary volcanics (Bernard et al. 1989; Wisner 1977). Rainfall, except in the hilly regions, is low and unreliable (Bernard et al. 1989). The precipitation pattern is bimodal, with long rains falling between March and May and short rains from October to December (Moore 1979). The soils are all generally of low fertility, and many are highly erodible. The dominant vegetation is dry bush with trees, and, in the higher areas, savannah with scattered trees (Ominde 1968). The “desirable” agricultural land has been cleared, leaving behind dry expanses of grazing land. The forest zone is now largely under cultivation, with shrubby secondary growth dominating non-cultivated areas.
The type of fish cages used was according to the BOMOSA cage specifications. The cage was formed by a top (1.20 × 0.94 m), a base of (0.90 × 0.90 m) and a height of 0.75 m. The frames of the cages were constructed from 5.1-cm diameter polyvinyl chloride pipes and covered with nylon net of mesh size of 1.4 cm (Waidbacher et al. 2006). The fish (Oreochromis niloticus) were stocked in 5 cages, and the number of fish per cage ranged from 140 to 150 with a mean weight ranging from 0.03 to 0.08 kg. They were fed twice a day (10:00 h and 16:00 h) with the help of automatic feeders at a daily feeding rate of 1.5% of body weight with a crude protein content of 24%.
Sampling
Water samples were collected biweekly at integrated depths of 1 m from the surface from March to August 2005 using a column sampler (1.12 m) developed by Boyd and Tucker (1992). The samples were collected in 1-l polyethylene containers, stored and transported on ice. In Ngeki, ten more samples were taken, that is, five from the fish cages and another five approximately 10 m away from the cages. During sampling, in situ measurements were made for water temperature and specific conductivity with HI 9060 and HI 8733—Hanna Instruments probes, while dissolved oxygen and pH were measured with DO-5509 and pH-201 Lutron metres. Transparency was measured with a Secchi disc. A plankton net (20 μm) was used to concentrate and collect living material for phytoplankton identification in the laboratory. For Ngeki, primary productivity measurements using the Winkler’s light–dark bottle method were done. Water samples were collected from 10 cm below the surface using a 10-l canister. After vigorous shaking, bottles were filled immediately and then duplicate transparent and dark bottles were exposed vertically at specific depths (0, 10, 25, 50, 75 and 120 cm) considering light attenuation in the reservoir. The incubation experiments were carried out around mid-day and lasted for 2 h. The oxygen content in the exposed bottles was fixed immediately by adding Winkler’s reagents consecutively and shaking vigorously (American Public Health Association 1995). The samples were then transported to the laboratory under cool conditions for further analysis.
Analysis of samples
Water-quality analyses were done according to standard procedures (American Public Health Association 1995). The samples were filtered through glass fibre filters (Whatman GF/F) and used for analysis of Nitrite–N (NO2–N), Ammonium–N (NH4–N), Nitrate–N (NO3–N) and soluble reactive phosphorus (SRP). The sulphanilamide method was applied to determine NO2–N, whereas the phenol–hypochlorite method, the sodium-salicylate method and the molybdate-blue method were used for NH4–N, NO3–N and SRP, respectively. Unfiltered samples were used to analyse total phosphorus (TP), total alkalinity and total hardness. For TP, the sample was first digested using persulphate digestion in the autoclave after which the samples were analysed according to the SRP protocol. Total alkalinity and total hardness were titrated using standard procedures (American Public Health Association 1995). Dry mass (DM) and ash mass (AM) were analysed by filtering a defined volume of water sample using pre-weighed filters (Ederol BM/C filters). For DM, filters together with material were dried at 95 ± 5°C until a constant weight was achieved. For the estimation of the AM mass (inorganic contents), DM filters were combusted in a muffle furnace for 2 h at 550°C. The weight loss on ignition gave an estimate of the organic content (OC).
