Abstract
The purpose of this study was to investigate the growth and physiological status of Litopenaeus vannamei subjected to one constant temperature (25°C) and four cyclical temperature change regimes (25 ± 1°C, 25 ± 2°C, 25 ± 3°C and 25 ± 4°C). The growth rates of shrimp at 25 ± 2°C or 25 ± 3°C were significantly higher than that at a constant temperature of 25°C. On the other hand, the growth rate in 25 ± 4°C regime was significantly lower than those in other regimes. The daily feed intake rate of shrimp at 25 ± 4°C was the lowest, and the food conversion efficiency was also significantly lower than those at 25 ± 2°C and 25 ± 3°C, respectively. The food conversion efficiency at 25 ± 2°C or 25 ± 3°C was significantly higher than those in other regimes. Thus, it can be inferred that the growth enhancement in the test shrimp at the suitable diel fluctuating temperatures was due to high food conversion efficiency. Studies of the physiological parameters showed that at 25 ± 4°C, the hemolymph glucose content of the test shrimp was the lowest, while the activity of PK in hepatopancreas was the highest, which indicated that the test shrimp at 25 ± 4°C was in a stressed condition. The hemolymph glucose content of the test shrimp at 25 ± 3°C was the highest, and the activity of HK in hepatopancreas was the lowest. These results indicated that the test shrimps at 25 ± 3°C were not in a stressed condition. Compared with the constant temperature regime, the expression of HSP70 in any of the four cyclical temperature change regimes was not significantly increased. The reason for this might be that the fluctuation amplitude of ± 4°C did not induce the increased expression of HSP70.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water temperature is one of the major environmental factors influencing growth and physiological status in aquatic ectotherms. In natural aquatic systems, water temperature fluctuates diurnally and seasonally, and the effect of fluctuating temperature on the growth of aquatic animals is well studied (Miao and Tu 1993, 1996; Tian 2001). Tian (2001) reported that suitable diel fluctuating temperature could enhance the growth of Fenneropenaeus chinensis Osbeck, and the oxygen consumption changes in shrimps were also discussed preliminarily. However, there is little information on the effects of cyclical temperature change on the growth and physiological status of Litopenaeus vannamei.
Once the environment changes, the activities of glycolytic enzymes are altered in order to produce more energy (Somero and Childress 1990). Glucose is the main material for producing energy in body, and the level of glucose is usually referred to evaluate the state of metabolism in aquatic animals by many researchers. Lourdes et al. (2006) suggested that the increased level of blood glucose in L. schmitti was an indicator of short-term stress. However, it was reported that the concentration of glucose in the hemolymph did not change under the following conditions: 4 weeks at high densities (Hall and van Ham 1998), 7 days in captive laboratory conditions (Sánchez et al. 2001) and 10 days exposed to a high temperature (Pascual et al. 2003). Glucose is also the pre-substrate of glycolysis, and the glucose content can affect the course of glycolysis. However, whether glucose can enter the course of glycolysis depends on the activity of hexokinase (HK, EC 2.7.1.1). Pyruvate kinase (PK, EC 2.7.1.40) is another key enzyme in the glycolytic pathway. This enzyme catalyzes the essentially irreversible transphosphorylation from phosphoenolpyruvate (PEP) and ADP to pyruvate and ATP (Valentini et al. 2002). In almost every cell type, PK controls the flux through the glycolytic pathway, together with the phosphofructokinase-1 (PFK) and hexokinase (Wang et al. 2005). But the information about key glycolytic enzymes in crustaceans, such as HK and PK, is still poor, although recently more information is becoming available due to the commercial and ecological importance of some crustacean species. For example, HK activity was detected in shore crab Pachygrapsus crassipes (Schatzkein et al. 1973), European green crab Carcinus maenas (Loret and Devos 1992) and shrimp L. vannamei (Rosas et al. 2001); while PK activity was detected in different organs in the spiny lobster Jasus edwardsii (Speed et al. 2001), the shrimp Farfantepenaeus paulensis (Lemos et al. 2003), the Dungeness crab Cancer magister (Guderley and Hochachka 1980) and the striped shore crab P. crassipes (Schatzkein et al. 1973). However, the relationship between the hemolymph glucose content and activities of the glycolytic enzymes is not well known in shrimps suffering diurnal fluctuating temperatures.
