Abstract
Cancer cell death is the utmost aim in cancer therapy. Anti-cancer agents can induce apoptosis, mitotic catastrophe, senescence, or autophagy through the production of free radicals and induction of DNA damage. However, cancer cells can acquire some new properties to adapt to anti-cancer agents. An increase in the incidence of apoptosis, mitotic catastrophe, senescence, and necrosis is in favor of overcoming tumor resistance to therapy. Although an increase in the autophagy process may help the survival of cancer cells, some studies indicated that stimulation of autophagy cell death may be useful for cancer therapy. Using some low toxic agents to amplify cancer cell death is interesting for the eradication of clonogenic cancer cells. Resveratrol (a polyphenol agent) may affect various signaling pathways related to cell death. It can induce death signals and also downregulate the expression of anti-apoptotic genes. Resveratrol has also been shown to modulate autophagy and induce mitotic catastrophe and senescence in some cancer cells. This review focuses on the important targets and mechanisms for the modulation of cancer cell death by resveratrol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer therapy is among the most challenging issues in medicine [1]. Treatment of most cancers remains a lingering problem that affects patients’ quality of life [2, 3]. Moreover, some aggressive cancers may lead to death during some months to years. Records show that the incidences of cancer and death are on the increase [4]. Furthermore, there is a growing number of people with side effects from cancer therapy [5]. While studies involving the identification of new drugs are ongoing, no drug has been approved for the eradication of cancer without recurrence. Although a very high dose of chemotherapy or radiotherapy may kill all cancer cells, normal tissue toxicity remains a major challenge [6]. Oncologists should use a limited dose of radiation or chemotherapy drugs as long as patients do not show any major side effects. In this condition, cancer researchers prefer to use the combination of various therapeutic modalities such as surgery with chemotherapy or radiotherapy, or both [7, 8].
The utmost aim in the various cancer therapy modalities is the induction of cell death in cancer cells. Ionizing radiation and some chemotherapy drugs induce cell death following the induction of massive DNA damage [9, 10]. Some cancer cells are known as cancer stem cells (CSCs), which have the ability to divide and generate new cancer cells. Usually, CSCs have a low mitotic index with a higher capability for the repair of DNA damage [11]. In most cases, DNA damage causes death through apoptosis or mitotic catastrophe. However, some other mechanisms are involved in cell death following DNA damage, including autophagy, mitophagy, senescence, necrosis, necroptosis, etc. The frequency of each of the mentioned mechanisms is highly dependent on the type of cells, type of treatment modality, and type of chemotherapy drug. Furthermore, the radiation dose plays a key role in the type of cell death following radiotherapy [12,13,14].
Administration of some low toxic adjuvants is an interesting strategy to increase the efficiency ratio of cancer therapy. Some herbal-derived agents are interesting because of their dual roles, including the protection of normal tissues against chemo/radiation toxicity and sensitization of cancer cells to anti-cancer drugs. Resveratrol has been shown to protect various types of cells/tissues against radiation or chemotherapy-induced toxicity. However, it has been shown to increase DNA damage accumulation and stimulate cell death signaling pathways in malignant cells. The dual role of resveratrol makes it an interesting adjuvant to use in cancer therapy. Furthermore, knowledge of how resveratrol can amplify cancer cell death may be interesting. In the present review, we aim to explain the various mechanisms of cell death following cancer therapy and the modulatory effects of resveratrol on cell death pathways.
Interactions in tumor
The tumor includes a diverse population of cells, including cancer and non-cancer cells. Indeed, cancer cells are one of the various types of cells within the tumor. In some tumors, such as a pancreatic tumor, the tumor microenvironment (TME) includes more than 90% of non-cancer cells, while cancer cells occupy less than 10% of TME [15]. Cancer cells within a tumor are under exposure to a wide range of molecules released by other immune and non-immune cells within TME. Within a tumor, there are several types of cells. Immune system cells such as CD4+ T lymphocytes, CD8+ T lymphocytes, regulatory T cells (Tregs), natural killer (NK) cells, and also macrophages are among the most important cells. However, tumor includes some other cells such as cancer-associated fibroblasts (CAFs), bone marrow-derived stem cells, endothelial cells, fibroblasts, etc. [16]. CD4+ T lymphocytes can proliferate towards CD8+ T lymphocytes, Tregs, or NK cells depending on the released cytokines and growth factors within TME. An increase in the level of IL-2 and interferon-γ (IFN-γ) can trigger CD4+ T lymphocytes to proliferate towards NK cells. IFN-γ can also trigger proliferation towards CD8+ T lymphocytes. However, increased levels of transforming growth factor-β (TGF-β) and T helper 2 (Th2) cytokines suppress NK cells and CD8+ T lymphocytes, while this leads to the proliferation of CD4+ T lymphocytes towards Tregs [17].
NK cells and CD8+ T lymphocytes are two main anti-cancer cells within TME. For killing cancer cells, CD8+ T lymphocytes should be exposed to antigens released by cancer cells. Macrophages and dendritic cells (DCs) detect and then carry released antigens towards CD8+ T lymphocytes. The presentation of antigens to CD8+ T lymphocytes leads to polarization of them towards the activated form of CD8+ T lymphocytes, which is known as cytotoxic CD8+ T lymphocytes (CTLs). NK cells and CTLs induce death in cancer cells through some mechanisms. The release of death signals such as Fas can induce upregulation of apoptosis initiator genes in cancer cells. Furthermore, the release of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and IFN-γ has the same consequences. The role of these signals in the induction of apoptosis will be discussed [18]. Immunologic cell death mechanisms, including necrosis and necroptosis, can release damage-associated molecular patterns (DAMPs), leading to the activation of CTLs and the release of death signals. Autophagic cell death may be able to release DAMPs and cause activation of anti-tumor immunity and induction of more cell death in cancer cells [17].
