Abstract
Precocity has been observed in fishes and crustaceans. However, mechanisms underlying precocity have not been well documented in crustaceans and are thought to be influenced by both genetic and environmental factors. Also, precocity is generally considered to have negative effects on crustaceans. The Chinese mitten crab Eriocheir sinensis, a catadromous species endemic to China, is a high valued commodity and in the recent past is being extensively cultured to meet the growing demand by the restaurant trade. The mitten crab is an ideal candidate for precocity studies because of their large size, distinct secondary sex characters, wide distribution and abundant availability from commercial farms. In this article, progress in several aspects of precocity of E. sinensis is reviewed, including the phenomena of precocity and its effect, identification of precocious crabs, factors related to precocity (temperature, salinity, light, nutrition, stocking density, and germplasm), relationships between precocity and neuro-endocrine system, steroid hormones or hepatopancreas, prevention and control methods of precocity. In addition, possible future directions for the study of precocity are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Precocity has been observed in fishes and crustaceans (O’Brien 1984; Amano et al. 1995; Jin and Xie 2001; Basavaraju et al. 2002; Jin et al. 2002; Okuzawa 2002; Begtashi et al. 2004). In fishes, precocity may benefit some fish farmers by enabling them to obtain fertilized eggs from precocious individuals of those species which need many years to reach puberty, such as sturgeons, but it may not be welcomed in species that exhibit short periods of quiescence before maturation for it may lead to growth depletion and be more susceptible to diseases (Okuzawa 2002; Begtashi et al. 2004; Felip et al. 2006). Precocity in crustaceans has not been widely studied.
The Chinese mitten crab Eriocheir sinensis, a catadromous species endemic to China, is a high valued commodity and in the recent past is being extensively cultured to meet the growing demand by the restaurant trade. The cultured production of mitten crab in China has increased from 232,400 tons in 2000 to 518,400 tons in 2008 (China Fishery Statistical Yeakbook 2009), with a commercial value of approximately US$ 2.2 billion in 2005 (FAO 2007). The mitten crab is an ideal candidate for precocity studies because of their large size, distinct secondary sex characters, wide distribution and abundant availability from commercial farms. The precocity of E. sinensis has drawn much attention from the aquaculture sector due to the importance of the recently emerged crab farming industry and the adverse economic effects of early maturation. Precocity is considered as a serious problem in juvenile crab culture (Liu 1998; Wu and Jiang 2000; Gu and Jiang 2002; Chen et al. 2003; Li et al. 2005; Wei et al. 2007; Wu et al. 2010). While the rate of precocity in wild crab populations is only 5–10%, but it may increase up to 18.2–98% under culture conditions (Zhang and Xu 2001). Precocious crabs have a low value due to their low growth rate, poor survival, and short life span (Chen and Shen 1999; Gu and Jiang 2002; Wei et al. 2007), which causes serious economic losses to farmers. In this study, progress in several aspects on precocity of E. sinensis is reviewed, including the phenomena of precocity and its effect, identification of precocious crabs, factors related to precocity, relationships between precocity and the neuro-endocrine system, steroid hormones or the hepatopancreas, prevention and control methods. In addition, the possible directions for the study of precocity in future are suggested.
Characters of precocity and the effects
The normal lifespan of female and male E. sinensis is about 24 and 22 months, respectively (Xu and Zhu 1994). Crabs that reach maturity 1 year earlier than the normal by the first autumn are called precocious crabs (Jin and Xie 2001). Therefore, the life span of female and male precocious crabs is about 12 and 10 months, respectively (Xu and Zhu 1994).
Overwintering survival rate and total survival rate of precocious crabs were reported to be significantly lower than that of normal crabs (Jin et al. 1998). If precocious crabs are reared in the second year, they grow slowly and most of them die off before June, with mortality up to 60–90% (Xu and Zhu 1994; Chen and Shen 1999). Due to unsuccessful molting, the high mortality was reported to be up to 94% or even more in some cases (Wang 1996). The reasons why precocious crabs do not molt successfully still remains unknown (Wang 1996; Jin and Li 1999). Precocious crabs usually weigh 15–50 g ind−1, much smaller than market size (≥150 g ind−1), and consequently have much lower commercial value.
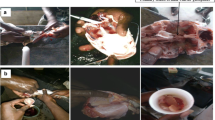
Because of the high rate of precocity in cultured juvenile crabs, it is important to improve the early identification of precocious crabs in practice. For identifying whether an E. sinensis is mature or not, the most accurate way is to observe the developmental stage of the gonad. Generally, the crab gonad development is divided into five stages: immature, pre-mature, close-to-mature, fully mature, and spent phases. Characteristics of each stage of ovarian development in mitten crab have been reported previously (Gu and He 1997). If the primary oocyte or primary spermatocyte could be observed in a year old female or male crab, respectively, then the crab can be considered as precocious (Li et al. 1998). However, this method requires autopsy observation and is inconvenient to crab farmers. As the morphological characters, especially secondary sex characters, are related to maturity, these are often used to identify maturity (Paul 1992; Li et al. 1998; Wang et al. 2001, 2007; Corgos and Freire 2006). The characteristics typically applied are as follows: (1) body color—the dorsal carapace of normal crabs is slightly yellow, also known as “yellow crab”; however, the dorsal carapace of sexually precocious crabs is dark green or blue with obvious stripes, also known as the “green crab” (Fig. 1). (2) Abdominal segment—the abdominal segment of mature female crabs reaches the pereiopod base and is surrounded by long and dense hair; the belly of mature males is protruded out of the segment breastplate (Fig. 1). (3) Appendages—the pereiopods have long and thick setae in mature crabs, and the pincers have very dense and long hair in mature male crabs (Fig. 1).
These observations of secondary sexual characteristics enable discrimination in mature crabs, but it is unable to identify crabs in early gonad development stages (stages II–III), leading to suggestions of alternative criteria. Du et al. (2000) proposed methods based on the density and length of setae inside the second step foot tarsus of males. Zhang et al. (2001) proposed the presence of visible medial setae, and the longest hair of the second pairs of pereiopod is more than 2 mm for mature male crabs, and the width of the fifth section from the ventral base is larger than the width of other sections for mature female crabs. In practice, if the above-mentioned identification methods can be combined, the identification will be much more accurate.
Factors related to precocity
The mechanism for crab precocity is very complex, and it is considered to be related to both endogenous and environmental factors (Du et al. 2000). The main external factors include ambient temperature, water quality (pH, salinity, and so on), water depth, stocking density, and nutrition. The internal factors are the genetic characteristics, including strain, sex, and so on. Reports are inconsistent about the dominant factors that lead to precocity. Du et al. (2000) based on studies on the effect of several factors (cumulative temperature days, nutrition and strain) in an orthogonal experiment suggested that genetic factors play a key role in causing precocity. However, it is widely accepted that over-nutrition and high cumulative temperature (cumulative temperature days = daily average temperature × experimental days = day degrees °C) are the main reasons for precocity (Wu and Jiang 2000; Chen et al. 2003). The effects of various factors on crab precocity are given in the following paragraphs.