Heat shock proteins also comprise a group of highly conserved proteins that are related to the function of resistance to adverse environmental conditions. The HSP70 family of proteins includes the constitutively expressed form (heat shock cognate 70, HSC70) and the stress-inducible form (HSP70) (Itoh and Tashima 1991). Generally, HSC70 acts to maintain the ordinary cellular environment, such as folding of newly synthesized proteins. HSP70 rescues intracellular proteins that are degenerated or aggregated under stressed conditions (Itoh and Tashima 1991). Compared with that at normal temperatures, the sublethal temperatures induced higher expression of heat shock proteins 70 in L. vannamei, indicating that there is a close relationship between the increased expression of HSP70 and the enhanced capacity of self-protection (Wu et al. 2006). When L. vannamei suffered the diel fluctuation temperature condition, how the HSP70 expressed and whether tolerance was strengthened as a result of HSP70 accumulation were not clear so far (Dong et al. 2008).
The purpose of this study was to compare the growth of L. vannamei at a constant temperature and fluctuating thermal regimes and to evaluate the effect of temperature fluctuation on physiological status of L. vannamei by determining the hemolymph glucose content, glycolytic enzyme activities and the expression of HSP70. These results may shed light on the growth and physiological status of L. vannamei under fluctuating temperature conditions.
Materials and methods
Experimental shrimp and acclimation
Litopenaeus vannamei juveniles with the average wet body weight of 5.068 ± 0.061 g (Mean ± SD) were obtained from Baorong Aquaculture Corporation, Qingdao, P. R. China. After transfer to the laboratory, shrimps were acclimated in tanks (170 cm × 75 cm × 35 cm; water volume: 450 L; salinity: 28–30 ppt; temperature: 25°C) for 10 days. Seawater was pre-filtered using a sand filter. One-half to two-thirds of the rearing water was exchanged daily by fresh seawater to ensure high water quality. During the acclimation period, shrimps were fed twice daily (at 6 a.m. and 4 p.m.) to satiation with a commercial feed (crude protein, 43.39 ± 0.22%; fat, 9.74 ± 0.30%; ash, 9.91 ± 0.05% and moisture, 8.41 ± 0.06%) manufactured by the Mawei Fishery Feed Co. Ltd., Fujian, China. A photoperiod of 14-h light:10-h dark was maintained.
Experimental design and facility
Experimental design: Thermal treatments consisted of one constant temperature (25°C) and four diel temperature fluctuations with daily means of 25°C. The amplitudes of temperature fluctuations (± Δt°C) were ±1, ±2, ±3 and ±4°C, respectively. There were five replicates for each thermal treatment, among which three were used for the comparision of the growth in L. vannamei and two for the evaluation of the physiological status. Twenty-five glass aquaria (45 cm × 25 cm × 30 cm, water volume: 35 L) were used for shrimp rearing. Each aquarium held seven individuals and was covered with a 5-mm thick screen to prevent the shrimp from jumping out. Experiments were started after feed deprivation 24 h in advance.
Temperature control system: The experiment was conducted in a room whose temperature was controlled at 25 ± 0.5°C using an air conditioner. For the constant and the fluctuating temperature treatments, the water temperature was regulated by a laboratory-designed temperature control system (Fig. 1). This system was composed of a programmed temperature controller, a temperature control tank, a heater, a refrigerator, a recirculation pump and a cold water reservoir. The actual temperature was calibrated daily with a mercury thermal meter to the nearest 0.2°C. Treatments with fluctuating temperatures were controlled by a pre-programmed temperature controller, which is specially designed to generate the needed temperature regimes by pumping the cold water and heating the water alternately. In order to ensure higher water quality, an absorbent cotton wool (0.5 cm thickness) was used to filter the water flow into the temperature control tank.