Mechanisms of cell death induction in cancer cells
As earlier mentioned, induction of cell death is the utmost aim in cancer therapy. The induction of cell death in cancers is dependent on the therapeutic modality. Radiotherapy and some chemotherapy drugs induce DNA damage [19]. The induction of massive DNA damage in targeted cancer cells leads to the accumulation of unrepaired DNA breaks. When cells are unable to repair damaged sites of DNA, cell death initiator enzymes will be activated [20]. Apoptosis is a common cell death mechanism following damage to the genetic content of cells. However, depending on cell type and damage severity, cancer cells may also undergo mitotic catastrophe, senescence, autophagic cell death, or necrosis [21]. Immune system cells including NK cells and CTLs can induce apoptosis or necrosis through the release of death signals. Telomere damage may lead to senescence. These are not the only ways for cell death following therapy. Cancer cells may undergo nutrition deprivation. Autophagic cell death is a common mechanism that causes the resistance of cells during stress conditions [22, 23]. In this section, we explain the mechanisms of cell death following cancer therapy.
Apoptosis
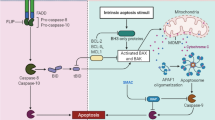
The induction of apoptosis is an important way for the immune system to kill pre-cancerous cells [24]. The interruption in the apoptosis pathways leads to the immune escape of pre-cancerous cells, which leads to the resistance of the tumor to therapy [1]. As earlier mentioned, immune cells can trigger apoptosis through the release of death signals such as FasL, and also cytokines such as TNF-α. TGF-β is a potent immunosuppressive cytokine that can induce apoptosis. However, it is also involved in resistance to therapy through the stimulation of proliferation, angiogenesis, and triggering tumor stiffness [25]. The induction of apoptosis is highly dependent on the expression of pro/anti-apoptotic genes such as p53, Bcl-2-associated X protein (Bax), and B-cell lymphoma 2 (Bcl-2) [26]. Usually, cancer cells with high expression of p53 and Bax are sensitive to radiotherapy and chemotherapy [27]. However, cancer cells with mutant p53 are more resistant to radiation/chemotherapy. After p53, mutant phosphatase and tensin homolog (PTEN) is the second frequently mutated tumor suppressor gene in cancers. Dysregulation in PTEN is associated with an abnormal upregulation of phosphoinositide 3-kinase (PI3K) [28]. PI3K is downstream of some growth factor receptors such as epidermal growth factor receptor (EGFR). An abnormal increase in the expression of PI3K is associated with the proliferation of cancer cells, resistance to apoptosis, and also the invasion of tumors [29]. Ceramide is another key regulator of apoptosis, especially following irradiation and generation of reactive oxygen species (ROS). Ceramide is a product of ROS interaction with lipid membrane [30].
Apoptosis occurs through two different pathways including extrinsic and intrinsic (mitochondrial) pathways. The initiation of apoptosis signaling within cells is dependent on a group of proteins known as caspase. The caspase proteins are involved in both the initiation and continuation of apoptosis signaling. Thus, caspases can be divided into two different classes including initiator caspases and effector caspases. The initiator caspases include caspases 8–11. However, caspases 2–7 and also 11–13 are known as effector caspases. The activated caspases for extrinsic or intrinsic pathways may be different, however, some caspases are involved in both pathways [31]. Caspase 3 (which is known as the irreversible point of apoptosis) is involved in both the mitochondrial and extrinsic pathways of apoptosis [32]. Upregulation of Fas ligand (FasL), TNF-related apoptosis-inducing ligand (TRAIL) and TNF receptor 1 (TNFR1) are associated with triggering both pathways of apoptosis [33]. However, the generation of ceramide and also damage to the mitochondria can stimulate the mitochondrial pathway of apoptosis [34, 35].
Autophagy
Autophagy occurs to eliminate damaged cells and organelles as well as unfolded proteins [36]. Autophagy is known as an important mechanism during starvation. The degradation of damaged cells is mediated by vesicles called autophagosomes. These vesicles contain enzymes for recycling cellular components for survival. The process of autophagy can be associated with resistance of cancer cells to death or induction of cell death [37]. Autophagy may cause cell death by itself or via stimulation of the apoptosis signaling pathways [38]. Due to the complicated effects of autophagy in both cell death and survival, an increase in autophagy may be associated with resistance or sensitization of cancer cells to anti-cancer drugs [39]. The autophagy process is under the control of some key proteins including the mammalian target of rapamycin (mTOR). This key protein causes phosphorylation and inactivation of autophagy protein (ATG). In some conditions such as damage to cells or starvation, inhibition of mTOR leads to upregulation of ATG and initiation of the autophagy process. It seems that the normal level of autophagy can act as a suppressor of tumorigenesis. It has been confirmed that lack of autophagic genes is associated with a high incidence of some cancers such as liver and breast malignancies [40]. However, on the other hand, an increase in autophagy during radiotherapy or chemotherapy may lead to tumor resistance [41].
Mitotic catastrophe
Mitotic catastrophe is a key mechanism for preventing genomic instability and the development of cancer [42]. Cells with wild-type p53 are able to arrest the cell cycle following exposure to genotoxic agents such as radiotherapy and chemotherapy. However, cells with mutant p53 (which is a sign of more than half of cancer cells) fail to induce cell cycle arrest at G1. Massive DNA damages following exposure to anti-cancer drugs lead to the accumulation of damaged DNA. Mitotic catastrophe is a mechanism that causes cell death with accumulated unrepaired DNA. Cell death can occur during first or later mitosis [43, 44]. Cell cycle arrest and initiation of mitotic catastrophe are highly dependent on DNA damage responses (DDRs). These responses are mediated by a wide range of regulatory genes such as ataxia telangiectasia-mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR), H2A histone family member X (H2AX), checkpoint kinases (Chk) including both Chk1 and Chk2, p53, p21, etc. [45]. Cells that undergo mitotic catastrophe may show signs of other cell death mechanisms such as apoptosis, autophagy, or senescence. Because of the important role of mitotic catastrophe in the prevention of genomic instability, an interruption of this pathway of cell death leads to an increased risk of carcinogenesis [42]. Furthermore, stimulation of mitotic catastrophe has been suggested to sensitize cancer cells to various anticancer therapy modalities such as chemotherapy and radiotherapy [45,46,47]. The mechanisms of mitotic catastrophe remain to be elucidated. Thus, targeting of mitotic catastrophe has been less studied for cancer therapy compared to apoptosis or autophagy.
