Abstract
Anoikis is a programmed cell death induced upon cell detachment from extracellular matrix. Anoikis resistance is a critical mechanism in tumor metastasis. Cancer cells deregulate and adapt their metabolism to survive in the absence of adhesion, spreading metastases to distant organs. These adaptations include abnormal regulation of growth factor receptors activating prosurvival signaling pathways, such as the Ras/ERK and PI3K/Akt pathways, and extracellular matrix remodeling, leading to metastasis by an increase of invasiveness and inhibiting anoikis. This study investigates the possible involvement of ECM components and signaling pathways in the regulation of resistance to anoikis in endothelial cells (EC). Endothelial cells submitted to stressful conditions by blocking adhesion to substrate (anoikis resistance) display an up-regulation of Ras/ERK and PI3k/Akt pathways by high expression of Ras, ERK, PI3K (p110α) and Akt (Thr 308). After ERK and PI3K inhibiting, all EC-derived cell lines studied showed lower growth, a decrease in invasive potential and a higher rate of apoptosis. Furthermore, anoikis-resistant cell lines display a decrease in the expression of fibronectin, collagen IV and hyaluronic acid and an increase in the expression of laminin, perlecan, αv, β3, α5 and β1 integrins subunits, hyaluronidades 1, 2 and 3 and metalloproteinases 2 and 9. These results indicate that the acquisition of anoikis resistance induced remodeling of the extracellular matrix and overexpression of the PI3K/Akt and Ras/ERK pathway components. Acquisition of resistance to anoikis is a potentially crucial step in endothelial cell transformation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attachment of a cell to the extracellular matrix (ECM) is vital for the proliferation and survival functions to be carried out efficiently. If a cell gets detached, apoptosis may be induced. Cell death triggered by lack of attachment to the ECM is defined as anoikis [1]. Anoikis acts as an important defense for the organism by preventing re-adhesion of detached cells to new matrices in incorrect locations and their dysplastic growth [2].
Dysregulation of anoikis, such as anoikis resistance, is a critical mechanism in tumor metastasis. Cancer cells rapidly develop several mechanisms to resist anoikis, progress towards malignancy and spread metastases to distant organs [3]. First, the cancer cells acquire a migratory and invasive phenotype, which allows them to degrade the ECM, invade through the basement membrane and enter into either the circulatory or lymphatic system. Later, these cells deregulate and adapt their metabolism to survive in the absence of adhesion to the ECM (anoikis resistance) [4].
Tumors produce several molecules that facilitate their proliferation, invasion and maintenance, especially proteoglycans. The syndecan-4, a heparan sulfate proteoglycan, can act as a co-receptor of growth factors and proteins of the extracellular matrix by increasing the affinity of adhesion molecules to their specific receptors [4, 5]. Changes in the expression of syndecan-4 have been observed in tumor cells, indicating its involvement in cancer. Acquisition of anoikis resistance induced syndecan-4 up-regulation in endothelial cells [6].
In addition to the expression of syndecan-4, anoikis resistance involves unregulated expression of growth factor receptors or components of their signaling pathways that inhibit cell death pathways and active cell survival pathways [5]. Abnormal regulation of growth factor receptors activates prosurvival signaling pathways, such as the Ras/ERK, PI3K/Akt, NF-κB, and Rho-GTPase pathways, leading to metastasis by inhibiting anoikis [5, 7, 8].
The Ras/extracellular signal-regulated kinase (Ras/ERK) and phosphatidylinositol 3-kinase/Akt (PI3K/Akt) signaling pathways are the chief mechanisms for controlling cell survival, differentiation, proliferation, metabolism, and motility in response to extracellular cues. These pathways can intersect to regulate each other and co-regulate downstream functions. The extent of this cross-talk and its significance in cancer therapeutics are targets of many studies [9].
Ras/ERK pathway is activated by growth factors, polypeptide hormones, neurotransmitters, chemokines, and phorbol esters, which signal through their cognate receptor tyrosine kinases (RTKs) and G protein-coupled receptors (GPCRs), or by direct activation of protein kinase C (PKC) [9,10,11].
Growth factors also activate the lipid kinase PI3K through either direct PI3K recruitment to the growth factor receptors or indirect recruitment involving the docking proteins insulin receptor substrate (IRS) or GRB2-associated binder (GAB). PI3K generates phosphatidylinositol 3, 4, 5 triphosphate (PIP3), which recruits the protein kinase Akt to the plasma membrane where it is activated by 3-phosphoinositide-dependent kinase 1 (PDK1) and the second mTOR complex, mTORC2. Akt phosphorylates many survival, proliferation and motility factors [9, 12].