Temperature
Growth and development of organisms are inseparable from temperature, and gonad maturation requires a certain degree of day degrees °C. It has been confirmed that temperature has a promotional effect on precocity in aquatic crustaceans. Huang and Wang (2000) found the maturity time of Daphnia magna was significantly shortened with increased temperature.
Zhang and Xu (2001) reported that the rate of precocity of juvenile E. sinensis reared under semi-intensive culture conditions was 18.2–22% in the Yangtze River basin, while that reared along Oujiang Estuary in south Zhejiang rose to 35%. They also reported that rate of precocity in no. 1 pool in Wenzhou with an average annual temperature of 18.2°C was 35%, while that in no. 2 pool in Shanghai with an average annual temperature of 15.8°C was 22%. Wang and Xia (1998) studied the effect of day degrees °C on precocity of E. sinensis. They decreased water temperature by irrigating the experimental paddy field with well water in the summer. No precocious crabs were found in cooler water in September, while the rate of precocity was 13% in the control group, suggesting day degrees °C effects crab precocity significantly.
However, the information about the accurate day degrees °C required for E. sinensis gonad maturation is very limited. Wang and Xia (1998) found the rate of precocity was as high as 13% in the control group with 2,731.5 day degrees °C, but no precious crabs were found in the lower temperature group of 2,597.5 day degrees °C during the experimental period (13th–12th, Jun–Sep). They suggested that the cumulative day degrees should not exceed 2,700 for juvenile culture. It has been reported that the growth rate increases with increasing water temperature until the optimum is reached and then declines with further temperature increases, following a bell-shaped curve (Ponce-Palafox et al. 1997). In regard to the mechanisms of temperature effects on precocity, some have suggested appropriate water temperature benefits juvenile crab metabolism and strengthens digestion and absorption of nutrients. Therefore, it enhanced growth and development of juvenile crabs, increased molting frequency, and resulted in early sexual maturation (Wang 1999; Wang et al. 2001).
Salinity
Eriocheir sinensis is a catadromous species, and its growth is closely related to salinity. In practice, higher rate of precocity was found in crabs cultured in coastal areas than those cultured in inland fresh waters (Wei et al. 2007), leading to the suggestion that high salinity to be one of the major reasons for precocity of the crab (Chen and Shen 1999). Zhang and Xu (2001) reported a rate of precocity of 18.2% when the crabs were reared in slightly salt water (0.1–0.3‰), while it rose up to 78% when crabs were reared in brackish water (3–7‰) and believed that high salinity could accelerate crab maturation. Gu and Jiang (2002) reported an exponential relationship between rate of precocity and water salinity when salinity was more than 10‰, rate of precocity could exceed 60%.
Although it had been documented that salinity could accelerate precocity, the mechanism was still poorly understood. There were two main viewpoints about the mechanism. In terms of the osmotic theory, some researchers assumed high salinity would slow down the relative growth rate between pre- and post-molting via affecting the rate of body expansion due to water absorption (Kwei 1978; Zhang and Xu 2001). Then, it shortened molting interval and increased molting frequency. Consequently, it resulted in a higher rate of precocity. On the other hand, oocytes need to absorb nutrients like vitellogenin during ovarian development, while Ca2+ in hemolymph had an important role in modulating vitellogenin production and absorption (Okumura 2006), and crabs could absorb Ca2+ from the environment during 48–96 h after molting (Wang et al. 2003). Therefore, one of the pathways for salinity affects was to promote the direct absorption of Ca2+ from water, which could be combined with vitellogenin and promote ovarian development, resulting in precocity. Wei et al. (2007) documented it by testing Ca2+ concentration in hemolymph of yearling crabs, and they presumed high salinity might facilitate the direct absorption of Ca2+ from water for molting of male E. sinensis then accelerate growth rate. Zhang and Xu (2001) pointed out that precocity of E. sinensis might also be an adaptation to high water salinity. Since mitten crab grows naturally in freshwaters, high salinity may be a stress to juvenile E. sinensis.
Light
Light is supposed to play an important role on crustacean growth and development, but related literature on the subject is limited, and even less so of light on crustacean precocity. Our previous study on the effects of different light intensity on precocity of E. sinensis indicated that light intensity had a significant effect on precocity (unpublished). However, Hoang et al. (2002) reported dim light (2 lx) favored ovarian maturation of prawns (Penaeus merguiensis de Man), and bright light (1,100 lx) had a strong inhibitory effect on developed ovaries. According to our data, we deemed the higher rate of precocity in brighter light group was mainly due to the effect of light intensity on crab locomotion for the crab favor dim light.
Nutrition
It is commonly accepted that nutrition, including quality and quantity, is one of the major factors that affect E. sinensis precocity (He et al. 1999; Wu and Jiang 2000; Du et al. 2000; Zhou and Geng 2001).
Protein: Protein is not only the basic component for life of an organism, but also a component of many active materials such as enzymes, hormones, antibodies, and so on. Appropriate protein level is favorable for crab growth, but too high a proportion of protein is considered to lead to precocity of crabs. Wang et al. (2001) reported rate of precocity in control groups (fed with wheat) was less than 5%, while that of test groups (fed with an artificial feed) were up to about 20%. They deemed high rate of precocity was caused by high protein level in the feed. In addition, dietary animal to plant protein ratio was also reported to have an effect on precocity. Zhu et al. (1999a) found rate of precocity of E. sinensis increased with increasing dietary animal to plant protein ratios.
The reason that high protein level, especially animal protein level would induce precocity is not very clear. Wang et al. (2001) believed crabs have strong metabolism ability in the warm season, and excessive intake of high protein feed would lead to fast growth. Excessive nutrients might deposit in the hepatopancreas and then be transferred to the gonad resulting in precocity. Furthermore, large amount consumption of high protein level might also cause great intake of cholesterol, a precursor of steroid hormones, which are optimal material for gonad maturity of juvenile crabs and would induce a higher rate of precocity (Wang and Xia 1998).
Lipid: Lipid, especially highly unsaturated fatty acids (HUFA) (e.g., DHA, EPA), plays an important role in crustacean reproduction (Cheng and Wang 2000). These not only serve as an energy source, but also are essential for vitellogen synthesis and embryo development, e.g., essential fatty acid (EFA), phospholipids, and pre-hormone. Therefore, these can promote sexual maturation of broodstock (Wouters et al. 2001a; Ai et al. 2003). It was reported that increased lipid content in crustacean hepatopancreas was a precondition for molting. That is, any nutritional factors that will speed the lipid accumulation in hepatopancreas could bring forward molting of crustaceans, and then induced precocity.