Senescence
Cellular senescence occurs in normal cells after some number of mitosis. A gradual shortening of telomere plays a key role in senescence. Cancer therapy drugs and also radiotherapy induce senescence in cancer cells [48]. This type of cell death occurs following DNA damage and some mutations such as deletion of the chromosome [49]. An increase in the induction of senescence is associated with increased cell death and suppression of cancer cell proliferation. Thus, the targeting of senescence has been suggested as a strategy for inhibition of tumor growth [50, 51]. To date, some mechanisms have been suggested for the initiation and progression of senescence [52]. Some cytokines and growth factors such as IL-1, IL-6, insulin-like growth factor binding protein 7 (IGFBP7), and TGF-β are able to trigger the induction of senescence-related genes such as NADPH oxidase 2 (NOX2) and NOX4. Some other genes such as p53 and PTEN tumor-suppressive genes are key triggers for the induction of senescence [52].
Necrosis
Necrosis may be observable following some other cell death mechanisms including mitotic catastrophe and apoptosis. Necrosis occurs following severe damages in DNA and other organelles such as mitochondria. Thus, it is predictable that using high doses of clastogenic agents such as ionizing radiation and some chemotherapy drugs induces necrosis. However, induction of low levels of DNA damage may not cause necrosis [53].
Resveratrol
Resveratrol is an antioxidant and anti-inflammatory agent that can be derived from some herbs and fruits. Resveratrol is a natural phenol that has been found in more than 70 plant species. It is supposed that resveratrol is produced by herbs in response to some pathogens such as fungi and bacteria. Furthermore, it may have protective effects in herbs against ultraviolet (UV) rays. Resveratrol can be found in high concentrations in red grapes [54]. Resveratrol was first isolated from a herbal root many years ago. However, most experiments aimed at discovering the potential beneficial effects of resveratrol have been performed in the last two recent decades. Experimental studies involving resveratrol have shown its interesting antioxidant effects, cardiovascular beneficial effects as well as anti-inflammatory and anti-cancer properties [55, 56].
Nowadays, resveratrol can be found as a product of some pharmaceutical companies. However, scientists are working to develop new forms of resveratrol with higher efficiency. Unfortunately, the natural form of resveratrol (that can be found in the market) has low bioavailability. Furthermore, due to the structure of resveratrol, its absorption is very low in the intestine. To resolve these challenges, scientists have developed new carriers and covers for resveratrol [57,58,59]. Chitosan as a carrier can increase the serum level of resveratrol for a longer time. This carrier can help reduce the fast infiltration of resveratrol in the kidney, thus enhances absorption by cells. Furthermore, some carriers like chitosan may increase the stability of resveratrol against some environmental conditions such as temperature and light. Another interesting property of carriers is that resveratrol can be tagged by some special molecules for drug delivery. Some experiments have revealed interesting results for targeting cancer cells using carriers [60]. Some other carriers and covers have been used to overcome low bioavailability and absorption of resveratrol. Nanoencapsulated forms are interesting for this aim. Liposomes, micelles, solid lipid nanoparticles, and polymeric nanoparticles are interesting for increasing bioavailability and absorption [61] (Fig. 1).
Resveratrol as an enhancer of apoptosis in cancer cells
Resveratrol has shown the ability to induce apoptosis through modulation of both pro-apoptotic and anti-apoptotic genes [62]. Furthermore, resveratrol can affect both intrinsic and extrinsic pathways of apoptosis [55]. In this section, we explain the modulatory role of resveratrol on the different targets that are involved in apoptosis induction in cancer cells.
Resveratrol as an enhancer of cytokine-mediated apoptosis
It has been shown that resveratrol induces immunologic cell death (necrosis and necroptosis), which leads to the release of pro-inflammatory cytokines such as IFN-γ and a reduction of immunosuppressive cytokines including TGF-β [63]. An increase in the release of IFN-γ following treatment with resveratrol increases the number of NK cells. The combination of resveratrol with IL-2 has shown a synergic effect for activation of NK cells, which may lead to improved efficiency in tumor suppression [64]. It seems that in addition to the direct apoptosis effect of resveratrol on cancer cells, it has an indirect effect through induction of immunologic cell death and triggering immune responses from Th2 to Th1 type. This effect is similar to a late immunologic response that can be induced following some combination therapies for the induction of a durable anti-tumor immunity [22].
Resveratrol inhibits the anti-apoptotic PI3K/AKT pathway
Resveratrol can target the PI3K/AKT pathway. Treatment of human colon cancer cells with resveratrol has shown a reduction in the PI3K expression. Inhibition of PI3K/AKT/mTOR and PI3K/AKT/FOXO pathways by resveratrol has been shown to potentiate apoptosis induction in cancer [65]. The downregulation of the PI3K/AKT signaling pathway by resveratrol can also attenuate epithelial to mesenchymal transition (EMT). As EMT is a key mechanism for tumor resistance, resveratrol may potentiate apoptosis in this way [66]. It seems that an increase in the expression of sirtuin-1 (Sirt-1) plays a key role in the suppression of PI3K/AKT and blunting EMT [67]. Radiosensitization and chemosensitization effects of resveratrol have been confirmed to be related to the suppression of EMT and PI3K signaling following upregulation of Sirt-1 [9, 68].