Additionally to net plankton identification, small forms were concentrated using membrane filtration. Living organisms were observed under a compound microscope (Olympus CH-2) to identify the various algae present with the aid of identification keys, including Wolowski (2002), John and Tsarenko (2002), Komarek (2003) and Kingston (2003). The relative abundances of living material were estimated on the Tansley scale (0 = not detected. 1 rare, 2 occasional, 3 frequent, 4 abundant, 5 dominant; Schaminée et al. 1995; Schils 2000). Algal biomass was estimated using the chlorophyll-a method (Wetzel and Likens 1991). A defined volume of water sample was filtered through a Whatmann GF/C filter by gentle vacuum filtration and the filter together with the phytoplankton stored in a freezer overnight so as to aid in the bursting of the cells. The phytoplankton and the filter were then homogenised in a mortar covered with about 8 ml of 90% aqueous acetone. The samples were transferred into screw-cap vial/centrifuge tube, the mortar rinsed with 90% acetone and the rinse added to the extraction slurry. The volume was adjusted to 12 ml with 90% acetone, and the sample was left for at least 8 h in the dark at 4°C for the chlorophyll-a extraction. After incubation, the sample was centrifuged in closed tubes for 10 min at 2,500 g. The clarified extract was decanted into a clean test tube. Light extinction was measured with a spectrophotometer with the sample placed in 1-cm cell cuvettes, at 663 nm. The phaeophytin effect was taken care of by acidifying the extract. The clarified extract absorbance will be read at 663 nm 5 min after adding 0.1 ml of 0.1 N HCl to the extract in the cuvette and gently agitating. Chl-a concentrations were calculated using the formulae, Chl-a (μg l−1) = 26.7 × V1 × (E663 − E663a)/V2 × Path, where, V1 = volume of extract, ml; V2 = volume of sample, l; Path = light path length or width of cuvette, cm; E663, E663a = absorbances of extracts before and after acidification, respectively.
Data analysis
Data were analysed by SPSS 12.0.1 statistical. One-way ANOVA was used to test for differences between the cages and open water for normally distributed data. Mann–Whitney U test was also used to test for differences in dissolved oxygen concentrations between the cages and open water. Pearson’s correlation tests were done to check for the presence of significant relationships between the water-quality parameters.
Results
The long rainy season lasted from March until May with a total of 370 mm of rainfall (Table 2). Water temperature of all the reservoirs ranged from 22 to 28°C depicting two main patterns: thermal gradients were formed during the rainy season, while isothermal conditions occurred in the dry season (Fig. 2). Accordingly, variations in oxygen concentrations over time and with depth were measured showing seasonal patterns in all the reservoirs and particularly Ngei where anoxic conditions occurred during the long rainy season (Fig. 2). A summary of the physical and chemical variables observed during this period is given in Table 3.
Ngeki’s overall average daily areal net primary productivity during the study period was 3.83 g O2 m−2 day−1 (Fig. 3). The highest daily areal net primary productivity occurred during the rainy season followed by a rapid decline going below the compensation point with a value of −0.99 g O2 m−2 day−1 at the beginning of June. This was later followed by a slight increase in productivity until the end of the study period. When comparing the cages’ areas with the open water zone, significant differences (P < 0.05, Mann–Whitney U test) in the DO concentration were noticed, with higher DO amounts in the open water. However, no significant differences were found for N, P and chlorophyll-a concentrations (Table 3). Temporal variations of SRP, TP, NO3–N, NO2–N, NH4–N and chlorophyll-a in the cage area were similar to the open water in Ngeki reservoir (Fig. 4). In all the reservoirs, temporal variations were observed for nutrients except NO3–N and NH4–N. Peak concentrations occurred in the rainy season with amounts decreasing to below detectable limits (SRP) in the dry season (Fig. 5).
Chlorophyta dominated the phytoplankton community especially at the start of the rainy season, while cyanobacteria prevailed with the onset of thermal gradient formation. Diatoms were notably absent in the rainy season, especially in Ngeki and Kavovi, but they were prevalent in the dry season (Table 4). In Ngei, the OC remained high throughout the study period, which was also confirmed by low Secchi depth values (Table 3).