Diagram of the fluctuating temperature control system (1, cool water reservoir; 2, cool water temperature sensor; 3, outlet pipe of refrigerator; 4, inlet pipe of refrigerator; 5, refrigerator; 6, temperature controller of cool water reservoir; 7, inlet pipe of experimental tank; 8, experimental tank; 9, Direction of flow; 10, outlet pipe of experimental tank; 11, temperature control tank; 12, pump; 13, water temperature sensor of temperature control tank; 14, temperature controller of temperature control tank; 15, heater)
Temperature fluctuating mode (using the temperature amplitudes of ±2°C as an example): The base temperature (25°C) was set at 0600 h and was increased gradually to a maximum of 27°C at 1,200 h, then it was decreased to a minimum of 23°C at 0000 h, followed by gradually increasing to the base temperature (25°C) again at 0600 h the next day (Fig. 2).
Experimental procedure and management
When the thermal acclimation was finished, experimental shrimps (mean wet body weight of 5.068 ± 0.061 g) were randomly chosen and weighed individually. They were then allocated into five aerated aquaria for corresponding temperature treatments. During the experiment, shrimps were fed to satiation twice daily (at 6 a.m. and 4 p.m.) with the commercial feed mentioned earlier. The uneaten feed and faces were separately collected into cups by siphoning within 3 h after each meal, allowed to settle down about 10 min and then dried at 65°C for further analysis. The exuviae (molted exoskeletons) were cleaned regularly. At the end of the experiment, shrimps from three aquaria of each treatment were collected after 24-h starvation, dried at 65°C and weighted individually for the growth analysis. Shrimps used for the physiological status analysis in each treatment were selected from the other two aquaria.
The experiment lasted for 45 days. During the course of the experiment, dissolved oxygen was maintained above 6.0 mg L−1, pH was around 7.8, ammonia (NH3 and NH4 +) was less than 0.24 mg L−1, salinity was 28–30 ppt. Photoperiod and water exchange rates were the same as those in the acclimation condition.
Sample preparation and assay for physiological status
At the end of the experiment, five inter-molt stage shrimps (Robertson et al. 1987) were selected for the evaluation of the physiological status. After drying the shrimp with paper towel, hemolymph (approximately 20 μl) was collected from the ventral sinus at the base of the first abdominal segment using a 1-ml syringe, which was pre-rinsed with a 5% sodium oxalate in isotonic saline-cooled anticoagulant solution (Mendoza 1992). Then, hemolymph was centrifuged at 3,000×g for 15 min at 4°C, and the supernatant fraction was collected and used for glucose assay immediately. After hemolymph was collected, shrimps were dissected rapidly on an ice plate. The hepatopancreas and muscle were quickly removed from each shrimp, frozen in liquid nitrogen and stored at −80°C for further analysis.
Glucose assay
Glucose was analyzed according to the manual of analyses kits (Shanghai Rongsheng Biotech Co., LTD, China) and the method described by Tiffany et al. (1972). Briefly, glucose from each sample was oxidized by glucose oxidase to produce gluconic acid and hydrogen peroxide. Then, with the help of peroxidase, hydrogen peroxide caused the coupling reaction between 4-aminoantipyrine and phenol to synthesize benzoquinones, which can be measured by spectrophotometry.
Enzyme assay
The hepatopancreas was homogenized on ice by a glass homogenizer for 2 min in 5 vol (w/v) of ice-cold 0.05 M PBS buffer (pH 7.5). The homogenate was centrifuged at 3,000×g for 10 min at 4°C. The supernatant fraction was collected for the measurement of enzyme activities. Activities of HK and PK were analyzed according to the manual of analyses kits (Nanjing Jiancheng Bioengineering Institute, China) and the method described by Tanaka et al. (1962) and Valentine et al. (1967), respectively.