Targeting of nuclear factor of κB (NF-κB)
Resveratrol can inhibit the expression of NF-κB at different levels. It can prevent phosphorylation and degradation of inhibitor of κB (IκB) [69]. TGF-β and some DAMPs are other important inducers of NF-κB. High mobility group box protein 1 (HMGB1) and some DAMPs such as IL-1 and oxidized DNA can trigger degradation of IκB and upregulation of NF-κB. Resveratrol can inhibit translocation of danger alarms like HMGB1 from damaged DNA, which causes the prevention of IκB degradation. Inhibition of signal transducer and activator of transcription 3 (STAT-3) can also be involved in the suppression of NF-κB by resveratrol [70]. The detailed results of NF-κB inhibition by resveratrol in cancer cells have been explained in Table 1.
Resveratrol and STAT-3
Treatment of some cancer cell lines with resveratrol has been shown to suppress the increased expression of STAT-3. Targeting of STAT-3 pathway by resveratrol led to inhibition of Bcl-2 and also cyclin D, which indicated an increase in apoptosis and reduction of proliferation [71, 72]. Similar results were observed in cells treated with both resveratrol and radiation [73].
Targeting of mitochondria by resveratrol
Some limited studies confirmed that resveratrol can trigger apoptosis via direct effects on the mitochondria. Treatment with resveratrol may stimulate oxidative phosphorylation (OXPHOS), leading to apoptosis. A high influx of calcium ions into cells following treatment with resveratrol may be involved in OXPHOS and apoptosis [74, 75].
Activation of p53 by resveratrol
Resveratrol can activate p53 through Sirt-1. Another pathway for activation of p53 by resveratrol is through domain-containing lysine methyltransferase 7/9 (SET7/9) [76]. In addition to apoptosis induction, p53 activation by resveratrol leads to stimulation of p21, which causes cell cycle arrest at the G1 phase. This effect of resveratrol is a sign of suppression of cancer cell proliferation [55] (Fig. 2).
Resveratrol as a regulator of autophagy
Modulation of autophagy has been suggested as a mechanism for the prevention and treatment of cancer by resveratrol [77]. It was suggested that resveratrol might change the metabolism of cancer cells to induce a condition similar to nutrient deprivation, thus increases autophagic cell death [78]. As mentioned earlier, mTOR plays a central role in the regulation of autophagy. Treatment of non-small cell lung cancer cells (A549) with resveratrol has been shown to cause cancer cell death through the mTOR pathway. This study showed that an increase in autophagy is responsible for cancer cell death, but not apoptosis. The induction of cell death was dependent on autophagic genes such as autophagy-related 5 (ATG5) and Beclin-1. The upregulation of these genes was observed when resveratrol caused an increase in the level of calcium ions within A549 cancer cells [79]. Increased phosphorylation of AMP-activated protein kinase (AMPK) and a reduction in the level of mTOR phosphorylation was suggested as pathways for autophagic cell death in cisplatin-resistant human oral cancer cells [80].
Some studies showed that resveratrol is able to induce both autophagy and apoptosis cell death mechanisms. Furthermore, some studies suggested that the initiation of apoptosis in the treated cancer cells is highly dependent on the autophagy process. Treatment of HT-29 and COLO 201 human colon cancer cells with resveratrol showed an apoptosis-autophagy relation. The incubation of these cells with resveratrol led to a remarkable increase in autophagy and its related genes. The induction of autophagy led to an increase in the induction of apoptosis, while inhibition of autophagy caused an absence of apoptosis. The results also showed that an increase in the level of ROS following treatment with resveratrol was responsible for initiating the autophagy process. These effects of resveratrol were dependent on both the time of incubation and the concentration of resveratrol [81].
A study showed that treatment of different types of cell lines with resveratrol led to induction of both autophagy and apoptosis, however, the percentage of each of these types of cell death was highly dependent on cell type [82]. In contrast to a previous study on colon cancer cells [81], induction of autophagy for several cervical cancer cell lines was not dependent on resveratrol concentration [82]. The relationship between the autophagy pathway and apoptosis has also been revealed for ovarian cancer cells. Targeting some autophagy mediators such as ATG5 was also shown to attenuate the induction of apoptosis [83]. Contrasting results were observed for another human ovarian cancer cell line, SKOV3. Treatment of this cell line showed an increase in autophagy, leading to the inhibition of apoptosis. The blockade of autophagy led to an increase in apoptosis [84].
An interesting strategy for cancer therapy is targeting mTOR. Rapamycin is the most common drug for targeting mTOR in cancers with the highly expressed mTOR. Mutation in PTEN and overexpression of PI3K have key roles in the aberrant upregulation of mTOR and inhibition of the autophagy process. Increased autophagy process in some cancers such as breast cancer following treatment with rapamycin is associated with tumor resistance to therapy and a higher probability of tumor recurrence. Furthermore, targeting of mTOR alone, led to overexpression of the AKT/mTOR signaling pathway. Therefore, a combination therapy for targeting autophagy and anti-apoptotic pathways can lead to improved efficiency of tumor response to therapy [85]. Resveratrol has been shown to suppress the activation of AKT following targeting of mTOR by rapamycin. This combination can augment the response of breast cancer cells to rapamycin [86]. This combination has also been shown to suppress the activation of autophagy and stimulates the induction of apoptosis [87] (Fig. 3).
Resveratrol as a regulator of mitotic catastrophe
Resveratrol has shown the ability to affect both DDRs and cell cycle progression in cancer cells, thus potentiates mitotic catastrophe. This regulatory effect of resveratrol has been confirmed for glioma cells. Indeed, glioma cells are able to resist the genotoxic effects of temozolomide following cell cycle arrest in G2. Treatment of glioma cells with resveratrol led to an increase in the phosphorylation of H2AX, ATM, and Chk2, however, it abrogated G2 cell cycle arrest. Accumulation of cancer cells in G2 is a mechanism for resistance to genotoxic agents because it provides more time for the repair of DNA damage. The abrogation of G2 cell cycle arrest in glioma cells may be a key mechanism for the induction of mitotic catastrophe [88].
