Abstract
The major cations and anions from lake water samples and its sources, including glacier snow, precipitation, stream, and swamp water in the Nam Co basin, central Tibetan Plateau, were studied. The concentrations of the major ions varied significantly in the five environmental matrices. Generally, the mean concentrations of most ions are in the order of lake water > swamp water > stream water > precipitation > snow. Rock weathering is the dominant process controlling the chemical compositions of the stream and swamp waters, with carbonate weathering being the primary source of the dissolved ions. The Nam Co lake water is characterized by high Na+ concentration and extremely low Ca2+ concentration relative to other ions, resulting from evapoconcentration and chemical precipitation within the lake. Comparison with the water chemistry of other lakes over the Tibetan Plateau indicated that Nam Co is located in a transition area between non-saline lakes and highly saline lakes. The relatively low concentration of total dissolved solids is possibly due to the abundant inflow of glacial meltwater and relatively high annual precipitation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Tibetan Plateau is the largest and highest plateau in the world, home to the tallest mountain range, the Himalaya, and the highest mountain, Mount Everest (Qomolangma). The plateau covers an area of about 2.5 × 106 km2, with an average elevation of over 4,000 m. Formed by tectonic plate collisions in the Cenozoic period, the uplift of the plateau began at least 50 Myear ago and reached its present altitude 35–15 Myear ago (Rowley and Currie 2006). The uplift of the plateau has greatly modified global climate (Kutzbach et al. 1989) and influenced monsoon intensity (Molnar et al. 1993). Climatically, the plateau lies at a critical junction of three climatic systems: the Indian monsoon, the East Asian Monsoon, and the cold polar airflow from the Siberian high pressure system. The human population density is sparse, and industrial activities remain minimal across most of the plateau, making it one of most remote and “pristine” regions in the world. However, concerns have been raised in recent years over the habitat loss due to excessive pasturing and unsustainable development, chemical contamination due to long-range transport of contaminants, and wide-scale systemic changes due to global climate change, all of which are threatening the unique and fragile alpine ecosystems and Tibetan culture.
As the rooftop of the world, the Tibetan Plateau is the source of many major rivers in Asia, including the Changjiang (Yangtze River), Huanghe (Yellow River), the Yarlung Zangbu-Brahmaputra, and the Lancangjiang-Mekong. In addition to those large exorheic river systems that cut through the plateau, there are also a vast number of endorheic rivers and streams, most of which are seasonal and supplied by glacial meltwater and summer precipitation. These endorheic rivers and streams form more than 1,500 alpine lakes in the basins of the plateau. Whereas much scientific attention has been given to those exorheic rivers (e.g., Chen et al. 2002, 2005), few studies are available on the water quality and quantity and their evolution of the alpine lakes of the plateau.
In June 2005, the Nam Co Station for Multisphere Observation and Research (NAMOR) was established by the Chinese Academy of Sciences in the southeast shore of Nam Co (Lake Namtso) to thoroughly study the geology, hydrology, chemistry, biology, and ecology of the largest lake in Tibet. As a pioneer research supported by NAMOR logistic assistance, Li et al. (2007a) studied the major ionic chemistry of precipitation in this region and indicated that the geochemistry of atmospheric deposition is mainly influenced by the regional continental environment. However, studies on Nam Co water quality and related geochemical processes, though critical for understanding this unique alpine lake system, are yet absent. Here, we report the first study of the major ion geochemistry of Nam Co and its source waters. This article is aimed at (a) providing a baseline dataset of major ion concentrations in a Tibetan lake basin and (b) investigating the geochemical processes that control the major ion chemistry.
2 Study Area and Methods
2.1 The Nam Co Basin
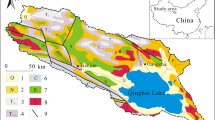
Nam Co (30°30′–56′ N, 90°16′–91°03′ E; 4,718 m a.s.l.) is the largest lake in Tibet, the second largest saline lake on the Tibetan Plateau, and one of the highest large lakes in the world (Fig. 1). The lake has a surface area of 1,920 km2 with a maximum depth of 122 m (Li et al. 2008). Located in the heart of the Tibetan Plateau, Nam Co drains a closed basin of about 1.06 × 104 km2 isolated by the Gandise Range in the south, the Nyainqen Tanglha Range in the southeast, and the Northern Tibetan Plateau hills in the north.