Discussion
Considering both the TP and chlorophyll-a concentrations obtained from this study and based on the classification criteria of Forsberg and Ryding (1980) and Wetzel (2001), all the three reservoirs were classified as hyper-eutrophic with a strong correlation between chlorophyll-a and TP (r = 0.781, n = 36, P < 0.01). To assess the impact of N and P from the nutrient input of cage farming, some estimations were done during this study period for Ngeki: initial total fish biomass was 60 kg and the daily feeding = 1.5% of body mass, which is 0.9 kg day−1 containing 1.65% P ≡ 14.85 g P and 3.84% N ≡ 34.56 g N. The volume of Ngeki was estimated with 40,000 m3, the duration of study period was 150 days. Therefore, the amount of P input in the whole reservoir per day is given with 0.37 μg l−1 or for 150 days with 55.5 μg l−1. In a similar way, the amount of N input during the study period was estimated at 129.6 μg l−1. However, only approximately 31% of N and P that is added through feed are removed at harvest (Holby and Hall 1991; Hall et al. 1990, 1992), the rest being released as fish faeces, urinary waste and uneaten feed (Troell and Norberg 1998). Using this ratio (31%), P and N converted into fish biomass is equivalent to 17.2 and 40.2 μg l−1, respectively. Therefore, 38.3 μg l−1 P and 89.4 μg l−1 N was released as waste into the reservoir during the study period. This nutrient input indicates the potential for further eutrophication. However, even with such an input, no significant differences in nutrient concentrations could be detected between the cages and open water. This could be attributed to a number of factors such as flushing, which may have occurred in the rainy season bringing about dilution and dispersal of accumulated waste. Wild fish were observed to be feeding on the unconsumed feed, which could additionally have limited any accumulation. The wild fish are harvested, though at a rather small scale, by the local people who may also contribute to minimising the impact of excess nutrients from waste food. The magnitude of any effect depends basically on the intensity of fish production, waste dispersion by currents and the assimilative capacity of any organic loading, N and P principally (Aguardo-Giménz and García-García 2004). A number of studies in coastal regions have indicated that where water circulation and tidal flushing are vigorous, there has been failure to detect any influence or have detected only transitory impacts on the water quality and food chain (Aure and Stiegebrandt 1990). To our knowledge, such studies however do not exist for small water bodies of the tropics.
Seasonality played an important role in the patterns of temperature gradients and oxygen concentration. Generally high water temperatures of the three reservoirs reflect tropical conditions, which are characterised by high solar radiation (Talling 2001; Wetzel 2001). This is also linked to high areal net primary productivity of tropical water bodies (Talling 2004), which caused super-saturation of oxygen especially in Ngei. In addition, the short fetch limited mixing to over-ride the prevailing strong oxygen gradient. Oxygen depletion can be seen as an indicator of elevated respiration and microbial activities. Such processes are most pronounced in shallow waters because of the greater ratio of sediment area to water volume and opportunities for vertical transport (Talling and Lemoalle 1998). The combined effect of these factors led to the anoxic conditions observed at greater depths. Under such conditions, NH3–N and hydrogen sulphide concentrations may rise to levels deleterious to organisms (Islam 2005). In addition to cage farming, which itself already incriminates these fragile ecosystems, pollution from surrounding areas might be another threat. One example is the generally higher conductivity, hardness and alkalinity recorded in Ngeki, which could be attributed to organic loading from the surrounding agricultural land. The higher values for total hardness originated probably from external Ca2+ loading of erosion from the agricultural farms, which included fertilisers such as, calcium ammonium nitrate (CAN). Lacoul and Freedman (2005) made a similar comparison in their survey of lentic Nepalese water bodies.
Variations in nutrient concentrations likewise were influenced by the changes of the hydrological regime. The exceptionally high TP concentrations (200–600 μg l−1) and continual presence of SRP throughout the study period in Ngei were most probably the result of internal loading enhanced by anoxic conditions. Similar concentrations have been observed in other hyper-trophic reservoirs and shallow lakes (Gonzalez et al. 2004; Kowalczewska-Madura and Gołdyn 2010; Song et al. 2010). The lack of an outlet also led to an exaggerated accumulation of nutrients with the reservoir acting as a sink. Interestingly, Kavovi had much lower concentrations of TP and SRP throughout the study period which could have resulted from the nutrient stripping done by the floating and emergent macrophytes (Brenner et al. 1999; Bachmann et al. 2002; Gereta et al. 2004). The presence of such macrophytes modifies the limnology through e.g., stabilising the water column, nutrient uptake and high respiration during the night and should be seriously considered when sighting locations for fish cage culture.