A study by Young et al. suggested that resveratrol may induce different cell death mechanisms at different times. They treated WR-21 cells (murine salivary cancer cells consisting of a mutated c-Ha-ras gene) with resveratrol and then evaluated cell death markers including both apoptosis and mitotic catastrophe. Interestingly, results showed that resveratrol causes a high expression of p53 and retinoblastoma (Rb) a few hours following treatment of cells. However, for later times, the expression of these genes returned near to baseline, while the appearance of mitotic catastrophe including the collapse of DNA and changes in the spindles were observed. These changes may indicate that although resveratrol induces apoptosis during the first few hours, it is able to trigger mitotic catastrophe in cancer cells that escaped apoptosis. This study has also shown a time-dependent property for the type of cell death that can be observed following treatment with resveratrol [89]. The resveratrol analogue trimethoxystilbene has also shown the ability to inhibit the proliferation of HeLa cancer cells through induction of mitotic catastrophe [90]. The induction of mitotic catastrophe in cancer cells following administration of resveratrol needs elucidation, especially using in vivo studies. As mitotic catastrophe may occur during some mitosis after DNA damage, the evaluation of time-dependence for induction of mitotic catastrophe is critical. As mitotic catastrophe is not an immunogenic cell death mechanism, it may be useful as a target for cancer therapy without induction of adaptive response by cancer cells. The induction of apoptosis alone may lead to the suppression of anti-tumor immunity following the release of immunosuppressive cytokines such as TGF-β [23, 25, 91]. The stimulation of mitotic catastrophe using some agents such as resveratrol may be useful for overcoming tumor resistance.
Resveratrol and senescence
As earlier explained, resveratrol is able to activate tumor suppressor genes including p53 and PTEN. However, it is able to blunt other mentioned stimulators of senescence such as TGF-β. Resveratrol has been shown to induce senescence in breast cancer cells through activation of the p53–p21 pathway. Activation of this p53/p21 and also p16/Rb pathways by resveratrol is associated with long-term cell cycle arrest and senescence in cancer cells [92]. Resveratrol-induced radiation-sensitive 9 (Rad9) gene expression is a mechanism that has been suggested as another mediator for senescence. Generation of ROS and induction of DNA damage are responsible for the upregulation of Rad9. Inhibition of Rad9 has been shown to reduce the induction of senescence following treatment with resveratrol. This may indicate that resveratrol triggers senescence through induction of reduction/oxidation, thereby inducing ROS overproduction in cancer cells that lead to DNA damage and cell death [93]. Increased ROS production by the mitochondria has been suggested as a mechanism for induction of senescence by resveratrol. The increase in ROS leads to upregulation of deleted in liver cancer 1 (DLC1), a tumor suppressor gene in the breast and lung cancer cells. It seems that DLC1 has a role in Sirt-1 upregulation by resveratrol, which can trigger cancer cell's apoptosis and senescence [94]. Upregulation of p53 following ROS overproduction is also involved in resveratrol-induced cancer cell senescence. Resveratrol is also able to induce the generation of ROS by NADPH oxidase enzymes. Upregulations of NOX1-3 and NOX5 have been confirmed following treatment of cancer cells with resveratrol [95]. However, the expression of NOXs is highly dependent on cell type and they may cause resistance of cancer cells to apoptosis [7].
The induction of senescence in cancer cells can be amplified using combination therapy modalities. Radiotherapy, which is the most potent clastogenic modality, can induce senescence following massive DNA damage in cancer cells. Treatment of PC-3 prostate cancer cells with resveratrol before irradiation showed that resveratrol can enhance the therapeutic efficiency of radiotherapy. Results indicated that the combination of radiotherapy and resveratrol can inhibit the proliferation of prostate cancer cells more effectively compared to irradiation alone. Furthermore, a dose-dependent response was observed for treatment with resveratrol. Irradiation or treatment with resveratrol alone had little effect on the activity of p53 and p21. However, the combined form of treatment led to a significant increase in the expression of both proteins. Increased expressions of p53 and p21 were associated with a significant reduction of proliferative enzymes, including cyclin D, cyclin B, and cyclin-dependent kinase 2 (CDK2). Results of this study showed that treatment with the combination of radiotherapy and resveratrol caused an increase in DNA damage and cell cycle arrest, leading to activation of both apoptosis and senescence in prostate PC-3 cancer cells [96] (Fig. 4).
Modulation of senescence in cancer cells by resveratrol. Resveratrol is able to induce senescence through activation of p53 and p21. Furthermore, stimulation of endogenous ROS production by mitochondria has a key role in the induction of senescence. As shown in this figure, senescence may occur with some other cell death mechanisms such as apoptosis and mitotic catastrophe
Resveratrol and necrosis
A study suggested that resveratrol cannot cause necrosis in glioma cancer cells [97]. However, some studies have revealed that resveratrol is able to induce necrosis in some cancer cells such as colon, breast, and prostate cancer cell lines [98,99,100,101]. Furthermore, a combination of resveratrol with radiation or hyperthermia has been shown to induce both necrosis and apoptosis in MCF-7 breast cancer cells [62] (Table 1).