Western blotting
HSP70 was measured as previously described by Dong et al. (2007). Protein concentration was determined according to Bradford (1976) with bovine serum albumin as standard. Gel electrophoresis of protein extracts was performed in 10% polyacrylamide gels (PAGE) according to the method described by Laemmli (1970). Equal amount of protein samples (20 μg) was loaded in each well and subjected to gel electrophoresis in the presence of 2-mercaptoethanol. Semi-dry electro-transfer onto PVDF-Immobilon membranes was performed according to Kyhse-Andersen (1984). Membranes were blocked and incubated with anti-HSP70 Ab (H5147, Sigma, USA) (diluted 1:1,000; both the HSC70 and the HSP70 could be incubated with it) for 2 h at 37°C. Then, the immune complexes were visualized by incubation with anti-mouse IgG (Horseradish peroxidase conjugated, HRP), followed by staining with diaminobezidine (DAB). Band intensity was quantified using GeneTools software (Syngene, USA).
From a shrimp in the normal water temperature regime, 20 μg of muscle protein was also loaded to each gel. The HSP70 level of this sample was designated as a standard for normalization. HSP70 levels in this study were expressed as values relative to the level of the standard sample (Relative unit, RU; %).
Data calculation and statistical analysis
Specific growth rate (SGRd, % day−1), daily feed intake rate (FId, % BW day−1) and food conversion efficiency (FCEd, %) were calculated using the following equations:
SGRd (% day−1) = 100 × (lnW2 − lnW1)/T
FId (% BW day−1) = 100 × F/[T × (W2 + W1)/2]
FCEd (%) = 100 × (W2 − W1)/F
where W 2 and W 1 are final and initial dry body weight (g), respectively. T is the feeding duration (d) and F is the total food intake (g).
Experimental data were analyzed by ANOVA using the software SPSS 11.0 (SPSS Inc., Richmond, CA, USA). Duncan’s multiple range tests were used to test the differences between treatments. P < 0.05 was accepted as the level of statistical significance.
Results
The growth of L. vannamei in different thermal regimes
The final body weight and specific growth rate (SGRd) of L. vannamei in different thermal regimes are shown in Table 1. It can be seen that the different amplitudes of temperature fluctuation (with an average temperature of 25°C) had various influences on the growth of the experimental shrimps. The final mean body weights of shrimps in 25 ± 2°C and 25 ± 3°C regimes were significantly higher than those in constant temperature (25°C) or other diel temperatures fluctuating regimes (P < 0.05). The final body weight of shrimps in 25 ± 4°C regime was significantly lower than those in other regimes. The SGRd of the shrimps in 25 ± 2°C regime was the highest and was significantly different from those in 25°C, 25 ± 1°C and 25 ± 4°C regimes (P < 0.05). SGRd of the shrimps in 25 ± 4°C regime was the lowest, and it was significantly lower than those in 25°C, 25 ± 2°C or 25 ± 3°C regimes (P < 0.05). Based on the studies of Miao and Tu (1996), the relationship between SGR and the thermal amplitude (TA) can be described as a cubic polynomial function:
SGR = 0.4196−0.1967TA + 0.2052TA2−0.0414TA3 (r 2 = 0.782, P < 0.001)
According to the equation, the optimal thermal amplitude (with a mean temperature of 25°C) for the growth of shrimp at sizes in the current experiment was estimated to be ±2.51°C.
The FId and FCEd of L. vannamei in different thermal regimes
The FId and FCEd of L. vannamei in different thermal regimes are shown in Table 1. The FId of the shrimps in 25 ± 4°C regime was significantly lower than that in 25°C regime (P < 0.05), but it was not significantly different from those in 25 ± 1°C, 25 ± 2°C and 25 ± 3°C regimes, respectively (P > 0.05). The FCEd of the shrimps in 25°C ± 2°C regime was the highest, which was close to that in 25°C ± 3°C regime, and was significantly higher than those in 25°C, 25 ± 1°C and 25 ± 4°C regimes, respectively (P < 0.05). The FCEd of the shrimps at 25 ± 4°C was significantly lower than that in 25°C, 25 ± 2°C and 25 ± 3°C regimes (P < 0.05).