The higher frequency for euglenophytes in Kavovi was attributed to the high amount of organic matter input originating from the cattle excreta, macrophytes and plant litter. This was further supported by the elevated NH4–N concentrations. Euglenoids such as Trachelomonas sp. have been found to occur more frequently in waters fringed with vascular plants, especially where an accumulation of plant litter occurs. In addition, they may reach bloom proportions on a regular basis in ponds and slow-flowing drainage ditches exposed to animal dung, although species diversity may be low in such nutrient-enriched situations (Kim and Sung 1996; Lee 2008; Rosowski 2003). In lakes enriched in nutrients, a persistently high mixing to euphotic depth ratio promotes the dominance of non-mucilaginous colonial Chlorococcales such as Scenedesmus, Pediastrum and Coelastrum (Shubert 2003)—a situation which was observed in Ngeki and Ngei where these taxa occurred abundantly. Diatoms, such as Navicula, Nitzschia and Asterionella appeared, in the cold dry season because of the low temperatures favourable for their high density. They often show optimal growth in well-mixed water columns (Bormans and Webster 1999) that were characteristic of this period. Colonial cyanobacteria like Microcystis and the large unicellular dinoflagellate Peridinium prevailed during the rainy season since they are favoured by their morphological and behavioural adaptations such as the density ‘reduction’ (mucilaginous colonies, gas vesicles) and motility (flagellates), which enabled them to have prolonged residence times in the upper irradiated waters (Reynolds 1984). Special attention has to be paid to Cyanobacteria blooms, which are symptomatic of eutrophication in productive lakes, since they may produce cyanotoxins (Chorus and Bartram 1999; Hudnell 2008). Cyanotoxins are toxic to vertebrates (Malbrouck and Kestemont 2006) and have been detected in muscle and liver tissues of certain fishes indicating their assimilation by the fish (Magalhães et al. 2001; Semyalo et al. 2010). The consumption of such contaminated fish can affect human health as this could cause serious gastrointestinal and neurological diseases (Magalhães et al. 2003). Hence, it is relevant to monitor the fish as well as the phytoplankton community so that the microcystin levels do not exceed the recommended WHO total daily intake (TDI) of 0.04 μg−1 kg−1 day−1 (Chorus and Bartram 1999).
Conclusions and recommendations
The reservoirs are suitable for cage culture, though the water quality in the reservoirs fluctuated in response to the seasonal changes. However, compared to the open water, some DO depletion was found in the cage area, which most probably was due to increased bacterial activity on the decomposing fish feed and waste. This should be taken into serious consideration when setting up potential resources for cage farming, because prolonged DO depletion may lead to massive fish kills. It was concluded therefore that the amount of diet offered to the fish is one crucial factor for cage farming, because too much food and/or too high feeding rates might cause severe DO depletion. Fish cage farming should be done with caution taking into account the critical periods when the anoxic conditions are most likely to occur to avoid fish kills. This may be counteracted by, for example, having lower fish-stocking densities or adjusting the cage depth so as to fit in the depth with good oxygen supply. The findings of this study clearly emphasise the advantage of a multi-level approach in the appropriate management of such reservoirs. These approaches should involve physical, chemical and biological factors so as to maximise the potential they have to offer as aquaculture resources and to optimise their productivity. Cost-effective monitoring that can be supported by modelling (FAO 2009) should be done to ensure that the optimal environmental conditions are maintained and also not to interfere with the other intended purposes of the reservoirs.
References
Aguardo-Giménz F, García-García B (2004) Assessment of some chemical parameters in marine sediments exposed to offshore cage fish farming influence: a pilot study. Aquaculture 242:283–296
Alongi DM, Chong VC, Dixon P, Sasekuman A, Tirendi F (2003) The influence of fish cage culture on pelagic carbon flow and water chemistry in tidally dominated mangrove estuaries of peninsular Malaysia. Mar Env Res 55:313–333
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington DC
Aure J, Stiegebrandt A (1990) Quantitative estimates of the eutrophication effects of fish farming on fjords. Aquaculture 90:135–156