Conclusion
In this review, we explained the cellular and molecular mechanisms involved in cell death induction in cancer by resveratrol. Resveratrol is a low toxic natural agent that has shown anti-cancer properties in a wide range of cancer types. However, it is important to achieve the best concentration of resveratrol for each cancer. There are still some concerns related to the clinical use of resveratrol. Low bioavailability and absorption in the intestine are properties of natural forms of resveratrol. However, new technologies, including nanotechnology can help improve the absorption and bioavailability of resveratrol. The main mechanism of anti-cancer drugs and also radiotherapy is the induction of cell death in cancer cells without remarkable side effects in normal tissues. Resveratrol has interesting properties that protect normal tissues/cells against the toxic effects of cancer therapy while sensitizing most cancer cells to radiation or chemotherapy. Resveratrol is able to activate p53 and p21, leading to cell cycle arrest and senescence. Furthermore, activation of p53 and PTEN as well as downregulation of PI3K can sensitize cancer cells to apoptosis. An important anti-cancer effect of resveratrol is the induction of endogenous ROS production in cancer cells. This effect of resveratrol could be mediated through some changes in the mitochondria membrane. Increased ROS generation by the mitochondria may lead to DNA damage and also some damages to other organelles, leading to apoptosis, mitotic catastrophe, autophagy, senescence, and possibly necrosis. Induction of each type of these cell death mechanisms is dependent on the upregulation or downregulation of some genes involved in cell death pathways such as p53, p21, Fas, PTEN, PI3K, Bcl-2, mTOR, etc. Activation of anti-tumor immune cells also has also a key role in the anti-tumor effect of resveratrol. An increase in the activity of NK cells and CD8+ T lymphocytes can cause the release of TNF-α, leading to induction of apoptosis. The effects of resveratrol on some cell death mechanisms such as mitotic catastrophe and necrosis need elucidation in future studies.
References
Goradel NH, Mohajel N, Malekshahi ZV et al (2019) Oncolytic adenovirus: a tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol 234:8636–8646
Haddadi Gh RA, Ma M-S, Hosseinzadeh M, Fardid R, Najafi M et al (2017) Hesperidin as radioprotector against radiation-induced lung damage in rat: a histopathological study. J Med Phys 42:25–32
Rezaeyan AFR, Haddadi Gh, Ma T, Hosseinzadeh M, Najafi M et al (2016) Evaluating radioprotective effect of hesperidin on acute radiation damage in the lung tissue of rats. J Biomed Phys Eng 6:165–174
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
O’flanagan CH, Smith LA, Mcdonell SB et al (2017) When less may be more: calorie restriction and response to cancer therapy. BMC Med 15:1–9
Azmoonfar R, Amini P, Yahyapour R et al (2020) Mitigation of radiation-induced pneumonitis and lung fibrosis using alpha-lipoic acid and resveratrol. Anti-inflamm Anti-allergy Agents Med Chem 19:149–157
Mortezaee K, Goradel NH, Amini P et al (2019) NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr Mol Pharmacol 12:50–60
Van Der Valk M, Van Etten B, Marijnen C et al (2020) Compliance, acute toxicity and postoperative complications of short-course radiotherapy followed by chemotherapy and surgery for high-risk rectal cancer. Results of the randomized RAPIDO-trial. Eur J Surg Oncol 46:e20
Ji K, Sun X, Liu Y et al (2018) Regulation of apoptosis and radiation sensitization in lung cancer cells via the Sirt1/NF-κB/Smac pathway. Cell Physiol Biochem 48:304–316
Lhuillier C, Rudqvist N-P, Elemento O et al (2019) Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med 11:40
Schulz A, Meyer F, Dubrovska A et al (2019) Cancer stem cells and radioresistance: DNA repair and beyond. Cancers 11:862
Ashrafizadeh M, Farhood B, Musa AE et al (2020) The interactions and communications in tumor resistance to radiotherapy: therapy perspectives. Int Immunopharmacol 87:106807
Farhood B, Aliasgharzadeh A, Amini P et al (2019) Radiation-induced dual oxidase upregulation in rat heart tissues: protective effect of melatonin. Medicina (Kaunas, Lithuania) 55:317
Yahyapour R, Shabeeb D, Cheki M et al (2018) Radiation protection and mitigation by natural antioxidants and flavonoids: implications to radiotherapy and radiation disasters. Curr Mol Pharmacol 11:285–304
Mortezaee K (2020) Immune escape: a critical hallmark in solid tumors. Life Sci 258:118110
Najafi M, Mortezaee K, Majidpoor J (2019) Stromal reprogramming: a target for tumor therapy. Life Sci 239:117049
Ashrafizadeh M, Farhood B, Musa AE et al (2020) Damage-associated molecular patterns in tumor radiotherapy. Int Immunopharmacol 86:106761
Wu J, Lanier LL (2003) Natural killer cells and cancer. Adv Cancer Res 90:127–156
Amini P, Ashrafizadeh M, Motevaseli E et al (2020) Mitigation of radiation-induced hematopoietic system injury by melatonin. Environ Toxicol 35:815–821
Nodooshan SJ, Amini P, Ashrafizadeh M et al (2020) Suberosin attenuates the proliferation of MCF-7 breast cancer cells in combination with radiotherapy or hyperthermi. Curr Drug Res Rev. https://doi.org/10.2174/2589977512666201228104528
Farhood B, Ashrafizadeh M, Hoseini-Ghahfarokhi M et al (2020) Targeting of cellular redox metabolism for mitigation of radiation injury. Life Sci 250:117570
Ashrafizadeh M, Farhood B, Musa AE et al (2020) Abscopal effect in radioimmunotherapy. Int Immunopharmacol 85:106663
Mortezaee K, Parwaie W, Motevaseli E et al (2019) Targets for improving tumor response to radiotherapy. Int Immunopharmacol 76:105847
Mortezaee K, Najafi M, Farhood B et al (2019) Genomic instability and carcinogenesis of heavy charged particles radiation: clinical and environmental implications. Medicina (Kaunas) 55:591