The glucose content and glycolytic enzyme activities in L. vannamei in different thermal regimes
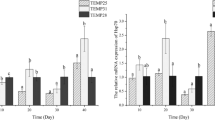
The glucose content and activities of HK and PK in L. vannamei from different thermal regimes are shown in Table 2. The glucose content of the shrimp’s hemolymph in 25 ± 3°C regime was the highest, and it was significantly different from the lowest value observed in the 25 ± 4°C regime (P < 0.05), but it was not significantly different from those in other regimes (P > 0.05). The HK activity in shrimp hepatopancreas in 25°C regime was the highest, and it was significantly different from that in the 25°C ± 3°C regime (P < 0.05), but it was not significantly different from those in other regimes (P > 0.05). The HK activity was the lowest in the 25 ± 3°C regime. The PK activity in the hepatopancreas of the shrimps in 25 ± 1°C regime was the lowest and was significantly different from those in other regimes (P < 0.05). There were no significant differences in the PK activity among other treatments (P > 0.05).
The expression of HSP70 in L. vannamei in different thermal regimes
The expression of HSP70 in the shrimp muscle under different thermal regimes is shown in Table 2. The expression level of HSP70 in 25 ± 2°C or 25 ± 3°C regimes was not significantly different compared to that in the 25°C regime (P > 0.05). However, the expression level of HSP70 in 25 ± 1°C and 25 ± 4°C regimes was significantly lower than that in the 25°C regime (P < 0.05).
Discussion
The effects of cyclical temperature changes on the growth of L. vannamei
Previous studies on the effects of the fluctuating temperature on the growth of aquatic animals are inconsistent. For example, the growth of both juvenile F. penicillatus and F. chinensis Osbeck increased under moderate diel fluctuating thermal regimes (Miao and Tu 1993; Tian 2001). However, Thorp and Wineriter (1981) reported that there was no significant difference on the growth of Procambarus a. acutus between the constant (17.5°C) and variable temperature regimes (a daily square-wave cyclic regime of 10–25°C, the average temperature was 17.5°C). Tian and Dong (2005) reported that the effect of temperature changes on the growth of the animals may be related to its average temperature, amplitude and temperature change mode with amplitude being the main reason. Results from this study showed that moderate diel fluctuating thermal regimes could accelerate the growth of L. vannamei. At an average temperature of 25°C, the temperature fluctuations of ±2 and ±3°C resulted in better growth than that at a constant temperature of 25°C. But the temperature fluctuation of ±4°C inhibited the growth of L. vannamei when compared with that at the constant temperature of 25°C. These results indicated that the effect of cyclical temperature changes on the growth of shrimp is closely related to the amplitude of temperature variation. Similar findings were also reported in F. chinensis (Tian 2001) and Cyprinus carpio (Konstantinov et al. 1990), respectively. Based on the cubic equation from this study, we found that the optimal thermal amplitude for the growth of L. vannamei at experimental sizes was estimated to be ±2.51°C with a mean temperature of 25°C, which was close to that reported by Tian (2001) in F. chinensis. So, the thermal amplitude of diel fluctuating temperatures should be under ±4°C in course of L. vannamei culture.
The mechanisms of the enhancement in the growth of shrimps at the suitable diel fluctuating temperatures are still ambiguous. Tian and Dong (2005) summarized reasons for the growth enhancement in shrimp at the suitable diel fluctuating temperatures as follows: higher food intake, lower metabolic rate and higher food conversion efficiency. In this study, the growth of shrimp in 25 ± 2°C or 25 ± 3°C regimes were higher than those under other regimes, but their FId were not significantly different from those in other regimes. Lower FCEd of shrimp in 25 ± 4°C regime compared to those in 25 ± 2°C and 25 ± 3°C regimes suggests that the growth enhancement of the shrimps at suitable diel fluctuating temperature was due to high food conversion efficiency.