The Nam Co Basin is located in a semi-arid to semi-humid area. The mean annual temperature is around −2 to 0°C (CAS 1982). The lake is ice-covered for 5 months, with a maximum ice thickness of 46 cm as observed by the Nam Co Station in 2007. The mean annual precipitation ranges from 300 to 410 mm (CAS 1982; Shao et al. 2004), and the mean annual evaporation is around 790 mm for the lake and 320 mm for its terrestrial basin (Zhu et al. 2004). Most of the precipitation occurs between June and October. As a closed-basin lake, the only loss of the water is through evaporation.
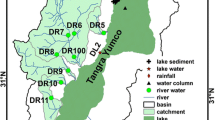
The major tributaries to the lake include the Angqu, Zhangaiqu, Gangyasangqu, Kongjinqu, and Niyaqu (Fig. 1). Most streams around the lake originate from high mountain glaciers. Streams in the south coast of Nam Co are short with steep falls, while streams in the west coast are relatively longer and flow through vast flat areas. The major supplies of these streams are glacial meltwater and precipitation. Wetlands are distributed mainly along the stream estuaries in the east and west coasts of the lake (Yuan et al. 2002).
The dominant soil type in the basin is alpine meadow soil. Most of the soils have a neutral to slightly alkaline pH (7–8) (Li et al. 2008). The basin is covered by alpine sedge mats consisting mainly of Kobresia pygmaea (Yuan et al. 2002). Geologically, the Nam Co basin is located on the Lhasa Terrain which was accreted during the Late Jurassic–Early Cretaceous, pervasively intruded by the Cretaceous–Tertiary Gangdese plutonic belt and overlain by volcanic rocks of equivalent age (Kapp et al. 2005). The southern and southeastern basins expose Cretaceous–Tertiary granitoids and orthogenises, Paleozoic to Cretaceous metasedimentary lithologies consisting of slate, phyllite, metaconglomerate, metalimestone, Cetaceous red sandstone, mudstone, and conglomerate, and Quaternary glacial, alluvial fan, fluvial, and lacustrine deposits (Kapp et al. 2005). The northern and western basins are underlain mainly by limestone, sandstone, quartz, and breccia. Some isolated broken hills stand close to the northeastern lakeshore; these hills are mainly composed of limestone, and some of the steeps are wave-eroded and developed into limestone caves. The bedrock geological setting of the Nam Co basin is shown in Fig. 1.
There are no industrial activities or urban development within the Nam Co basin, except for some small Tibetan villages. The basin is, however, a popular pasturing area. Degradation and desertification have been reported in the Pelgon County north of the lake due to natural causes and excess pasturing (Yuan et al. 2002).
2.2 Sampling and Sample Analysis
Stream, swamp, and Nam Co Lake surface water samples were collected in August and September, 2005, throughout the Nam Co Basin (Fig. 1) for the analysis of major cations and anions.
A total of 48 precipitation samples were collected on NAMOR during August 2005–July 2006. A 152-cm deep snow pit was sampled at 5 cm intervals on May 21, 2006 at the col of the Zhadang glacier (5,800 m a.s.l.), 50 km southwest of the station (Fig. 1). The major ion chemistry of the precipitation and snow pit samples have been reported and discussed by Li et al. (2007a), and here, we incorporated the data of mean ionic concentrations into this article to impel the discussion. Stream, swamp, and lake surface water samples were collected in pre-cleaned polyethylene bottles at sites shown in Fig. 1. The bottles were rinsed three times with sample water. Great caution was taken to minimize potential contamination during the sampling process.
All the samples were kept frozen and immediately transported to the laboratory of the Institute of Tibetan Plateau Research, CAS. Upon arrival in the lab, all the samples were melted at the room temperature and analyzed for major ions. Analysis of major cations (Ca2+, Mg2+, Na+, K+, and NH4 +) was carried out by ion chromatography (IC) using a Dionex ISC 2000 ion chromatograph, an IonPac CS12A column, 20 mM methanesulfonic acid (MSA) eluent, and CSRS suppresser. Major anions (Cl−, NO3 −, and SO4 2−) were analyzed by Dionex ISC 2500 ion chromatograph, using an IonPac AS11-HC column, 25 mM KOH eluent, and ASRS suppresser. The detection limits were 1 μg l−1 for all ions and analytical precision was within 5%. Analysis of field blanks showed that contamination during the sampling procedure, transportation, and treatment were not significant.