These pathways are important on the tumourigenesis, maintenance of phenotype and protection from apoptosis, and are further regulated by cell–ECM interaction. Binding of integrins and ECM molecules transmit signals from the extracellular environment to the intracellular network, as well as signals from the intracellular network to extracellular environment. Alterations in integrins expression or in ECM composition can change the transmission mediated by integrin-activated signaling molecules, such as focal adhesion kinase (FAK), phosphatidylinositol 3-kinase (PI3K), and members of the extracellular signal-regulated kinase 1 and 2/mitogen activated protein (ERK1 and 2/MAP) kinase family, also altering cell proliferation, migration, and apoptosis [3, 13, 14].
The ECM is the first barrier to developing metastasis. Conversely, extracellular molecules also contribute in a critical way to the progress of cancer cell invasion. These molecules can be: (a) part of the ECM, (b) secreted in the ECM, (c) secreted but also attached on the cell surface, (d) cell membrane proteins such as receptors [15].
ECM consists of several proteins and glycoproteins (including collagen, laminin, fibronectin, vitronectin), in addition to glycosaminoglycans (GAGs) such as hyaluronan (HA) and proteoglycans such as perlecan, with different physical and biochemical properties that regulate cell growth, survival, motility, differentiation and apoptosis by ligating specific receptors such as integrin receptors [16,17,18,19,20]. Integrins are mediators of cell–ECM and cell–cell interaction that can transduce biochemical signals both into and out of cells [21].
Matrix degradation is central to tumor development. Enzymes that degrade extracellular matrix are abundant in tumors. The most significant enzymes in ECM remodeling are metalloproteinases (MMPs) [22]. The MMP family are best known for their ability to cleave components of the ECM but they also can cleave other proteinases, proteinase inhibitors, latent growth factors, chemotactic molecules, growth factor binding proteins, cell surface receptors and cell–cell adhesion molecules [23, 24]. ECM proteoglycans and glycoproteins are also targeted by MMPs and ADAMTS, a disintegrin and metalloproteinase with thrombospondin domains. Additionally, the various GAG polysaccharide chains can be modified or removed by enzymes that specifically target them, changing ECM function. For example, hyaluronidases (HYALs), a class of enzymes that predominantly degrade HA and its production can be increased or suppressed in some malignant conditions compared with normal tissues [22, 25].
These and other results led us to investigate the possible involvement of ECM components and signaling pathways in the regulation of resistance to anoikis in endothelial cells. We now report that anoikis-resistant endothelial cells display remodeling of the extracellular matrix and overexpression of the PI3K/Akt and Ras/ERK pathways components.
Methodology
Reagents and antibodies
Rabbit polyclonal antibody fibronectin, rabbit polyclonal antibody laminin, rabbit polyclonal antibody collagen IV and goat anti-Rat IgG H&L (Alexa Fluor® 594) secondary antibody were purchased from Abcam (Cambridge, MA, USA). Rat monoclonal antibody heparan sulfate proteoglycan (perlecan) clone A7L6, rabbit monoclonal antibody Phospho-Akt (Thr 308), rabbit monoclonal antibody PI3K p110α and rabbit monoclonal antibody Phospho-ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) were acquired from Merck Millipore (Danvers, MA, USA). Mouse monoclonal antibody β-Actin, rabbit monoclonal antibody MMP2, rabbit monoclonal antibody MMP9, rabbit monoclonal antibodies integrins αv, β3, α5, β1 and HRP-linked goat anti-rat IgG secondary antibody were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Mouse monoclonal antibody HYAL-1, rabbit polyclonal antibody HYAL-2, rabbit polyclonal antibody HYAL-3, mouse monoclonal antibody GAPDH and rabbit polyclonal antibody H-Ras were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). HRP-linked goat anti-mouse IgG and HRP-linked goat anti rabbit IgG secondary antibodies were obtained from Sigma-Aldrich (St Louis, MO, USA). Goat anti-rabbit IgG (H + L) highly cross-adsorbed labeled with Hilyte Fluor 594 secondary antibody was obtained from Anaspec (Fremont, CA, USA). Specific inhibitor of PI3K, LY294002 (2-(4-Morpholinyl)-8-phenyl-4H-l-benzopyran-4) was purchased from Merck Millipore (Danvers, MA, USA).
Cell culture
Endothelial cell line derived from rabbit aorta (EC) [26], EC transfected with EJ-ras oncogene (EJ-ras EC) [27] and EC resistant to anoikis (Adh1-EC and Adh2-EC) [6] were maintained in F12 medium (Sigma-Aldrich, St. Louis, USA) supplemented with 10% fetal calf serum (FCS) (Athena—Campinas, Brazil) at 37 °C, 2.5% CO2.
RT-PCR
For RT-PCR, total RNA was isolated from the endothelial cells with the Trizol-reagent from Invitrogen (Carlsbad, USA) and quantitated by spectrophotometer. RT-PCR was prepared using TaqMan® Reverse Transcription Reagents kit (Applied Biosystems, Foster, USA), according to the manufacturer’s instructions.