The effects of cyclical temperature changes on the glucose content and activities of glycolytic enzymes in L. vannamei
Some researchers had found that glucose levels during long-term stress would not be as pronounced as during short-term stress (Hall and van Ham 1998; Sánchez et al. 2001; Pascual et al. 2003). In this study, the glucose level in shrimps at 25°C was not significantly different from those in other regimes and is close to those reported for other studies in long-term stressed conditions. An adaptation or acclimation to repeated stressor could explain the absence of high glucose concentration in stressed L. vannamei hemolymph (Laurence et al. 2006). In 25 ± 3°C regime, the hemolymph glucose content was the highest, its HK activity in hepatopancreas was the lowest and the PK activity was moderate. The moderate PK activity indicates that the metabolism of the shrimps in 25 ± 3°C regime was normal. The lowest HK activity in 25 ± 3°C regime suggested that the amount of glucose phosphorylated in the shrimp’s hepatopancreas was decreased, and there was no metabolic compensation of glycogen in hepatopancreas, which may facilitate other organs to make use of glucose. The glucose content of the shrimps in 25°C regime was 1.527 ± 0.145 mmol L−1, which is close to the normal glucose content in L. schmitti (Lourdes et al. 2006). The HK activity of the shrimps in 25°C regime was the highest among all regimes, but it was only significantly different from that in 25 ± 3°C regime. Allert et al. (1991) suggested that the activity of HK correlates with the flow of glucose. Thus, high HK activity in the shrimps under 25°C regime may suggest that the flow of glucose was high at that condition. The PK activity of the shrimps at 25°C was close to that at 25 ± 3°C, suggesting that the increased glucose flow at 25°C did not join in the process of glycolysis to produce more energy, but instead, it was transformed into glycogen. Couto et al. (2008) suggested that the increased activity of PK was related to the elevation of glycolysis. In this study, the high PK and HK activities of the shrimps at 25 ± 4°C suggested that the glycolysis in these shrimps was at a high level. Considering the low growth rate at 25 ± 4°C, it was reasoned that the elevation of glycolysis at 25 ± 4°C might be caused by the temperature amplitude, which, in turn, might have a negative impact on the shrimps and results in a stressed condition. Hence, the glucose in the shrimps at 25 ± 4°C was consumed and resulted in decreased concentrations in their hemolymph. The PK activity of the shrimps at 25 ± 1°C was significantly lower than those in other regimes, suggesting that the glycolysis in these shrimps dropped. The reason for this might be that the overall metabolism of shrimp in this regime was low.
The effects of cyclical temperature changes on the expression of HSP70 in L. vannamei
Heat shock proteins protect other proteins from unfolding, assist refolding of denatured protein or target them for degradation. These functions of heat shock proteins are based on heat stress-induced reactions (Morimoto et al. 1994; Frydman and Höhfeld 1997). Thus, the expression of HSPs is always considered an indicator of heat stress (Feder and Hofmann 1999). The synthesis and chaperone function of HSPs require energy consumption, which means there was an “investment-income” trade-off between the heat tolerance and the energy allocated for growth and reproduction (Somer 2002). Therefore, induction of the expression of heat shock proteins needs certain inducing temperature (Sanders et al. 1991; Tomanek and Somero 2000). In this study, the expression of heat shock protein 70 (HSP70) in four cyclical temperature change regimes was not significantly increased when compared with that in the constant temperature regime. The reason for this might be the fluctuation amplitude of ±4°C that did not induce the increased expression of HSP70. This finding is in accordance with that reported for the sea cucumber Apostichopus japonicus Selenka (Dong et al. 2008). The HSP70 family of proteins includes the constitutively expressing form (heat shock cognate 70; HSC70) and the stress-inducible form (HSP70). Generally, HSC70 acts to maintain the ordinary cellular environment such as folding of newly synthesized proteins (Itoh and Tashima 1991; Zeniya