The alkalinity was calculated by the difference between the sum of all the cations and that of the anions (in equivalents). Since the pH of stream and swamp water are generally lower than 8.8, CO3 2− can be neglected. However, the pH of lake water (around 9.4) is highly alkaline (Li et al. 2008), hereby the contribution of CO3 2− in the alkalinity cannot be neglected. The concentrations of HCO3 − and CO3 2− in lake water are calculated from the total carbonate concentration based on the following equation:
Assuming the pH of all lake samples is 9.4, the HCO3 − and CO3 2− should account for ~89.5% and ~10.5% of total carbonate (Qian and Ma 2005). The concentration of the total dissolved solids (TDS) was calculated by summing up all the ions including the estimated concentration of HCO3 − and CO3 2−.
3 Results and Discussion
The dissolved ions were present in a wide range of concentrations in the five environmental matrices (Table 1). In general, the mean concentrations of most ions are in the order of lake water > swamp water > stream water > precipitation > snow.
3.1 Chemistry of the Atmospheric Precipitation
The TDS of precipitation and glacier snow in the Nam Co Basin is very low, compared with that of stream, swamp, and lake waters. The cations are dominated by Ca2+, and anions by HCO3 −. As suggested by Li et al. (2007a), major ion concentrations in precipitation were higher than those in snow pit samples, due to the relatively higher local aerosol inputs (Cong et al. 2007). The precipitation chemistry in the Nam Co basin is mainly influenced by the regional environment, dominated by crustal dust aerosols. Wind blown lake salt aerosols also affect precipitation chemistry, especially in summer. Sea salt contribution is the least due to the long distance of transport.
3.2 Stream and Swamp Water
Stream and swamp waters in the Nam Co basin showed similar major ion compositions (Table 1). On an equivalence basis, Ca2+ and Mg2+ were the dominant cations, accounting for ~86% of total cations in stream water and ~87% in swamp water; Ca2+ alone accounted for 63% and 67% of total cations in stream and swamp water, respectively. Anions were dominated by HCO3 − and SO4 2− which constituted ~98% of total anions in stream water and ~97% in swamp water; HCO3 − alone accounted for 82% and 91% of total anions in stream and swamp water, respectively. When pooling stream and swamp waters together, HCO3 − correlated significantly with Ca2+ and Mg2+ (r = 0.95 and 0.84, respectively), and a close correlation was also found between Ca2+ and Mg2+ (r = 0.81).
The weathering of rock-forming minerals, with a minor contribution from atmospheric and anthropogenic sources, is the major source of dissolved ions in most aquatic systems (Stallard and Edmond 1983). Gibbs (1970) suggested that a simple plot of TDS versus the weight ratio of Na+/(Na+ + Ca2+) could provide information on the relative importance of three major natural mechanisms controlling surface water chemistry: (1) atmospheric precipitation; (2) rock weathering, and (3) evaporation and fractional crystallization. The composition of the swamps and most streams in the Nam Co drainage basin falls in the rock-weathering domain, suggesting that their chemical compositions are mainly controlled by rock weathering (Fig. 2). A few stream water samples fall outside the boomerang region that encompasses most surface waters in the world. These stream waters are characterized by relatively low TDS content due to their glacial meltwater source.