Quantitative real-time PCR (qPCR)
For quantitative PCR (qPCR), 2 µg of total RNA per sample was reverse transcribed to cDNA with TaqMan® Reverse Transcription Reagents kit (Applied Biosystems, Foster, USA) and then 500 ng of cDNA was analyzed using the Maxima SYBR Green qPCR Master Mix Kit (Thermo Fisher Scientific Inc., Rockford, USA) with a ABI 7500 real-time PCR instrument (Applied Biosystems, Foster, USA), according to the manufacturer’s instruction, respectively. The primers are used as follow: Fibronectin Fwd (5′-CCTATGGAGAAACAGGAGGAAATAG-3′) and Fibronectin Rev (5′-CAGCGTACACAGTGATGGTATAG-3′); Collagen IV Fwd (5′ GGTGATGCTGCCAGTGAGTT-3′) and Collagen IV Rev (5′- CTACCTGGCTCACCCTTTGG-3′); Perlecan Fwd (5′-GCCTCGAGCACCTCTCGGAACT 3′) and Perlecan Rev (5′-CTCAGGAGACGA CCTGGGCAGT-3′); Integrin β1 Fwd (5′- ACGGGAAACTTGGTGGTATT-3′) and Integrin β1 Rev (5′- GTG GGC AAT AGA AGG GTA GTC-3′); PI3K Fwd (5′- AGCAAGCGCCCCAAACTGGAA3′) and PI3K Rev (5′-TGCAGGCCACTGGTGAAGCAA-3′); Akt Fwd (5′-CACATAGCAGGGGCACCTTCCG-3′) and Akt Rev (5′-AGGGTGAAAGGTGGCGAGGGG-3′); ERK Fwd (5′-AGTTCTTGACCCCTGGTCCT3-′) and ERK Rev (5′-AATGGGTGACACACACAGGG-3′); Ras Fwd (5′-ACAGTGCAATGAGGGACCAGTA 3-′) and Ras Rev (5′-GTATAGAAGGCATCAACACC-3′); GAPDH Fwd (5′-CGCTTCGCTCTCTGCTCCTCC-3′) and GAPDH Rev (5′-TGGTGACCAGGCGCCCAATAC-3′). PCR was performed at 95 °C for 15 s and 60 °C for 60 s for 40 cycles. GAPDH was used as an internal standard to normalize mRNA levels for differences in sample concentration and loading. Fold changes in the expression of each target mRNA relative to GAPDH was calculated based on the threshold cycle (Ct) as 2 − Δ(ΔCt), where ΔCt = Ct target-Ct GAPDH and Δ(ΔCt) = ΔCtAdh-EC − ΔCtEC. The post-amplification melting curve analysis was performed to confirm whether the nonspecific amplification was generated from primer-dimers. Quantitative PCR reactions were performed in triplicate and repeated three times.
Western blotting
Endothelial cells treated and untreated with LY294002 (Merck Millipore) 3 μM for 1 h were lysed in buffer [50 mM Tris–HCl, pH 8, 1% Triton, 150 mM NaCl, 0.5% Deoxycholate and Halt Protease and Phosphatase Inhibitor Cocktail (Thermo Fisher Scientific Inc.—Rockford, USA)] for 2 h at 4 °C. The homogenates were spun at 14,000 rpm for 15 min and the supernatant was collected. Proteins were quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc.—Rockford, USA). Equal amounts of protein extracts (40 µg) were subjected to 10% SDS-PAGE and blotted onto a PVDF membrane (Thermo Fisher Scientific Inc.—Rockford, USA). After blocking, the membrane was incubated with the primary antibody (rabbit anti-fibronectin 1: 1000; rabbit anti-integrin αv, α5, β1 and β3 1: 1000; rabbit anti-laminin 1: 1000; rabbit anti-collagen IV 1:1000; rat anti-perlecan 1:1000; rabbit anti-MMP2 and MMP9 1: 1000; mouse-anti HYAL-1; rabbit anti-HYAL-2 1:1000; goat anti-HYAL-3 1:1000; rabbit anti-PI3K (p110α) 1:500; rabbit anti-phospho-Akt (Thr 308) 1:1000; rabbit anti-phospho-ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) 1:1000; rabbit anti-H-Ras 1:5000; mouse anti-GAPDH 1:500 and mouse anti-beta actin 1:1000) diluted in blocking buffer overnight at 4 °C and followed by incubation with secondary antibodies (1:10,000) for 1 h at room temperature. The samples were detected by enhanced chemiluminescence using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc., Rockford, IL, USA). An Alliance mini photodocumentation system from UVITEC (Cambrige, UK) was used to scan the films and UVIBAND MAX v1503b software (UVITEC—Cambrige, UK) was used to measure the amount of protein detected by each antibody. The experiments were performed in duplicate and repeated twice.