Cholesterol, a neutral lipid, is a synthetic steroid hormone precursor and plays an important role in crustacean endocrine and reproduction. Crustaceans cannot synthesize cholesterol in vivo, so they must get it from diets (Zhu et al. 1999b). It has been tested that hepatopancreas was the major tissue for cholesterol storage in immature crab. Cholesterol would be transferred to the gonads when it accumulated to a certain degree in the hepatopancreas. Somatic growth and reproduction have been known to be antagonistic (Charmantier et al. 1997). It is commonly accepted that nutrients must be available for metabolism first, and then for growth and finally for reproduction in nature (Bray and Lawrence 1992); i.e. once the nutrient or energy needs for metabolism and growth are met, the surplus nutrient or energy will be stored or used for reproduction. Zhu et al. (1999b) studied the effect of cholesterol levels in diets on crab precocity and found when cholesterol was above a certain level, the rate of precocity would increase with cholesterol level. They presumed that high rate of precocity was the result of frequent molting induced by high cholesterol content in diets. Sun et al. (2001a) deemed that precocity induced by cholesterol may be attributed to two aspects. Firstly, high cholesterol content might cause abnormal increment of the molting hormone (MH), consequently promoting molting, growth and gonad development of crabs. MH might also act on gonad development directly. Secondly, excessive accumulation of cholesterol might directly facilitate the synthesis and secretion of gonadotropin, methyl farnesoid, 17-β estradiol, testosterone and other steroid hormones, which stimulate fast gonadal development and finally leading to precocity.
Crustaceans have a limited ability to synthesize phospholipids and need to get it from the diet. Phospholipids have an important role on crustacean nutrition metabolism (Teshima 1997) and are considered to have an important role on lipid transition from hepatopancreas to gonad when gonads began to develop (Wouters et al. 2001b). Therefore, absence of phospholipids will induce triglyceride deposition in the hepataopancreas, which cannot be transmitted out in time and lead to an excessive accumulation of neutral lipids in hepatopancreas, causing early molting and precocity.
Fatty acids in diets, especially C18:2, C20:5 (EPA), C22:6 (DHA), and other long-chain polyunsaturated fatty acids (PUFA) are essential fatty acids for crustaceans and have a significant impact on crustaceans growth, reproduction, and molting (D’Abramo 1997). Hepatopancreas is the main storage and processing organ for fatty acids. Chen et al. (2003) found fatty acid composition of hepatopancreas in precocious crab was different before and after precocity and speculated that C20:5n-3 may be mainly involved in molting and membrane construction and C22:6n-3 involved in ovarian development and yolk deposition. It had been documented that dietary PUFA was preferentially used as a component of phospholipid synthesis in vivo (Cheng and Wang 2000). After lipid absorption from diets with a high PUFA content, most PUFAs were used for synthesis of phospholipids used for muscle and other tissue growth after being timely transferred out from the hepatopancreas. Thus, fat accumulation in the hepatopancreas would not increase in a short time and extend the molting cycle. It may have a certain effect on prevention of precocity.
Calcium: Calcium is one of the basic elements of the exoskeleton. According to Michele (1999), Ca2+ is assimilated into the crustacean body through infiltration and food. A proportion of calcium is deposited in the epidermis and tissues, and the rest is transported to various tissues through the hemolymph, and in the cells participates in various physiological and biochemical functions which are closely related to calcium and calcium-related protein (yolk protein) synthesis. In practice, crabs reared in ponds where quicklime is used as insecticide become precocious easily, leading to the suggestion that high Ca to be one of the main reasons for crab precocity (Wu and Jiang 2001). Other researchers found crabs can absorb Ca2+ directly from environment during 48–96 h after molting (Wang et al. 2003). Therefore, high concentration of Ca2+ in the environment and (or) tissue fluid may strengthen secretion of eyestalk neuropeptide hormone in crabs. Wu and Jiang (2001) found that hemolymph calcium concentration in female precocious crabs was significantly higher than that of normal buckle-size female crabs, and 17β-estradiol concentration in hemolymph of precocious crabs was significantly higher than that of normally developed crabs. Therefore, they speculated a certain intrinsic link existed between hemolymph Ca2+ concentration and ovarian development, and Ca2+ had a promotional role in ovarian development.
Vitamin: Vitamin requirements of E. sinensis is little known and impacts on precocity even less. It is documented that vitamins A, E, C, and astaxanthin could effectively improve the reproductive performance of shrimps (Cahu et al. 1995; Pangantihon-Kuhlmann et al. 1998), but comparable reports in crabs are unknown. Liu (1998) reported the absence of ascorbic acid (VC) would lead to precocity of about 14% female during juvenile cultivation, and VC content in normal juvenile crab hepatopancreas and muscle was 1–2 times higher than that in precocious juvenile crabs, and rate of precocity was negatively correlated to VC content. However, the mechanism for precocity caused by the absence of VC remains unclear, and it needs further study.
Stocking density
He et al. (1999) found that the rate of precocity decreased slightly as stocking density increased. Li et al. (2007) studied the effect of different stocking densities on crab yield in paddy fields and found that the rate of precocity decreased significantly with increasing stocking densities. The main reason may be the relatively lower amount of nutrition available for crabs grown at high density, for the crab body weight in high-density group was significantly lower. In conclusion, high stocking densities would affect food intake, thereby affecting growth and development of crabs.
Germplasm and gender
It was commonly accepted that precocity of mitten crab was mainly caused by exorbitant accumulative temperature and excess nutrients during juvenile stage. Some other factors, such as inbreeding, broodstock miniaturization are also considered to be related to precocity. Wang et al. (2001) found the rate of precocity in males and females (17.20 and 16.06%, respectively) from the Yangtze River population was significantly higher than that from the Liaohe River population. Zhang et al. (2001) compared the effect of temperature, feed, and crab strains on sexual precocity and demonstrated that strain was the main factor, and rate of precocity in different strains differed significantly from each other.
Wang et al. (2001) found rate of precocity of female crabs was significantly higher than that of male crab in both Yangtze River and Liaohe River population, suggesting that gender also can affect precocity of crabs.
The relationships between neuro-endocrine system and precocity
The responses of organism to environmental changes are often categorized as primary, secondary and tertiary responses. The primary response is the release of hormones into circulatory system, which then triggers secondary responses that can include series of physiological and biochemical changes, when the organism would show more external and macroscopic responses, named as tertiary responses, such as basic behavior and growth (Pickering 1981). Crab precocity is considered as the result of synergical effects of environmental and intrinsic factors. In order to control precocity in crabs from neuro-endocrine perspective, it is necessary to know the mechanisms of neuro-endocrine system on precocity. The X-organ-sinus gland complex in the eyestalk in crustaceans is equivalent to the hypothalamus pituitary system in vertebrates, with the function of regulating physiological processes, such as gonad development, ripening, spermiation, and spawning (Keller 1992). Many have studied these endocrine tissues or organs and related issues by the biopsy, histochemistry, electron microscopy, immunology, and molecular cloning techniques. Some have reviewed the effect of morphology, structure, and function of neuro-endocrine organs such as mandibular organ, eyestalk neuro-endocrine system, androgenic gland, Y-organ, brain or thoracic ganglion and ovarian on precocity (Cai 1998; Yuan et al. 2004; Li et al. 2005), and no attempt will be made at present to review these except to focus on the relationships between neuro-endocrine organ or tissue and precocity of crabs.