Plot of TDS versus weight ratio of Na+/(Na+ + Ca2+) of water samples in the Nam Co basin (after Gibbs 1970)
The dominant control of rock weathering in the major ion chemistry of the streams and swamps in the Nam Co basin provides a means of probing into the weathering reactions in the basin, as weathering of different parent rocks (e.g., carbonate, silicate) yields different combinations of dissolved cations and anions in solution (Garrels and Mackenzie 1971; Stumm 1992). The high concentration of HCO3 − and its positive correlation with Ca2+ and Mg2+ as discussed above indicate carbonate weathering as a major source of HCO3 −, Ca2+, and Mg2+. Stoichiometrically, the carbonate weathering reactions demand that carbonate-derived Ca2+ and Mg2+ should be equal to the carbonate-derived HCO3 −. A plot of (Ca2+ + Mg2+) versus HCO3 − (Fig. 3a) shows that in most stream and swamp samples, (Ca2+ + Mg2+) is close to HCO3 − with an average equivalent ratio of 1.08. The average equivalent ratios of Ca2+/HCO3 − (0.8) and (Ca2+ + Mg2+)/(HCO3 − + SO4 2−) (0.9) are also close to 1, indicating these ions likely resulted from corresponding carbonates (e.g., calcite and dolomite) and sulfates (e.g., gypsum). Na+ and K+ are most likely derived from sulfate minerals and silicates. The (Ca2+ + Mg2+) versus total cations (Fig. 3b) show that most of the points are close to the equiline, i.e., 1:1 with an average ratio of 0.85. The relatively high contribution of (Ca2+ + Mg2+) to the total cations and the very low contribution of (Na+ + K+) to the total cations (Fig. 3c) suggest that carbonate weathering is the major source of the ions, while the contribution from silicate weathering is minor (Pandey et al. 1999; Sarin et al. 1989). To study the relative importance of two major proton-producing reactions—carbonation and sulfide oxidation, the equivalent ratio of (HCO3 −/HCO3 − + SO4 2−), called the C-ratio (Brown et al. 1996), is calculated. Generally, a C-ratio of 1.0 would signify carbonic acid weathering involving pure dissolution and acid hydrolysis, consuming protons come from atmospheric CO2. Conversely, a ratio of 0.5 would suggest coupled reactions involving the weathering of carbonates by protons derived from sulfide oxidation. For the stream and swamp waters in the Nam Co basin, the C-ratio is always higher than 0.6, signifying that carbonic acid weathering is the major proton producer.
The dominant role of carbonate weathering in controlling chemical composition of inflow waters is further supported by the wide distribution of carbonate rocks in the Nam Co basin (Fig. 1).
3.3 Lake Water
The major ion composition of the Nam Co lake water is characterized by HCO3 − and SO4 2− as the dominant anions, and Na+ and Mg2+ as the dominant cations; Ca2+ was extremely low compared with other cations. On an equivalence basis, Na+ accounted for ~62% of total cations and HCO3 − accounted for ~65% of total anions. Furthermore, low standard deviations of major ion concentrations indicate the lake surface water was mixed well. The result is in general agreement with the previous survey conducted by Zhao et al. (2003), which reported that all the soil samples around the lakeshore were carbonate-rich sandstone and conglomeration.
Dissolved salts in the lake water can be derived from a variety of physical, chemical, and biological processes in the drainage basin. Three sources have been named by Berner and Berner (1996) as the possible causes of dissolved salts into the lake waters, which include (1) sea salts carried by the atmospheric transport and deposited in lakes, (2) weathering of silicate, carbonate, evaporite, and sulfide minerals in the drainage basin, and (3) anthropogenic input. As discussed earlier, the contribution of sea salts to ion composition of precipitation is of least importance due to the long distance transport. This, together with the very low annual precipitation rate (300–410 mm), suggests that the sea salts play a negligible role in the ion composition of the Nam Co lake water. Anthropogenic impact is also minimal, due to the sparse population and almost non-existent industrial activities in the area. Additionally, airborne substances and underground water can also contribute to the ionic load in the lake. Although no data is available, we suspect their contributions are minor. The geochemical evolution in evaporative lakes without river outlets is primarily controlled by inflow composition, selective removal processes of dissolved species, and concentration processes in the lake basin (Yan et al. 2002). Inflow streams around Nam Co are the major supply of the lake water and thus contribute most of the ionic load of the lake water. Compared with the stream and swamp waters in the basin, the Nam Co lake water is characterized by the enrichment of Na+ and depleted in Ca2+. The enrichment of Na+ in lake water has been observed in many evaporative lakes in Tibetan Plateau (e.g., Wang and Dou 1998; Yang et al. 2003). The mean annual evaporation in Nam Co basin is approximately 790 mm (Zhu et al. 2004), which is twice the mean annual precipitation. In addition, the water balance of Nam Co in the last 15 years was negative as estimated by the records of lake sediment (Mügler et al. 2008). Salinity in Nam Co lake water has been increasing in the last 200 years and has experienced an intensive increase since the middle of 1960s, as inferred from the increasing trend of the lake water conductivity, which is reconstructed based on its relationship with the diatom in the lake sediments. This is mainly due to the significant evaporation resulting in the negative water balance (Wang et al. 2006). Therefore, we conclude that the enrichment of Na+ is mainly due to the evapoconcentration. The depletion of Ca2+ is another notable characteristic of lake water, most likely due to the selective removal processes operating in the lake (e.g., Banks et al. 2004). Since Nam Co lake water has reached a high pH and salinity, the mineral precipitation (such as bio-induced Ca-carbonate) would be the most important selective removal process (Yan et al. 2002). This is supported by the study carried out by Li et al. (2008), in which carbonates are the major components of the lacustrine sediments from Nam Co. Additionally, the lake sediment is enriched of monohydrocalcite, which is linked to an alkaline environment and the presence of bacteria, algae, diatoms, and ostracodas (Li et al. 2008). The calculation of saturation indices (1–1.2) of calcite also signify that lake waters are significantly supersaturated with respect to calcium carbonate, indicating that the HCO3 − concentrations are high enough to facilitate carbonate mineral precipitation. Thus, the depletion of Ca2+ in the lake water is likely due to its removal by carbonate precipitation and biological activities.