et al. 1995). On the other hand, HSP70 is an inducible form of HSP70 family protein that functions to rescue intracellular proteins that are degenerated or aggregated under stressed conditions (Itoh and Tashima 1991; Zeniya et al. 1995). In this study, the expression level of HSP70 represented the combined levels of both HSC70 and HSP70. Results showed that there were certain differences for the expression of HSP70 among the five regimes under the sub-inducing temperatures. The reason for this might be that the contents of HSC70 changed under different thermal regimes. Laurence et al. (2006) reported that protein contents in hemolymph, hepatopancreas and muscle decreased in the stressed conditions. The metabolism of shrimps at 25 ± 1°C and 25 ± 4°C might be affected by an adverse effect, but this effect may not be enough to induce high-level expression of HSP70. Consequently, the presumably decreased total protein contents in shrimps at 25 ± 1°C and 25 ± 4°C may account for the significantly low HSP70 expression in these two regimes compared with that at 25°C. There was no apparent relationship between the growth of the test shrimps and the expression level of HSP70 at different diel fluctuating temperatures in this experimental condition. In spite of the result that no increase in HSP70 expression with thermal amplitudes within ±4°C was revealed in the present experiment, it might be expected that HSP70 expression will be considerably enhanced at one threshold thermal amplitude for L. vannamei. It is unclear so far, and future study should be conducted.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem 72:248–254
Couto A, Enes P, Peres H et al (2008) Effect of water temperature and dietary starch on growth and metabolic utilization of diets in gilthead sea bream (Sparus aurata) juveniles. Comp Biochem Physiol 151A:45–50
Dong YW, Ji TT, Dong SL (2007) Stress responses to rapid temperature changes of the juvenile sea cucumber (Apostichopus japonicus Selenka). J Ocean Univ China (English Edition) 6:275–280
Dong YW, Dong SL, Ji TT (2008) Effect of different thermal regimes on growth and physiological performance of the sea cucumber Apostichopus japonicus Selenka. Aquaculture 275:329–334
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress responses. Annu Rev Physiol 61:243–282
Frydman J, Höhfeld J (1997) Chaperones get in touch: the hip-hop connection. Trends Biochem Sci 22:87–92
Guderley H, Hochachka PW (1980) Catalytic and regulatory properties of muscle pyruvate kinase from Cancer magister. J Exp Zool 212:461–469
Hall MR, Van Ham EH (1998) The effects of different types of stress on blood glucose in the giant tiger prawn Penaeus monodon. J World Aquac Soc 29:290–299
Itoh H, Tashima Y (1991) The stress (Heat shock) proteins. Int J Biochem 23:1185–1191
Konstantinov AS, Zdanovich VV, Tikhomirov DG (1990) The effect of temperature fluctuations on metabolic rate and energetics of juvenile fish. J Ichthyol 30:38–47
Kyhse-Andersen J (1984) Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods 10:203–209
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laurence M, Elena P, Ángel I et al (2006) Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 258:633–640
Lemos D, Salomon M, Gomes V et al (2003) Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): effects of development and temperature. Comp Biochem Physiol 135B:707–719
Loret SM, Devos PE (1992) Hydrolysis of G-6P by a microsomal specific phosphatase and glucose phosphorylation by a low Km hexokinase in the digestive gland of the crab Carcinus maenas. Variations during the moult cycle. J Comp Physiol B 162:651–657
Lourdes PJ, Tania RR, Laida R et al (2006) Changes in metabolic and immunological variables of wild and pond-reared southern white shrimp Litopenaeus schmitti adult males during continuous reproductive activity. Aquaculture 252:591–597
Mendoza R (1992) Etude de la vitellogenèse et de sa stimulation chezles crevettes péneidés pardes facteurs hétérologues et homologues. Doctoral Thesis, Université de Bretagne Occidentale, Brest, France. p 200
Miao S, Tu S (1993) Modling effect of thermal amplitude and stocking density on growth of redtail shrimp, Penaeus penicillatus (Alock). Bull Zool Acad Sin 32:253–264
Miao S, Tu S (1996) Modeling effect of thermal amplitude on growing Chinese shrimp Penaeus chinensis (Osbeck). Ecol Model 88:93–100