X-organ-sinus gland complex, XO-SG
The XO-SG, located in eyestalk, is the main neuro-endocrine control center in crustaceans. Hormones secreted by it include gonad-inhibiting hormone (GIH), molt-inhibiting hormone (MIH), crustacean hyperglycemic hormones (CHH), red pigment concentration hormone (RPCH), pigment dispersion hormone (PDH), etc., (Keller 1992; Okumura and Aida 2001; Sun et al. 2000; Yuan et al. 2004). These hormones are stored in and released from the sinus gland in the form of particles. They regulate crab gonadal development, molting, carbohydrate metabolism, and adaptations to the environment and are closely related to reproductive endocrinology.
Sun et al. (2001b) compared the structure of the sinus gland in precocious and normal crabs. Their observations showed that the sinus gland morphology in precocious crab did not change significantly, but the release of neurosecretory substances in a variety nerve twigs distinctly changed, in particular, no secretion was observed in type V nerve twigs in the sinus gland of precocious crabs. These phenomena indicate that certain environmental factors may affect the sinus gland neuro-peptide hormone storage and release, thereby inducing the precocity in juvenile crabs. Luo et al. (1990) found that removal of eyestalk would cause endocrines to change (MIH and GIH withdrawal, while 20-hydroxyecdysone (20-HE) increases), subsequently stimulate oocyte growth and accelerate ovarian development. Okumura and Aida (2001) found prawn molting hormone levels in hemolymph rose rapidly, and molt interval was significantly shorter than that in control group after removal of eyestalk. Similarly, Kang et al. (1998a, b) reported that eyestalk removal would stimulate the transfer of nutrients stored in hepatopancreas to testis, which may lead to precocity. Zhang and Wang (2006) found protein content in hepatopancreas and gonad showed an increase tend after eyestalk removal, while protein content in other organs declined sharply, and protein types in various organs obviously changed after removal of eyestalk.
Mandibular organ, MO
The MO is located at the base of the large jaw mandibular tendon, and its main secretion is methyl farnesoate (MF), and plays an important regulatory role in growth and development of crustaceans (Homola and Chang 1997; Cai 1998). It has been confirmed that the large jaw mandibular gland secretion of MF would increase during oocyte development and oogenesis in crustaceans, leading to the suggestion that MF has a catalytic role on ovarian development (Huberman 2000). Zhao and Lu (2003) found MF synthesis rate in precocious female and male crabs was about two times that in normal in September. They pointed out that environmental factors and over-nutrition caused early development of the mandibular organ in precocious crabs, which causes a large amount of gonadotrophin (e.g. MF) synthesis and secretion, and subsequently stimulate the ovaries or testes mature.
Y organ
Y-organ, epithelial endocrine gland, is an important endocrine organ of crustaceans. Crustacean molting was regulated by molting hormone (MH) and MIH secreted by X-organ-sinus gland complex. According to Luo et al. (1990), the 20-HE concentration in hemolymph will increase following the removal of the eyestalk. Apparently, eyestalk removal curtailed the inhibition of MIH on Y-organ and promoted the secretion of MH. It is also suggested that the rate of molting hormone synthesis and (or) secretion in Y-organ of was the main mechanism in controlling the content of molting steroid hormone in hemolymph (Huberman 2000).
Relationship between steroid hormone and precocity
Steroid hormones, such as progesterone, estradiol and testosterone, received extensive attention in crustaceans in recent years. Couch (1987) found progesterone concentration in mandibular gland always maintained at a relatively high level, and it had no relation with ovarian development or maturation. Progesterone was assumed to be secreted by the mandibular gland and could be transformed into estradiol in the reproductive season. Estradiol and other steroid hormones regulate vitellogenesis and oocyte growth in oviparous vertebrates. Some reports pointed out these hormones were also involved in the regulation of vitellogenesis and oocyte growth in crustaceans and speculated that estradiol and ovarian development were intrinsically related (Rodríguez et al. 2002a, b; Zapata et al. 2003), this being also confirmed for E. sinensis (Wei et al. 2005). Zapata et al. (2003) tested the effect of several hormones in Chasmagnathus granulata, in vivo and in vitro, and found 17α-progesterone could promote ovarian growth in every period of the reproductive cycle of C. granulata. Kang et al. (1998a, b) found exogenous 17α-methyl testosterone had a promotional effect on juvenile testis development, while 17β-estradiol had an inhibition effect on juvenile testis development. Wei et al. (2005) believed that one of the reasons for precocity in female E. sinensis yearling was the secretion level of estradiol reached the level that of a 2-year-old, and promoted early gonadal development and maturation. In addition, Wei et al. (2007) found the increase in estradiol content in hemolymph has a dose-dependent relationship with salinity and pointed out that precocity caused by salinity was stimulated by the physiological processes of increasing estradiol content in hemolymph. It further confirmed that elevation of estradiol content under high salinity was one reason for precocity.
The active substance related to molting and maturation of oocytes in secretion is a steroid hormone, 20-HE. The circulating titer of 20-HE was found to vary along the molt cycle of shrimps. Luo et al. (1990) found the 20-HE content in hemolymph changed significantly in the entire molt cycle, which could sharply decline from the highest in the molt preparation period (127 pg μl−1) to the lowest in post-molt (4.0 pg μl−1). Luo et al. (1990) also reported that the 20-HE content in hemolymph rose continuously in early oocyte growth stage then rapidly declined after puberty molt. Similar results were reported by Jiang et al. (1992). They determined the 20-HE content in puberty molt crabs at different phases (1–2 days pre-molting, molting, post-molting) under artificial conditions, which indicated that 20-HE could induce molting periodically. Injection of exogenous 20-HE had a promotional effect on ovarian weight gain (Luo et al. 1990). Therefore, 20-HE also has an important role on sexual maturation.
Methyl farnesoate (MF), secreted by mandibular organs, has roles in both growth and reproductive processes (Laufer et al. 1987). It was also reported to have stimulatory effects on ovarian maturation of red swamp crayfish Procambarus clarkii (Laufer et al. 1998). Stimulatory effects of MF on molting had been reported in crustaceans, in vitro and in vivo (Tamone and Chang 1993; Homola and Chang 1997). Some authors documented injection of MF had a clear promotion effect on ovarian growth in red swamp crayfish (Laufer et al. 1998; Rodríguez et al. 2002a, b). MF also plays an important regulatory role in growth and development of crabs (Homola and Chang 1997; Cai 1998).
The relationship between the hepatopancreas and precocity
Hepatopancreas is not only the digestive gland, but also is the main storage organ of energy reserves of crustaceans. During the process of sexual maturation, hepatopancreas is considered to be the major energy source for the development of the gonad (Wei and Bao 1999). Wen et al. (2001) found hepatopancreatic lipid concentration increased with maturity of the ovaries, peaked at early maturing (29.9%), and decreased during the subsequent period to spawning (16.7%), while the muscular and hemolymph lipid concentration did not change markedly during ovarian development. Wu and Jiang (2000) found total protein concentration in hepatopancreas of precocious crabs was significantly lower than that of normal crabs. Chen et al. (2003) reported that the hepatic lipid content of precocious crabs was significantly lower than that of normal crabs, and the fatty acid composition in hepatopancreas was different before and after precocity. These results suggest the possible movement of hepatic nutrients to the ovaries during maturation. It has also been observed that the hepatosomatic index became smaller, while gonad index became larger all the time during gonadal development of mitten crab, and the variation in gonad index was closely related to that of the hepatosomatic index, which further confirmed that the energy resource for gonadal development mainly came from the hepatopancreas (Li and Guo 2001; Chen et al. 2003).