Table 2 compares major ion concentrations between Nam Co and other lakes over the Tibetan Plateau. Lake Duoqing Co, Pumoyong Co, Chen Co, Kongmo Co, and Yanzho are located in the southern Tibetan Plateau, Lake 1/2, Buji Co, Hang Co, Cuoe, Cuomorong, and Seling Co are close to Nam Co, while Lake Qinghai, Qingshuihu, Kusaihu, and Tuosuhai lie in the northern part of the Tibetan Plateau. TDS in the Nam Co water is low–intermediate among all the lakes. Generally, lakes with higher TDS have Na+ and Cl− as the dominant ions, while Ca2+ and SO4 2− are the major ions in lakes with lower TDS. Ion composition patterns vary with TDS, while TDS tends to increase with the latitude. Southern lakes contain mostly fresh water with lower TDS, while northern lakes tend to be alkaline with higher TDS. It has been recognized (CAS 1982) that on large scale, there is a climatic pattern transition from warm-wet to cold-dry from the south to the north over the Tibetan Plateau, mainly resulting from the precipitation gradient induced by the decreasing influence of the Indian summer monsoon. Thus, lakes exhibit transitions in the order of fresh water lakes–saline lakes–highly saline lakes (CAS 1984). Nam Co is geographically located in the transition area between non-saline lakes and highly saline lakes, and accordingly displays intermediate TDS. Nevertheless, local climatic conditions and environment within basins play a significant role on geochemistry of lake water. TDS in Nam Co is relatively low compared with the nearby lakes. This is possibly due to the abundant inflow supplies from glacial meltwater (CAS 1984) and relatively high annual precipitation.
3.4 Ions Evolution in the Nam Co Lake Basin
Ternary diagrams of cations and anions (Fig. 4) were plotted to evaluate the evolution of solutes from the glacier snow to streams and to lake water in the Nam Co basin.
As shown in Fig. 4, Ca2+ is abundant in the glacier snow, due to the dry deposition of dust aerosols on the glacier surface, especially during winter and spring (Li et al. 2007a). Meanwhile, calcium is the major element in the aerosols collected in the glacier area in the Nam Co basin even during summer season as suggested by Li et al. (2007b). The relative abundance of TDS experienced a sharp increase during its evolution from the glacier snow to the stream water (Table 1), due to the release of solutes from the stream beds. This is understandable because the relative ionic abundance increases rapidly during its evolution from glacier snow to proglacial water (e.g., Fortner et al. 2005). Besides, streams were mostly sampled in the lower reaches and downstream variations of ionic load generally display an increase trend (e.g., Singh and Hasnain 2002). The runoff is rich in Ca2+ and Mg2+ before it empties into the lake where their concentrations decrease due to the chemical precipitation in the lake. On the contrary, Na+ and K+ are relatively low in the stream water but are the dominant cations in the lake water due to the evapoconcentration and the Ca2+ removal. The ternary diagram of anions, however, shows relatively little difference in various waters due to the predominance of HCO3 − in all the waters. The relative abundance of HCO3 − increases when the meltwater flows through the bedrocks, resulting from the rock weathering as discussed earlier. The relative abundance of SO4 2− and Cl− increases during the evolution of stream to lake water, signifying the salinity trend of lake water.