Morimoto RI, Tissieres A, Georgopoulos C (1994) The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N. Y
Pascual C, Sánchez A, Sánchez A et al (2003) Haemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: the effect of an extreme temperature. Aquaculture 218:637–650
Robertson L, Bray WA, Leung-Trujillo JR et al (1987) Practical molt staging of Penaeus setiferus and Penaeus stylirostris. J World Aquac Soc 18:180–185
Rosas C, Cuzon G, Gaxiola G et al (2001) Metabolism and growth of juveniles of Litopenaeus vannamei: effect of salinity and dietary carbohydrates level. J Exp Mar Biol Ecol 259:1–22
Sánchez A, Pascual C, Sánchez A et al (2001) Hemolymph metabolic variables and immune response in Litopenaeus setiferus adult males: the effect of acclimation. Aquaculture 198:13–28
Sanders BM, Hope C, Pascoe VM et al (1991) Characterization of stress protein response in two species of Collisella limpets with different temperature tolerances. Physiol Zool 64:1471–1489
Schatzkein FC, Carpenter HM, Rogers MR et al (1973) Carbohydrate metabolism in the striped shore crab, Pachygrapsus crassipes: I. The glycolytic enzymes of gill, hepatopancreas, heart and leg muscles. Comp Biochem Physiol B 45:393–405
Somer GH (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integ Comp Biol 42:780–789
Somero GN, Childress JJ (1990) Scaling of atp-supplying enzymes, myofibrillar proteins and buffering capacity in fish muscle: relationship to locomotory habit. Exp Biol 149:319–333
Speed SR, Baldwin J, Wong RJ et al (2001) Metabolic characteristics of muscles in the spiny lobster, Jasus edwardsii, and responses to emersion during simulated live transport. Comp Biochem Physiol B 128:435–444
Tanaka KR, Valentine WN, Miwa S (1962) Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood 19:267–295
Thorp JH, Wineriter SA (1981) Stress and growth response of juvenile crayfish to rhythmic and arrhythmic temperature fluctuations. Arch Environ Contam Toxicol 10:69–77
Tian XL (2001) Effect of diel fluctuation temperature on growth of Chinese shrimp, Fenneropenaeus chinensis Osbeck, and its bioenergetic mechanisms. PhD thesis, Ocean university of Qingdao, China (in Chinese)
Tian XL, Dong SL (2005) The research of effects of temperature fluctuation on growth of aquatic animals. Chin J Appl Ecol 16:1780–1785 (in Chinese)
Tiffany TO, Jansen JM, Burtis CA et al (1972) Enzymatic kinetic rate and end-point analyses of substrate, by use of a GeMSAEC fast analyzer. Clin Chem 18:829–840
Tomanek L, Somero GN (2000) Time course and magnitude of synthesis of heat-shock proteins in congeneric marine snails (Genus Tegula) from different tidal heights. Physiol Biochem Zool 73:249–256
Valentine WN, Oski FA, Paglia DE et al (1967) Hereditary hemolytic anemia with hexokinase deficiency. Role of hexokinase in erythrocyte aging. N Engl J Med 276:1–11
Valentini G, Chiarelli LR, Fortin R et al (2002) Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia. J Biol Chem 277:23807–23814
Vondracek B, Cech JJ, Buddington RK (1989) Growth, growth efficiency and assimilation efficiency of the Tahoe sucker in cyclic and constant temperature. Environ Biol Fish 24:151–156
Wang Y, Zou S, Zhang Y (2005) Modern animal biochemistry, 3rd edn. High Education Press of China, Beijing, pp 466–490 (in Chinese)
Wu R, Xie S, Sun Y (2006) High level prokaryotic expression of heat shock protein 70 in Litopenaeus vannamei. J Fish Sci China 13(2):305–309 (in Chinese)
Zeniya A, Otaka M, Itoh H et al (1995) Induction and intracellular localization of a 72-kDa heat shock protein in rat gastric mucosa after water-immersion stress. J Gastroenterol 30:572–577
Acknowledgment
This work was supported by the Chinese National Natural Science Foundation (Grant No. 30571441), Key Project of Scientific and Technical Supporting Programs funded by Ministry of Science and Technology of China (Grant No. 2006BAD09A07) and the Project under the Major State Basic Research of China (Grant No. 2009CB118706).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, B., Wang, F., Dong, S. et al. The effects of cyclical temperature changes on growth and physiological status of Litopenaeus vannamei . Aquacult Int 18, 921–932 (2010). https://doi.org/10.1007/s10499-009-9314-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-009-9314-y