However, Hasek and Felder (2005) reported that the hepatopancreas net weight did not decrease over the course of ovarian development in two groups of grapsoid (Armases cinereum and Sesarmanr. Reticulatum). These authors concluded that the lipid used for ovarian maturation was from increased dietary intake and not from the stored lipid in the hepatopancreas. These differences could be species or different nutritional conditions.
Prevention and control methods of precocity
Precocious crabs have no culture value in the second year. Precocious crabs become unedible and lose their commercial value when the gonad degenerates from April in the following year. As such, it is very important to prevent and control precocity occurrence. In practice, farmers have explored a number of prevention measures based on their own understanding on the reasons for precocity of crabs. To sum up, the main prevention measures were as follows. (1) To prevent exorbitant day degree temperatures, larval stocking time is delayed and hydrophytes planted and pond water level increased to reduce water temperature; (2) Prevention of over-nutrition by using proper feeds and improving feeding strategies. The feeding strategy commonly adopted is termed “two ends precision, middle rough”; this means feeding juvenile crabs with high quality and excess feed during spring, early summer and late fall, and feeding a lower quality diet when water temperature is high in summer and early fall; (3) For germplasm quality, farmers considered it was important to select large sized crabs (250 g ind−1 or larger) as broodstock and select different strains for parental hybridization. It was reported that the measures had achieved some success (Zhang et al. 2001).
Problems and perspective
Surplus nutrients, exorbitant temperature days, and degradation of germplasm were considered as the main reasons for precocity in mitten crab. However, some viewpoints lacked experimental data. Although some experiments were carried out to confirm that low-nutrition or low-temperature days would prevent precocity, these results indicated the final size of crabs was very small, with an average weight of only about 3 g ind−1 or even smaller. This meant that crabs were farmed under abnormal rearing conditions with long-term stagnation in growth. It was reported that rate of precocity was almost 0% when body size was smaller than 7 g ind−1, and the high incidence of precocity occurred when body size reached 10 g ind−1 (Zhang et al. 2001). Therefore, it is meaningless to compare the rate of precocity of crabs reared under abnormal conditions with that of normally reared crabs in practice.
Precocious crabs cause great losses to farmers due to their small size and low survival rate. Therefore, it is very important to prevent or reduce the occurrence of precocity. On the other hand, making use of the limited commercial value of precocious crabs is also of importance. According to Jin et al. (1998), the Baoan Lake Fisheries (Daye, Hubei, China) benefited from precocious crabs by marketing (50–100 g ind−1) during the off-season. At present, precocious crabs are generally slaughtered or released immediately after capture. In fact, a number of small edible crabs were also reared in some countries as important commercial species, such as Aegla urugayana (Aeglidae) (Viau et al. 2006), with a 15–17 mm carapace length at the time of maturation. It is said that small E. sinensis were consumed as barbecue food at night markets in some coastal cities in China. If consumer demand could be generated for popularising different sizes and stages of mitten crab, economic loss from precocity can be reduced significantly.
The mechanisms of precocity are extremely complex. In order to establish effective prevention and control methods of precocity, it needs cooperative work on the following aspects:
Neuro-endocrinology With the purpose of revealing mechanisms for precocity and control it by neuro-endocrine regulation, basic and useful information on structure, micro-structure, and function of tissues or organs of E. sinensis neuro-endocrine system have been unraveled (Sun et al. 2000, 2001c, 2003a, b, 2004; Wei et al. 2002; Wang et al. 2006). However, there are still many areas need further study, such as: (1) Hormone, biological, chemical detection, and analysis of chemical properties. The concentration of hormones in crustacean is very low, usually 10−9–10−12g ml−1 (Cai 1998). Thus far, only a few hormones can be determined by current detection methods. Therefore, it is necessary to research and establish more sensitive and convenient methods. There are still quite a number of hormones, of which the chemical properties need to be analyzed and explored for their analogs or antagonist for controlling precocity. (2) Regulation mechanism of hormones. Hormones regulate and control molting, reproductive and other physiological activities in organism, but also are regulated by other hormones or neurotransmitters. Up to now, only the function of various neuro-endocrine organs is approximately known, but the role of neuro-endocrine organs and hormones in precocity process is still not very clear. (3) Application of neuro-endocrine hormones or its analogs in aquaculture. Based on well-understood mechanisms of endocrine regulation, how to use hormones to control sexual precocity is an interesting research topic with practical value. A range of applied research is still needed in topics such as accurate hormone application dosage, application method, and effective timing of dosing need further study.
Molecular biology Genetic research on E. sinensis developed slowly compared with other aquaculture species in China. Early researches focused on two fields including taxon confusion and different population identification (Wang et al. 1995; Tang et al. 2003). Some studied precocity using molecular biology technologies on genetic variation in wild, intensive reared, and precocious individual mitten crabs (Li et al. 2003) or cDNA library of E. sinensis ovary (Ma et al. 2003a, b).
Studying sexual precocity at gene level is still in its infancy. In order to regulate and control precocity at gene level, there is much to be done, including the functions of related genes (including biological, cellular, and developmental function), gene expression analysis, and mutation detection. Investigating these genes and their functions may shed light on those physiological and biochemical studies related to growth and reproduction. In common carp, sterile triploids were considered as a potential solution to precocity (Basavaraju et al. 2002). So it is possible to cultivate new varieties of anti-precocious by hybridization and suitable genetic engineering. In addition, carrying out genomics research not only contributes to heredity and breeding, but also is conducive to explore the possibility of shortening the breeding cycle.
Nutrition Excess nutrient was widely considered as one of the main reasons that caused precocity in E. sinensis. On the other hand, the small size of precocious crabs was suspected to be mainly due to nutritional imbalances under intensive culture (Cheng and Wang 2000). In natural waters (such as lakes, with abundant floating macrophytes and benthic organisms), precocious individual are larger than pond cultured, up to 50–100 g ind−1 (Jin et al. 1998). This indicates that if proper nutrition is provided in intensive culture, crabs could be larger and of a marketable size (≥150 g ind−1) even if precocious. However, the nutrient requirements of mitten crab are not fully known, apart from the information on protein, lipid, and carbohydrates requirements. Micro nutrient requirements of mitten crab need to be investigated enabling the formulation of a balanced wholesome diet. On the other hand, it is necessary to investigate optimum feeding levels and strategies for E. sinensis, which will help to set up more scientific and reasonable feeding management strategies. The two aspects will reduce feed costs and sexual precocity caused by poor-proportioned nutrients or improper feeding strategies.