Bachmann WR, Horsburgh AC, Hoyer VM, Mataraza KL, Canfield ED Jr (2002) Relations between trophic state indicators and plant biomass in Florida lakes. Hyd 470:219–234
Bernard FE, Campbell DJ, Thom DJ (1989) Carrying capacity of the eastern ecological gradient of Kenya. Nat Geog Res 5:399–421
Bormans M, Webster TI (1999) Modelling the spatial and temporal variability of diatoms in the River Murray. J Plank Res 21:581–598
Boyd CE, Tucker CS (1992) Water quality and pond soil analyses for Aquaculture. Auburn, Alabama
Brenner M, Keenan LW, Miller SJ, Schelske CL (1999) Spatial and temporal patterns of sediment and nutrient accumulation in shallow lakes of the Upper St. Johns River Basin, Florida. Wet Eco Mgt 6:221–240
Cho CY, Bureau DP (2001) A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac Res 32:349–360
Chorus I, Bartram J (eds) (1999) Toxic Cyanobacteria in water: a guide to their public health consequences, monitoring and management. Spon Press, London
DeSilva SS (1998) Tropical mariculture. In: DeSilva SS (ed) Current status and prospects, 1st edn. Academic Press, San Diego
FAO (2009) Fisheries and Aquaculture Technical Paper, 527. Environmental impact assessment and monitoring in aquaculture. Requirements, practices, effectiveness and improvements. Fisheries and Aquaculture Department, Rome
Forsberg C, Ryding SO (1980) Eutrophication parameters and trophic status indices in 30 waste-receiving Swedish lakes. Archiv für Hydr 89:189–207
Gereta E, Mwangomo E, Wolemski E (2004) The influence of wetlands in regulating water quality in the Seronera River, Serengeti National Park, Tanzania. Wet Eco Mgt 12:301–307
Gonzalez JE, Ortaz M, Peñaherrera C, de Infante A (2004) Physical and chemical features of a tropical hypertrophic reservoir permanently stratified. Hydrobiologia 522:301–310
Guo LG, Li JZ (2003) Effects of nitrogen and phosphorous from fish cage culture on the communities of a shallow lake in middle Yangtze river basin of China. Aquaculture 226:201–212
Hall POJ, Andersson LG, Holby O, Kollberg S, Samuelsson MO (1990) Chemical and mass balances in a marine fish cage farm. I. Carbon. Mar Eco Prog Ser 61:61–73
Hall POJ, Holby O, Kollberg S, Samuelsson MO (1992) Chemical fluxes and mass balances in a marine fish cage farm. IV. Nitrogen. Mar Eco Prog Ser 89:81–91
Holby O, Hall POJ (1991) Chemical fluxes and mass balances in a marine cage farm. II. Phosphorus. Mar Eco Prog Ser 70:263–272
Hudnell HK (2008) Cyanobacterial harmful algal blooms: state of the science and research needs, 1st edn. Springer, New York
Islam SM (2005) Nitrogen and phosphorus budget in coastal and marine cage aquaculture and impacts of effluent loading on ecosystem: review and analysis towards model development. Mar Poll Bull 50:48–61
John DM, Tsarenko PM (2002) Order Chlorococcales. In: John DM, Whitton BA, Brook AJ (eds) The freshwater algal flora of the British Isles, an identification guide to freshwater and terrestrial algae, 1st edn. Cambridge University Press, Cambridge
Kim TJ, Sung MB (1996) Seasonal changes of the Euglenoid Species and the Biomass in the Kumgang River. Algae (The Kor J of Phy) 11:375–379
Kingston CJ (2003) Araphid and Monoraphid Diatoms. In: Wehr JD, Sheath RG (eds) Freshwater algae of North America, ecology and classification, 1st edn. Academic Press, San Diego
Komarek J (2003) Coccoid and Colonial Cyanobacteria. In: Wehr JD, Sheath RG (eds) Freshwater Algae of North America, ecology and classification, 1st edn. Academic Press, San Diego
Kowalczewska-Madura K, Gołdyn R (2010) Models of phosphorus turn-over in a hypertrophic lake: the Lake Swarzędzkie case study. Oceanol Hydrobiol Stud 39:21–33
Lacoul P, Freedman B (2005) Physical and chemical limnology of 34 lentic waterbodies along a tropical-to-alpine altitudinal gradient in Nepal. Int Rev Hydrobiol 90:254–276
Lee RE (ed) (2008) Phycology. Cambridge University Press, Cambridge
Liti D, Cherop L, Munguti J, Chhorn L (2005) Growth and economic performance of Nile tilapia (Oreochromis niloticus) fed on two formulated diets and two locally available feeds in fertilised ponds. Aquac Res 36:746–752
Magalhães FV, Soares MR, Azevedo OFMS (2001) Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Jeneiro, Brazil): ecological implication and human health risk. Toxicon 39:1077–1085
Magalhães FV, Marinho MM, Domingos P, Oliveira CA, Costa MS, Azevedo OL, Azevedo OFMS (2003) Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brasil, RJ). Toxicon 42:289–295
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Env Tox Chem 25:72–86