4 Conclusions
This study represents the first effort in establishing a database for the major ion concentrations of the Nam Co lake and its sources. The mean concentration of almost all the major ions in the various environmental matrices is of the order lake water > swamp water > stream water > precipitation > glacier snow. The major ion geochemistry of various water matrices are summarized in Table 3. The geochemistry of the atmospheric precipitation in the Nam Co area is predominantly influenced by the regional crustal aerosols. Chemical compositions of stream and swamp waters are controlled by rock weathering particularly by the weathering of carbonates which is abundant in the basin. The lake water is characterized by enrichment of Na+ and depletion in Ca2+, due to the evapoconcentration effect and Ca2+ removal by chemical precipitation and biological activities.
References
Banks D, Parnachev VP, Frengstad B, Holden W, Karnachuk OV, Vedernikov AA (2004) The evolution of alkaline, saline ground- and surface waters in the southern Siberian steppes. Appl Geochem 19(12):1905–1926. doi:10.1016/j.apgeochem.2004.05.009
Berner EK, Berner RA (1996) Global environment: water, air, and geochemical cycles. Prentice Hall, New Jersey
Brown GH, Sharp M, Tranter M (1996) Subglacial chemical erosion: seasonal variations in solute provenance, Haut Glacier d’Arolla, Valais, Switzerland. In: Collins D (ed) Annals of glaciology, vol 22. Proceedings of the international symposium on glacial erosion and sedimentation. Annals of glaciology, pp 25–31
CAS (1982) Physical geography of Xizang (Tibet). Science Press, Beijing
CAS (1984) Rivers and Lakes of Xizang(Tibet). Science Press, Beijing
Chen JS, Wang FY, Xia XH, Zhang LT (2002) Major element chemistry of the Changjiang (Yangtze River). Chem Geol 187(3–4):231–255. doi:10.1016/S0009-2541(02)00032-3
Chen JS, Wang FY, Meybeck M, He DW, Xia XH, Zhang LT (2005) Spatial and temporal analysis of water chemistry records (1958-2000) in the Huanghe (Yellow River) basin. Glob Biogeochem Cycles 19(3). doi:10.1029/2004GB002325
Cong ZY, Kang SC, Liu XD, Wang GF (2007) Elemental composition of aerosol in the Nam Co region, Tibetan Plateau, during summer monsoon season. Atmos Environ 41(6):1180–1187. doi:10.1016/j.atmosenv.2006.09.046
Fortner SK, Tranter M, Fountain A, Lyons WB, Welch KA (2005) The geochemistry of supraglacial streams of Canada Glacier, Taylor Valley (Antarctica), and their evolution into proglacial waters. Aquat Geochem 11(4):391–412. doi:10.1007/s10498-004-7373-2
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. Norton, New York
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. doi:10.1126/science.170.3962.1088
Ju JT, Zhu LP, Wang Y, Xie MP, Peng P, Zhen XL Wang JB (in press) Preliminary research on composition, spatial distribution, and environmental significance of water ions in Pumayum Co lake and its catchment, South Tibet. J Geogr Sci
Kapp JLD, Harrison TM, Kapp P, Grove M, Lovera OM, Lin D (2005) Nyainqentanglha Shan: a window into the tectonic, thermal, and geochemical evolution of the Lhasa block, southern Tibet. J Geophys Res Solid Earth 110(B8). doi:10.1029/2004JB003330
Kutzbach JE, Guetter PJ, Ruddiman WF, Prell WL (1989) Sensitivity of climate to late Cenozoic uplift in southern Asia and the American West: numerical experiments. J Geophys Res 94(D15):18393–18407. doi:10.1029/JD094iD15p18393
Li C, Kang SC, Zhang QG, Kaspari S (2007a) Major ionic composition of precipitation in the Nam Co region, Central Tibetan Plateau. Atmos Res 85(3–4):351–360. doi:10.1016/j.atmosres.2007.02.006
Li CL, Kang S, Cong ZY (2007b) Elemental composition of aerosols collected in the glacier area on Mt. Nyainqêntanglha, Tibetan Plateau, during summer monsoon season. Chin Sci Bull 52(24):3436–3442. doi:10.1007/s11434-007-0445-0