Abbreviations
- PUFA:
-
Polyunsaturated fatty acids
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosehexaenoie acid
- VC:
-
Ascorbic acid
- MF:
-
Methyl farnesoate
- MO:
-
Mandibular organs
- MH:
-
Molting hormone
- GIH:
-
Gonad-inhibiting hormone
- MIH:
-
Molt-inhibiting hormone
- CHH:
-
Crustacean hyperglycemic hormone
- RPCH:
-
Red pigment concentrating hormone
- PDH:
-
Pigment dispersing hormone
- 20-HE:
-
20-Hydroxyecdysone
- XO-SG:
-
X-organ-sinus gland
- MTXO:
-
Medulla terminalia X-organ
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
- RAPDs:
-
Random amplified polymorphic DNAs
References
Ai C, Li S, Wang G, Lin Q (2003) Research advancement on requirements of broodstock nutrition for shrimps and crabs. J Oceanogr Taiwan Strait 22(2):254–261 (in Chinese with english abstract)
Amano M, Hyodo S, Kitamura S, Ikuta K, Suzuki Y, Urano A, Aida K (1995) Short photoperiod accelerates preoptic and ventral telencephalic salmon GnRH synthesis and precocious maturation in underyearling male masu salmon. Gen Comp Endocrinol 99:22–27
Basavaraju Y, Mair GC, Mohan KHM, Pradeep Kumar S, Keshavappa GY, Penman DJ (2002) An evaluation of triploidy as a potential solution to the problem of precocious sexual maturation in common carp, Cyprinus carpio, in Karnataka, India. Aquaculture 204:407–418
Begtashi I, Rodríguez L, Moles G, Zanuy S, Carrillo M (2004) Long-term exposure to continuous light inhibits precocity in juvenile male European sea bass (Dicentrarchus labrax, L.). I. Morphological aspects. Aquaculture 241:539–559
Bray W, Lawrence A (1992) Reproduction of Penaeus species in captivity. In: Fast AW, Lester LJ (eds) Marine shrimp culture: principles and practices. Elsevier, Amsterdam, pp 93–170
Cahu C, Cuzon G, Quazuguel P (1995) Effect of highly unsaturated fatty acids, α-tocopherol and ascorbic acid in broodstock diet on egg composition and development of Penaeus indicus. Comp Biochem Physiol 112A:417–424
Cai S (1998) A review of crustacean endocrinology. J Fish China 22:28–31 (in Chinese with english abstract)
Charmantier G, Charmantier-Daures M, Van Herp F (1997) Hormonal regulation of growth and reproduction in crustaceans. In: Fingerman M, Nagabhushanam R, Thompson MF (eds) Recent advances in marine biotechnology. Endocrinology and reproduction, vol 1. Science Publishers, New Hampshire, pp 109–162
Chen X, Shen J (1999) Observations of precocity of one year old mitten crab. Fish Sci Technol Inf 26:28–31 (in Chinese)
Chen Z, Cheng Y, Wang W (2003) Changes of hepatopancreas index, lipid content and fatty acid composition in Eriocheir sinensis during precocity. J Fish China 27:57–61 (in Chinese with english abstract)
Cheng Y, Wang W (2000) Lipid nutrition of s and precocity of Chinese mitten crab Eriocheir sinensis. Sci Fish Farming 6:39–40 (in Chinese)
China Fishery Statistical Yearbook (2009) China fishery statistical yearbook 2008. Fisheries Bureau, Department of Agriculture of China, Beijing (in Chinese)
Corgos A, Freire J (2006) Morphometric and gonad maturity in the spider Maja brachydactyla: a comparison of methods for estimating size at maturity in species with determinate growth. J Mar Sci 63:851–859
Couch EF (1987) Changes in estradiol and progesterone immunoreactivity in tissues of the lobster, Homarus americanus with developing and immature ovaries. Comp Biochem Physiol 67A:765–770
D’Abramo LR (1997) Triacylglycerols and fatty acids. In: D’Abramo LR, Conklin DE, Akiyama DM (eds) Crustacean nutrition: advances in world aquaculture. World Aquaculture Society, pp 71–84
Du X, Zhang D, Zhao J, Zheng W (2000) Preliminary analysis on precocious phenomenon of mitten crab, Eriocheir sinensis reared in ponds. J Dalian Fish Univ 15:254–258 (in Chinese with english abstract)
FAO (2007) The state of world fisheries and aquaculture 2006. FAO, Rome, p 180
Felip A, Zanuy S, Carrillo M (2006) Comparative analysis of growth performance and sperm motility between precocious and non-precocious males in the European sea bass (Dicentrarchus labrax, L.). Aquaculture 256:570–578
Gu Z, He L (1997) Histological and cytological observation on the development cycle of (Eriocheir sinensis) ovary. Oceanol Limnol Sin 28:138–145 (in Chinese with english abstract)
Gu X, Jiang J (2002) Preliminary study on the effect of salinity on precocious maturation of Chinese mitten-handed crab Eriocheir sinensis. Mar Fish 24:22–24 (in Chinese with english abstract)
Hasek B, Felder D (2005) Biochemical composition of ovary, embryo, and hepatopancreas in the grapsoid crabs Armases cinereum and Sesarma nr. reticulatum (Crustacea, Decapoda). Compar Biochem Physiol 140B:455–463
He Z, Yin J, Zhu Y (1999) Co-relation between density nutrition and growth prematuration of young mitten crab. Fish Sci Technol Inf 26:73–76 (in Chinese with english abstract)
Hoang T, Barchiesis M, Lee SY, Keenan CP, Marsden GE (2002) Ovarian maturation of the banana prawn, Penaeus merguiensis de Man under different light intensities. Aquaculture 208:159–168
Homola E, Chang E (1997) Methyl farnesoate: crustacean juvenile hormone in search of functions. Comp Biochem Physiol 117B:347–356
Huang X, Wang W (2000) The effects of temperature and salinity on growth and reproduction of Daphnia magna. J Shanghai Fish Univ 1:15–20 (in Chinese with English abstract)
Huberman A (2000) Shrimp endocrinology. A review. Aquaculture 191:191–208
Jiang R, Tan Y, Wu J (1992) Changes of hemolymph 20-hydroxyecdysone, 17β-oestradiol and testosterone levels in Eriocheir Sinensis. J Fish China 16:101–105 (in Chinese with english abstract)
Jin G, Li Z (1999) Biology of mature 0+ crabs, Eriocheir Sinensis (II): sexual behavior, overwintering habit and molting possibility. J Lake Sci 11:172–176 (in Chinese with english abstract)
Jin G, Xie P (2001) The growth patterns of juvenile and precocious Chinese mitten crabs, Eriocheir sinensis (Decapoda, Grapsidae), stocked in freshwater lakes of China. Crustaceana 13:261–273
Jin G, Li Z, Fang R, Liu H (1998) Cultural ecology of mature yearling Chinese mitten-handed crab, Eriocheir Sinensis (Crustacea, Decapoda), and its fishery evaluation. Acta Hydrobiol Sin 22:143–147 (in Chinese with English abstract)
Jin G, Xie P, Li Z (2002) The precocious Chinese mitten crab: changes of gonad, survival rate, and life span in a freshwater lake. J Crustacean Biol 22:411–415