McGhie TK, Crawford CM, Mitchell IM, O′Brien D (2000) The degradation of fish-cage waste in sediments during fallowing. Aquaculture 187:331–336
Moore TR (1979) Rainfall erosivity in East Africa. Geog Ann Phy Geog 61a:147–156
Ngugi CC, Manyala JO (2004) Aquaculture extension services in Kenya. In: FAO Fisheries Department (ed) Aquaculture extension services in Sub-Saharan Africa Fisheries Department Circular–C1002. FAO, Italy
Ominde SH (1968) Land and population movements in Kenya. Northwestern University Press, Illinois
Pawar V, Matsuda O, Yamamoto T, Hashimoto T, Rajendran N (2001) Spatial and temporal variations of sediment quality in and around fish cage farms: a case study of aquaculture in Seto Inland Sea, Japan. Fish Sci 67:619–627
Reynolds CS (1984) The ecology of freshwater phytoplankton. In: Reynolds CS (ed) Cambridge studies in ecology, 1st edn. University Press, Cambridge
Rosowski RJ (2003) Photosynthetic Euglenoids. In: Wehr JD, Sheath RG (eds) Freshwater algae of North America, ecology and classification, 1st edn. Academic Press, San Diego
Schaminée JHJ, Weeda EJ, Westhoff V (1995) De vegetatie van Nederland. Deel 2. Opulus Press, Uppsala, Leiden 357 pp
Schils T (2000) Short report: macroalgal assemblages of the Socotra Archipelago, Yemen. In: Hariri KI, Krupp F (eds) Conservation and sustainable use of biodiversity of Socotra Archipelago. Marine habitat, biodiversity and fisheries surveys and management. Report of phase IV. Senckenberg Research Institute, Germany 12 pp
Semyalo R, Rohrlack T, Naggawa C, Nyakairu WG (2010) Microcystin concentrations in Nile Tilapia (Oreochromis niloticus) caught from Murchison Bay, Lake Victoria and Lake Mburo: Uganda. Hydrobiologia 638:235–244
Shubert EL (2003) Non-motile Coccoid and Colonial Green Algae. In: Wehr JD, Sheath RG (eds) Freshwater algae of North America, ecology and classification, 1st edn. Academic Press, San Diego
Song X, Liu Z, Yang G, Chen Y (2010) Effects of resuspension and eutrophication level on summer phytoplankton dynamics in two hypertrophic areas of Lake Taihu, China. Aquat Ecol 44:41–54
Talling JF (2001) Environmental controls on the functioning of shallow tropical lakes. Hydrobiologia 458:1–8
Talling JF (2004) Interrelation of diel and seasonal change, involving summer thermal stratification, weather variables and a mobile dinoflagellate in a productive English lake. Hydrobiologia 524:215–227
Talling FJ, Lemoalle J (eds) (1998) Ecological dynamics of tropical inland waters. Cambridge University Press, Cambridge
Tomaszek JA, Koszelnik P (2003) A simple model of nitrogen retention in reservoirs. Hydrobiologia 504:51–58
Troell M, Berg H (1997) Cage fish farming in the tropical Lake Kariba, Zimbabwe: impact and biological changes in sediment. Aquac Res 28:527–544
Troell M, Norberg J (1998) Modelling output and retention of suspended solids in an integrated salmon-mussel culture. Ecol Mod 110:65–77
Waidbacher H, Liti MD, Fungomeli M, Mbaluka KR, Munguti MJ, Straif M (2006) Influence of pond fertilisation and feeding rate on growth performance, economic returns and water quality in a small-scale cage-cum-pond integrated system for production of Nile tilapia (Oreochromis niloticus L.). Aquac Res 37:594–600
Wetzel GR (ed) (2001) Limnology, lake and river ecosystems. Academic Press, San Diego
Wetzel GR, Likens EG (eds) (1991) Limnological analyses. Springer, New York
Wisner B (1977) Man-made famine in eastern Kenya: the interrelationship of environment and development. In: O’Keefe P, Wisner B (eds) Landuse and development. African Environment Special Report 5, London
Wolowski K (2002) Order Euglenales. In: Whitton BA, Brook AJ (eds) The freshwater algal flora of the British Isles, an identification guide to freshwater and terrestrial algae, 1st edn. Cambridge University Press, Cambridge
Acknowledgments
We thank the Austrian Academy of Sciences for funding this project and the BOMOSA pilot project (EU contract no. 032103) for granting us free access to the plots and for all the assistance rendered to be able to successfully carry out this work. Special thanks to Gerold Winkler for his support in publishing this work. We also acknowledge and appreciate Paul T. Yillia for his constructive suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaggwa, M.N., Liti, D.M. & Schagerl, M. Small tropical reservoirs and fish cage culture: a pilot study conducted in Machakos district, Kenya. Aquacult Int 19, 839–853 (2011). https://doi.org/10.1007/s10499-010-9403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9403-y