Farhood B, Hoseini-Ghahfarokhi M, Motevaseli E et al (2020) TGF-β in radiotherapy: mechanisms of tumor resistance and normal tissues injury. Pharmacol Res 155:104745
Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P et al (2020) Targets for protection and mitigation of radiation injury. Cell Mol Life Sci 77:3129–3159
Mortezaee K, Narmani A, Salehi M et al (2021) Synergic effects of nanoparticles-mediated hyperthermia in radiotherapy/chemotherapy of cancer. Life Sci 269:119020
Ashrafizadeh M, Zarrabi A, Samarghandian S et al (2020) PTEN: what we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol 881:173226
Ashrafizadeh M, Najafi M, Ang HL et al (2020) PTEN, a barrier for proliferation and metastasis of gastric cancer cells: from molecular pathways to targeting and regulation. Biomedicines 8:264
Presa N, Gomez-Larrauri A, Dominguez-Herrera A et al (2020) Novel signaling aspects of ceramide 1-phosphate. Biochim Biophys Acta (BBA) 1865:158630
Larsen BD, Sørensen CS (2017) The caspase-activated DN ase: apoptosis and beyond. FEBS J 284:1160–1170
D’arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Kretz A-L, Von Karstedt S, Hillenbrand A et al (2018) Should we keep walking along the trail for pancreatic cancer treatment? Revisiting TNF-related apoptosis-inducing ligand for anticancer therapy. Cancers 10:77
Cao K, Tait SW (2018) Apoptosis and cancer: force awakens, phantom menace, or both? Int Rev Cell Mol Biol 337:135–152
Jardim FR, De Rossi FT, Nascimento MX et al (2018) Resveratrol and brain mitochondria: a review. Mol Neurobiol 55:2085–2101
Galati S, Boni C, Gerra MC et al (2019) Autophagy: a player in response to oxidative stress and DNA damage. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5692958
Miller DR, Thorburn A (2021) Autophagy and organelle homeostasis in cancer. Dev Cell 56:906–918
Maiuri MC, Zalckvar E, Kimchi A et al (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Smith AG, Macleod KF (2019) Autophagy, cancer stem cells and drug resistance. J Pathol 247:708–718
Yun CW, Lee SH (2018) The roles of autophagy in cancer. Int J Mol Sci 19:3466
Ashrafizadeh M, Zarrabi A, Orouei S et al (2021) MicroRNA-mediated autophagy regulation in cancer therapy: the role in chemoresistance/chemosensitivity. Eur J Pharmacol 892:173660
Vitale I, Galluzzi L, Castedo M et al (2011) Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 12:385–392
Adjemian S, Oltean T, Martens S et al (2020) Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis 11:1–15
Prokhorova EA, Egorshina AY, Zhivotovsky B et al (2020) The DNA-damage response and nuclear events as regulators of nonapoptotic forms of cell death. Oncogene 39:1–16
Portugal J, Mansilla S, Bataller M (2010) Mechanisms of drug-induced mitotic catastrophe in cancer cells. Curr Pharm Des 16:69–78
Kobayashi D, Oike T, Shibata A et al (2017) Mitotic catastrophe is a putative mechanism underlying the weak correlation between sensitivity to carbon ions and cisplatin. Sci Rep 7:1–8
Mc Gee MM (2015) Targeting the mitotic catastrophe signaling pathway in cancer. Mediat Inflamm 2015:146282
Gewirtz DA, Holt SE, Elmore LW (2008) Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol 76:947–957
Wunderlich R, Ruehle P-F, Deloch L et al (2017) Interconnection between DNA damage, senescence, inflammation, and cancer. Front Biosci 22:348–369
Campisi J, Kim S-H, Lim C-S et al (2001) Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol 36:1619–1637
Nardella C, Clohessy JG, Alimonti A et al (2011) Pro-senescence therapy for cancer treatment. Nat Rev Cancer 11:503–511
Acosta JC, Gil J (2012) Senescence: a new weapon for cancer therapy. Trends Cell Biol 22:211–219
Amaravadi RK, Thompson CB (2007) The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res 13:7271–7279
Salehi B, Mishra AP, Nigam M et al (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6:91
Ashrafizadeh M, Taeb S, Haghi-Aminjan H et al (2020) Resveratrol as an enhancer of apoptosis in cancer: a mechanistic review. Anti-cancer Agents Med Chem. https://doi.org/10.2174/1871520620666201020160348
Mortezaee K, Najafi M, Farhood B et al (2020) Resveratrol as an adjuvant for normal tissues protection and tumor sensitization. Curr Cancer Drug Targets 20:130–145
Ahmadi Z, Mohammadinejad R, Ashrafizadeh M (2019) Drug delivery systems for resveratrol, a non-flavonoid polyphenol: emerging evidence in last decades. J Drug Deliv Sci Technol 51:591–604
Ashrafizadeh M, Ahmadi Z, Farkhondeh T et al (2020) Resveratrol targeting the Wnt signaling pathway: a focus on therapeutic activities. J Cell Physiol 235:4135–4145
Ashrafizadeh M, Javanmardi S, Moradi-Ozarlou M et al (2020) Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy: an updated review on resveratrol. Biosci Rep. https://doi.org/10.1042/BSR20200257
Senthil Kumar C, Thangam R, Mary SA et al (2020) Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym 231:115682
Summerlin N, Soo E, Thakur S et al (2015) Resveratrol nanoformulations: challenges and opportunities. Int J Pharm 479:282–290
Amini P, Nodooshan SJ, Ashrafizadeh M et al (2020) Resveratrol induces apoptosis and attenuates proliferation of MCF-7 cells in combination with radiation and hyperthermia. Curr Mol Med. https://doi.org/10.2174/1566524020666200521080953
Zhang Y, Yang S, Yang Y et al (2019) Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect Agents Cancer 14:27
Chhabra G, Singh CK, Amiri D et al (2021) Recent advancements on immunomodulatory mechanisms of resveratrol in tumor microenvironment. Molecules (Basel, Switzerland) 26:1343
Chen Q, Ganapathy S, Singh KP et al (2010) Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE 5:e15288
Wang Z, Wu L, Tong S et al (2016) Resveratrol suppresses the epithelial-to-mesenchymal transition in PC-3 cells by down-regulating the PI3K/AKT signaling pathway. Anim Cells Syst 20:77–85