Kang X, Sun H, Mi Y, Liu B, Liu L, Wang S (1998a) Studies on the changes of liver structure and amino acid of Eriocheir sinensis by eyestalk ablation. Donghai Mar Sci 16:116–121 (in Chinese with english abstract)
Kang X, Liu Z, Zhou K, Wang S (1998b) Primary study of exogenous steroid hormone effect on the testis development of the Chinese mitten-handed crab, Eriocheir sinensis (Crustcea, Decapoda). Donghai Mar Sci 16:39–44 (in Chinese with english abstract)
Keller R (1992) Crustacean neuropetides: structure, function and comparative aspects. Experimentia 48:439–448
Kwei E (1978) Size composition growth and sexual maturity of Callinectes latimanus (Rath.) in two Ghanaiin lagoons. Zool J Linn Soc 64:151–175
Laufer H, Borst D, Baker F, Carrasco C, Sinkus M, Reuter CC, Tsai LW, Schooley DA (1987) Identification of a juvenile hormone-like compound in a crustacean. Science 235:202–205
Laufer H, Biggers W, Ahl J (1998) Stimulation of ovarian maturation in the crayfish Procambarus clarkii by methyl farnesoate. Gen Comp Endocrinol 111:113–118
Li Y, Guo Y (2001) Comparative study of gonad and hepatopancreas index changes of Yangtse River and Liaohe River mitten crab (Eriocheir sinensis). J Hydroecol 21:10–12 (in Chinese)
Li C, Wang C, Li S (1998) Discrimination analysis on precocity of Chinese mitten crab Eriocheir sinensis. Fish Sci Technol Inf 25:73–76 (in Chinese with english abstract)
Li H, Hou L, Wei F (2003) RAPD analysis of wild, farmed, and premature mitten-handed crab (Eriochei r sinensis) in Liaohe River. Fish Sci 22:1–3 (in Chinese with english abstract)
Li Y, Kang X, Zhao X, Wen X, Han L (2005) Intrinsic factors of precocity in mitten-handed crab Eriocheir sinensis. Fish Sci 24:34–36 (in Chinese with english abstract)
Li XD, Dong SL, Lei YZ, Li YH (2007) The effect of stocking density of Chinese mitten crab Eriocheir sinensis on rice and seed yields in rice-culture systems. Aquaculture 273:487–493
Liu J (1998) To absorb the vitamin C from the young crabs of Eriocheir sinensis and overcome pubertas praecox. J Nanjing Agric Tech Coll 14:11–15 (in Chinese with english abstract)
Luo R, Wang Y, Cao M (1990) The role of hemolymph 20-hydroxyecdysone in molting and oocyte development of the crab Eriocheir sinensis. Acta Zool Sin 36:157–164 (in Chinese with english abstract)
Ma C, Zhou K, Guo Y, Wang Y, Pan H, Zhao N, Wang C (2003a) Construction of a RACE cDNA library of mitten crab ovary. Chinese J Zool 38:14–16 (in Chinese with english abstract)
Ma C, Zhou K, Guo Y, Wang Y, Pan H, Zhao N, Wang C (2003b) Construction on subtractive cDNA library of mitten crab ovaries. Zool Res 24:53–56 (in Chinese with english abstract)
Michele G (1999) Calcium homeostasis in crustacea: the evolving role of bronchial, renal, digestive and hypodermal epithelia. J Exp Zool 283:620–640
O’Brien J (1984) Precocious maturity of the majid crab, Pugettia producta, parasitized by the rhizocephalan barnacle, Heterosaccus californicus. Biol Bull 166:384–395
Okumura T (2006) Effects of cyclic nucleotides, calcium ionophore, and phorbol ester on vitellogenin mRNA levels in incubated ovarian fragments of the kuruma prawn Marsupenaeus japonicus. Gen Comp Endocrinol 148:245–251
Okumura T, Aida K (2001) Effects of bilateral eyestalk ablation on molting and ovarian development in the giant freshwater prawn, Macrobrachium rosenbergii. Fish Sci 67:1125–1135
Okuzawa K (2002) Puberty in teleosts. Comp Biochem Physiol 26:31–41
Pangantihon-Kuhlmann M, Millamena O, Chern Y (1998) Effect of dietary astaxanthin and vitamin A on the reproductive performance of Penaeus monodon broodstock. Aqua Living Resourc 11(6):403–409
Paul A (1992) A review of size at maturity in male tanner (Chionoecetes bairdi) and king (Paralithodes camtschaticus) crabs and the methods used to determine maturity. Am Zool 32:534–540
Pickering A (1981) Introduction: the concept of biological stress. In: Pickering AD (ed) Stress and fish. Academic Press, pp 38–48
Ponce-Palafox J, Martinez-Palacios CA, Ross LG (1997) The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp Penaeus vannamei, Boone, 1931. Aquaculture 157:107–115
Rodríguez EM, López Greco LS, Medesani DA, Laufer Hans FM (2002a) Effect of methyl farnesoate, alone and in combination with other hormones, on ovarian growth of the red swamp crayfish, Procambarus clarkii, during vitellogenesis. Gen Comp Endocrinol 125:34–40
Rodríguez EM, Medesani DA, López Greco LS, Laufer Hans FM (2002b) Effects of some steroids and other compounds on ovarian growth of the red swamp crayfish, Procambarus clarkii, during early vitellogenesis. J Exp Zool 292A:82–87
Sun J, Liu A, Chen J, He B, Wang X, Guo S, Wang Y (2000) Cytology and culture of neurosecretory cell in the eyestalk of Eriocheir sinensis. Acta Hydrobiol Sin 24:347–381 (in Chinese with english abstract)
Sun J, Liu A, He B, Lu J, Xiang J (2001a) Effects of several steroids on ICa from neurosecretory cells in the eyestalk of Eriocheir sinensis. Oceanol Limnol Sin 32:627–634 (in Chinese with english abstract)
Sun J, Liu A, Du Y, Lu J, Xiang J, Ma W (2001b) Microstructure and ultrastructure of the sinus gland in the eyestalk of Eriocheir sinensis. Acta Zool Sin 47:27–31 (in Chinese with english abstract)
Sun J, Liu A, He B, Lu J, Xiang J (2001c) Patch clamp study on the potassium channels in the cultured MTXO neurons in Eriocheir sinensis. Acta Zool Sin 47(monograph):87–91 (in Chinese with english abstract)
Sun J, Gao C, Xiang J (2003a) Study on the GABA gated channels in the neurosecretory cells of MTXO in the eyestalks of Eriocheir sinensis. Prog Biochem Biophys 30:129–134 (in Chinese with english abstract)
Sun J, Gao C, Xiang J (2003b) Real-time monitor of secretion activity in neurosecretory cells in the eyestalk of Chinese mitten-handed crab Eriocheir sinensis. Oceanol Limnol Sin 34:552–557 (in Chinese with english abstract)
Sun J, Gao C, Xiang J (2004) Research on the glutamate gated channels in the neurosecretory cells of MTXO in the eyestalke of Eriocheir sinensis. Acta Hydrobiol Sin 28:648–652 (in Chinese with english abstract)
Tamone S, Chang E (1993) Methyl farnesoate stimulates ecdysteroid secretion from Y-organs in vitro. Gen Comp Endocrinol 89:425–432
Tang BP, Zhou KY, Song DX, Yang G, Dai AY (2003) Molecular systematics of the Asian mitten crabs, genus Eriocheir (Crustacea: Brachyura). Mol Phylogenet Evol 29:309–316