Li CL, Kang SC, Wang XP, Ajmone-Marsan F, Zhang QG (2008) Heavy metals and rare earth elements (REEs) in soil from the Nam Co Basin, Tibetan Plateau. Environ Geol 53:1433–1440. doi:10.1007/s00254-007-0752-4
Li M, Kang S, Zhu L, You Q, Zhang Q, Wang J (2008) Mineralogy and geochemistry of the Holocene Lacustrine sediments in Nam Co, Tibet. Quaternary Int 187(1):105–116. doi:10.1016/j.quaint.2007.12.008
LIZIG (1979) Qinghai Lake monograph of the 1961 expedition. Science Press, Beijing
Molnar P, England P, Martinod J (1993) Mantle dynamics, uplift of the Tibetan plateau, and the Indian monsoon. Rev Geophys 31(4):357–396. doi:10.1029/93RG02030
Mügler I, Sachse D, Werner M, Xu B, Wu G, Yao T, Gleixner G (2008) Effect of lake evaporation on δD values of lacustrine n-alkanes: a comparison of Nam Co (Tibetan Plateau) and Holzmaar (Germany). Org Geochem 39(6):711–729. doi:10.1016/j.orggeochem.2008.02.008
Pandey SK, Singh AK, Hasnain SI (1999) Weathering and geochemical processes controlling solute acquisition in Ganga headwater-Bhagirathi river, Garhwal Himalaya, India. Aquat Geochem 5(4):357–379. doi:10.1023/A:1009698016548
Qian H, Ma ZY (2005) Hydrogeology and geochemistry. Geological Press, Beijing
Rowley DB, Currie BS (2006) Palaeo-altimetry of the late Eocene to Miocene Lunpola basin, central Tibet. Nature 439(7077):677–681. doi:10.1038/nature04506
Sarin MM, Krishnaswami S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering processes and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009. doi:10.1016/0016-7037(89)90205-6
Shao ZG, Meng XG, Zhu DG, Wang J, Yang CB, Han JE et al (2004) Variation of precipitation in Nam Co, Tibet, since the late Pleistocene and its environmental response. J Geom 10(4):337–343 (in Chinese)
Singh AK, Hasnain SI (2002) Aspects of weathering and solute acquisition processes controlling chemistry of sub-Alpine proglacial streams of Garhwal Himalaya, India. Hydrol Process 16(4):835–849. doi:10.1002/hyp.367
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: the influence of the geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688. doi:10.1029/JC088iC14p09671
Stumm W (1992) Chemistry of the solid–water interface. Wiley, New York
Wang SM, Dou HS (1998) Directory of lakes in China. Science Press, Beijing
Wang R, Yang XD, Zhu LP (2006) Environmental changes of Nam Co, Xizang, during the past 200 years. Quaternary Sci 26(5):791–798 (in Chinese)
Yan JP, Hinderer M, Einsele G (2002) Geochemical evolution of closed-basin lakes: general model and application to Lakes Qinghai and Turkana. Sediment Geol 148(1–2):105–122. doi:10.1016/S0037-0738(01)00212-3
Yang XD, Kamenik C, Schmidt R, Wang SM (2003) Diatom-based conductivity and water-level inference models from eastern Tibetan (Qinghai-Xizang) Plateau lakes. J Paleolimnol 30(1):1–19. doi:10.1023/A:1024703012475
Yuan J, Gao JX, Lu XG, Chen KL (2002) Assessment of wetland resources in Lake Nam Co and Counter measures for conservation and rational use. Resour Sci 24(4):29–34 (in Chinese)
Zhao XT, Zhu DG, Yan FH, Wu ZH, Ma ZB, Mai XS (2003) Climatic change and lake-level variation of Nam Co. Xiang since the last interglacial stage. Quaternary Sci 23:41–52 (in Chinese)
Zhu DG, Meng XG, Zhao XT, Shao ZG, Yang CB, Ma ZB et al (2004) Evolution and climatic change of Nam Co of Tibet and an ancient large lake in the northern Tibetan Plateau since the late Pleistocene. Geol China 31:269–277 (in Chinese)
Acknowledgments
We thank Zhiyuan Cong and the staff of NAMOR for their assistance in field logistics. This study was supported by the National Natural Science Foundation of China (40771187), the National Basic Research program of China (2005CB422004), the “Talent Project” of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Kang, S., Wang, F. et al. Major Ion Geochemistry of Nam Co Lake and its Sources, Tibetan Plateau. Aquat Geochem 14, 321–336 (2008). https://doi.org/10.1007/s10498-008-9039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-008-9039-y