Chai R, Fu H, Zheng Z et al (2017) Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep 16:8037–8044
Jin X, Wei Y, Liu Y et al (2019) Resveratrol promotes sensitization to Doxorubicin by inhibiting epithelial–mesenchymal transition and modulating SIRT1/β-catenin signaling pathway in breast cancer. Cancer Med 8:1246–1257
Xu L, Botchway BO, Zhang S et al (2018) Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Front Neurosci 12:690
Mortezaee K, Najafi M, Farhood B et al (2019) NF-kappaB targeting for overcoming tumor resistance and normal tissues toxicity. J Cell Physiol 234:17187–17204
Kotha A, Sekharam M, Cilenti L et al (2006) Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther 5:621
Li D, Wang G, Jin G et al (2019) Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int J Mol Med 43:630–640
Baek SH, Ko J-H, Lee H et al (2016) Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 23:566–577
Ma X, Tian X, Huang X et al (2007) Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca 2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem 302:99–109
Sareen D, Darjatmoko SR, Albert DM et al (2007) Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol 72:1466–1475
Liu Z, Wu X, Lv J et al (2019) Resveratrol induces p53 in colorectal cancer through SET7/9. Oncol Lett 17:3783–3789
Tian Y, Song W, Li D et al (2019) Resveratrol as a natural regulator of autophagy for prevention and treatment of cancer. Onco Targets Ther 12:8601–8609
Kueck A, Opipari AW, Griffith KA et al (2007) Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol Oncol 107:450–457
Zhang J, Chiu J, Zhang H et al (2013) Autophagic cell death induced by resveratrol depends on the Ca(2+)/AMPK/mTOR pathway in A549 cells. Biochem Pharmacol 86:317–328
Chang C-H, Lee C-Y, Lu C-C et al (2017) Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: a key role of AMPK and Akt/mTOR signaling. Int J Oncol 50:873–882
Miki H, Uehara N, Kimura A et al (2012) Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int J Oncol 40:1020–1028
García-Zepeda SP, García-Villa E, Díaz-Chávez J et al (2013) Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur J Cancer Prev 22:577–584
Lang F, Qin Z, Li F et al (2015) Apoptotic cell death induced by resveratrol is partially mediated by the autophagy pathway in human ovarian cancer cells. PLoS ONE 10:e0129196
Wang H, Peng Y, Wang J et al (2018) Effect of autophagy on the resveratrol-induced apoptosis of ovarian cancer SKOV3 cells. J Cell Biochem 120:7788–7793
Yu J, Parkhitko A, Henske EP (2011) Autophagy: an ‘Achilles’ heel of tumorigenesis in TSC and LAM. Autophagy 7:1400–1401
He X, Wang Y, Zhu J et al (2011) Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett 301:168–176
Alayev A, Sun Y, Snyder RB et al (2014) Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle (Georgetown, Tex.) 13:371–382
Filippi-Chiela EC, Thomé MP, Bueno e Silva MM et al (2013) Resveratrol abrogates the Temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the Temozolomide-induced senescence in glioma cells. BMC Cancer 13:147
Young LF, Martin KR (2006) Time-dependent resveratrol-mediated mRNA and protein expression associated with cell cycle in WR-21 cells containing mutated human c-Ha-Ras. Mol Nutr Food Res 50:70–77
Traversi G, Fiore M, Percario Z et al (2017) The resveratrol analogue trimethoxystilbene inhibits cancer cell growth by inducing multipolar cell mitosis. Mol Carcinog 56:1117–1126
Farhood B, Goradel NH, Mortezaee K et al (2019) Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal 13:3–16
Giménez-Bastida JA, Ávila-Gálvez MÁ, Espín JC et al (2019) Conjugated physiological resveratrol metabolites induce senescence in breast cancer cells: role of p53/p21 and p16/Rb pathways, and ABC transporters. Mol Nutr Food Res 63:1900629
Chen K-Y, Chen C-C, Chang Y-C et al (2019) Resveratrol induced premature senescence and inhibited epithelial-mesenchymal transition of cancer cells via induction of tumor suppressor Rad9. PLoS ONE 14:e0219317
Ji S, Zheng Z, Liu S et al (2018) Resveratrol promotes oxidative stress to drive DLC1 mediated cellular senescence in cancer cells. Exp Cell Res 370:292–302
Li B, Hou D, Guo H et al (2017) Resveratrol sequentially induces replication and oxidative stresses to drive p53-CXCR2 mediated cellular senescence in cancer cells. Sci Rep 7:208
Fang Y, Demarco VG, Nicholl MB (2012) Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci 103:1090–1098
Sayd S, Thirant C, El-Habr EA et al (2014) Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev Rep 10:103–113
Alobaedi OH, Talib WH, Basheti IA (2017) Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac J Trop Med 10:400–408
San Hipólito-Luengo Á, Alcaide A, Ramos-González M et al (2017) Dual effects of resveratrol on cell death and proliferation of colon cancer cells. Nutr Cancer 69:1019–1027
Scifo C, Cardile V, Russo A et al (2004) Resveratrol and propolis as necrosis or apoptosis inducers in human prostate carcinoma cells. Oncol Res 14:415–426
Scifo C, Milasi A, Guarnera A et al (2005) Resveratrol and propolis extract: an insight into the morphological and molecular changes induced in DU145 cells. Oncol Res 15:409–421
Acknowledgements
Supported by Cooperative Foundation of Shaoyang University.
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparing first draft. The scientific edition performed by MN. All authors wrote and approved the article.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human and/or animal participants
This article does not contain human or animal studies performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Li, M., Tang, C. et al. Targeting of cancer cell death mechanisms by resveratrol: a review. Apoptosis 26, 561–573 (2021). https://doi.org/10.1007/s10495-021-01689-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01689-7