Teshima S (1997) Phospholipds and sterols. In: D’Abramo L, Conklin D, Akiyama D (eds) Crustacean nutrition: advances in world aquaculture. World Aquaculture Society, pp 85–107
Viau V, López Greco L, Bond-Buckup G, Rodríguez EM (2006) Size at the onset of sexual maturity in the anomuran crab, Aegla uruguayana (Aeglidae). Acta Zool (Stockholm) 87:253–264
Wang H (1996) Methods to distinguish “yellow crab” from “green crab” and their mortality of molting. Fish Sci Technol Inf 23:41–43 (in Chinese with english abstract)
Wang Y (1999) Effect of seasonal changes of temperature on growth and development of juvenile mitten crab Eriocheir sinensis. J Aquacult 4:19–20 (in Chinese)
Wang D, Xia D (1998) Study on the reasons and prevention methods of precocity of Eriocheir sinensis. Fish Sci 17:17–20 (in Chinese)
Wang D, Yu W (1995) A comparative study on the isozymes in Eriocheir sinensis from Changjiang and Liaohe Rivers. J Liaoning Univ (Nat Sci) 22:79–81 (in Chinese with an english abstract)
Wang C, Li S, Li C, Zhao J (2001) Observation and analysis on appearing difference of precocious crab of Yangtze population and Liaohe population cultured in ponds. J Lake Sci 13:57–62 (in Chinese with english abstract)
Wang S, Wei Y, Shen D (2003) Calcium and phosphorus levels in the muscle, hepatopancreas and carapace of Eriocheir sinensis in different stages of moulting cycle. J Fish China 27:219–224 (in Chinese with english abstract)
Wang X, Sun J, Yang W (2006) Isolation and activity of a crustacean hyperglycemic hormone (CHH) from Eriocheir sinensis. J Fish China 30:151–155 (in Chinese with english abstract)
Wang W, Cheng Y, Li Y (2007) The biology of Chinese mitten crab. Fish Sci Technol Inf 34:25–29 (in Chinese)
Wei Z, Bao C (1999) Comparison of gonad and hepatopancreas index of pool cultured and lake discharged mitten crab Eriocheir sinensis. Freshwat Fish 29:16–17 (in Chinese)
Wei R, Qiu G, Lou Y (2002) Nerve terminal types of sinus gland and neurosecretory cell types of X-organ in Eriocheir sinensis. Zool Res 23(3):226–232
Wei W, Wei H, Liu Q (2005) Effect of estradiol in hemolymph and gonad on precocity of Eriocheir sinensis. J Fish China 29:862–865 (in Chinese with english abstract)
Wei W, Wu J, Wei H (2007) Physiological mechanism of precociousness influenced by salinity in juvenile Eriocheir sinensis. J Fish Sci China 14:275–280 (in Chinese with english abstract)
Wen XB, Chen LQ, Ai CX, Zhou ZL, Jiang HB (2001) Variation in lipid composition of Chinese mitten-handed crab, Eriocheir sinensis during ovarian maturation. Comp Biochem Physiol 130B:95–104
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001a) Penaeid shrimp broodstock nutrition: an up dated review on research and development. Aquaculture 202:1–21
Wouters R, Piguave X, Calderon J, Sorgeloos P (2001b) Ovarian maturation and haemolymphatic vitellogenin concentration of Pacific white shrimp Litopenaeus vannamei (Boone) fed increasing levels of total dietary lipids and HUFA. Aquacult Res 32:573–582
Wu J, Jiang X (2000) The relationship of the total protein levels in the hemolymph and hepatopancreas with precociousness of Eriocheir sinensis. J Fish China 24:306–311 (in Chinese with english abstract)
Wu J, Jiang X (2001) The relationships between Ca2+, 17β-estradiol levels in the hemolymph and precociousness of Eriocheir sinensis. J Fish China 25:112–115 (in Chinese with english abstract)
Wu X, Chang G, Cheng Y, Zeng C (2010) Effects of dietary phospholipids and highly unsaturated fatty acid on the gonadal development, tissue proximate composition, lipid class and fatty acid composition of precocious Chinese mitten crab, Eriocheir Sinensis. Aquacult Nutr 16:25–36
Xu X, Zhu Z (1994) Probe into the occurrence, harm, identification and prevention of precocity of Chinese mitten crab Eriocheir sinensis. Freshwat Fish 24:3–6 (in Chinese)
Yuan C, Cui Q, Zhao C (2004) Prospective of neuro-endocrine regulation on precocity of Chinese mitten crab Eriocheir sinensis. J Hydroecol 24:11–14 (in Chinese)
Zapata V, López Greco LS, Medesani D, Rodríguez EM (2003) Ovarian growth in the crab Chasmagnathus granulata induced by hormones and neuroregulators throughout the year: In vivo and in vitro studies. Aquaculture 224:339–352
Zhang K, Wang X (2006) Studies on variation of protein and changes of its content in hepatopancreas and gonad of Eriocheir sinensis by eyestalk ablation. J Shanxi Agri Univ 26:128–131 (in Chinese with english abstract)
Zhang L, Xu Q (2001) Studies on sex maturity and early maturity of mitten crab (Eriocheir sinensis) in natural and farming water. Fish Sci Technol Inf 28:106–111 (in Chinese with english abstract)
Zhang D, Du X, Zhao J, Zheng W, Wei F, Zhang S, Zhang Z (2001) Study on the occurrence reasons and control methods of mitten crab precocity. Freshwat Fish 31:36–39 (in Chinese)
Zhao W, Lu J (2003) The relationship between hormone biosynthesis of mandibular organ and precociousness in Eriocheir sinensis. J Fish China 27:289–294 (in Chinese with english abstract)
Zhou K, Geng L (2001) Research development of nutritional requirements and sexual precocity of the Chinese mitten-handed crab Eriocheir sinensis (Crustacea, Decapoda). J Baoding Teach Coll 14:7–10 (in Chinese with english abstract)
Zhu Y, Wang J, Zhang G, He Z (1999a) Effects of dietary cholesterol level and protein content on growth and sexual precocity of juvenile Chinese mitten crab Eriocheir sinensis. Fish Mod 3:3–6 (in Chinese)
Zhu Y, Zhang G, Wang J, Chen H (1999b) Suitable ratio between animal and vegetable protein in formulated pellet and its co-relation with growth and pre-maturation of young crab. Fish Sci Technol Inf 26:21–25 (in Chinese with english abstract)
Acknowledgments
The present study was financially supported by the National Scientific and Technological Supporting Program of China (2007BAD37B03 and 2006BAD03B02) and the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-YW-N-47-06). We also thank the two anonymous reviewers who provided useful comments for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Li, Z., Liu, J. et al. Advances in precocity research of the Chinese mitten crab Eriocheir sinensis . Aquacult Int 19, 251–267 (2011). https://doi.org/10.1007/s10499-010-9400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9400-1