Immunocytochemistry
Cells were cultured on 13.0 mm diameter glass coverslips (5 × 103 cells/coverslip) in 24-well plates for 2 days followed by fixation with 4% formaldehyde in PBS (pH 7.4) for 20 min at room temperature. The cells were washed four times with PBS and once with PBS containing 0.1 M glycine. They were then permeabilized with PBS containing 1% BSA and 0.01% saponin for 15 min at room temperature. After this step, the cells were washed with PBS and incubated with rabbit anti-fibronectin (1:200), rabbit anti-laminin (1:200), rat anti-perlecan (1:200), rabbit anti-collagen IV (1:200), rabbit anti-PI3K (p110α) (1:200), rabbit anti-phospho-Akt (Thr 308) (1:200), rabbit anti-phospho-ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) (1:200) and rabbit anti-H-Ras (1:200) diluted in PBS or PBS containing 0.01% saponin for 1 h at room temperature. After washing, the cells were incubated with goat anti-rabbit labeled with Hilyte Fluor 594 (1:300) or goat anti-rat labeled with Alexa Fluor® 594 (1:300) and DAPI (1:10,000) in PBS containing 0.01% saponin for 20 min at room temperature. The cells were washed and mounted in Fluoromount-G and examined using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems, Wetzlar, DE). Each figure shown in the “Results” corresponds to the best of two experiments.
Cell growth analysis
Equal numbers (5 × 104) of EC, EJ-ras EC, Adh1-EC and Adh2-EC cells, treated and untreated with U0126 (Merck Millipore) 10 µM for 2 h or LY294002 (Merck Millipore) 3 μM for 1 h, were added into the different cell culture dishes and maintained in F12 medium (Sigma-Aldrich, St. Louis, USA) supplemented with 10% fetal calf serum (FCS) (Athena, Campinas, Brazil) at 37 °C, 2.5% CO2. Every two days, cells were removed from the dishes, washed with PBS and counted using a Neubauer chamber. Triplicate dishes were used for each time point (0, 2, 4, 6 and 8 days). Each experiment was repeated three times.
Annexin V-FITC/PI staining assay
Apoptosis was assessed by the binding of annexin V conjugated with fluorescein isothiocyanate (FITC) to phosphotidylserine, which is externalized to the outer leaflet of the plasma membrane early during induction of apoptosis. Briefly, EC cells and its derived clones (1 × 106), treated and untreated with U0126 (Merck Millipore) 10 µM for 2 h or LY294002 (Merck Millipore) 3 μM for 1 h, were resuspended in the binding buffer provided in the annexin V-FITC apoptosis detection kit II (BD Pharmingen, San Diego, CA, USA) and reacted with 5 μL of annexin V-FITC reagent and 5 μL of propidium iodide (PI) for 30 min at room temperature in the dark. Stained cells were analyzed by flow cytometry (FACSCalibur—Becton, Dickinson and Co, Franklin Lakes, NJ, USA) using the FlowJo program (Tree Star Inc., Ashland, OR, USA). Each experiment was repeated three times.
Invasion assay
The invasion assays were performed on 24-well plates using Polycarbonate Transwell of 8 µm pore size (Corning Inc. Lowell, MA, USA) coated with ECL cell attachment matrix (entactin, collagen IV and laminin) (1 mg/mL) (Merck Millipore, Danvers, MA, USA). Briefly, the coated inserts were hydrated with warm F12 without serum in 2.5% CO2 at 37 °C for 2 h. A total of 5 × 104 endothelial cells, treated and untreated with U0126 (Merck Millipore) 10 µM for 2 h or LY294002 (Merck Millipore) 3 μM for 1 h were seeded into the upper chamber containing F12 without serum and the lower chamber contained F12 with 10% FCS. The cells were incubated at 37 °C in 2.5% CO2 for 48 h. Non-invading cells were removed from the upper membrane by scrubbing with a cotton swab. Invading cells were fixed with 4% formaldehyde at room temperature for 5 min and methanol (p.a. 99.8%) for 20 min, stained with DAPI (1:10,000) dissolved in PBS for 20 min in darkness and visualized under an inverted microscope Axio Observer Z1 (Zeiss, Jena, Germany). The results were measured by counting the total number of nuclei stained with DAPI. ImageJ software (Bethesda, MA, USA) was used in the quantification and analysis of images. Each experiment was repeated three times.
Extraction and quantification of hyaluronic acid (HA)
Determination of HA was performed using a highly specific, highly sensitive “ELISA-like” fluorimetric method that employed a biotinylated HA-binding protein and europium-labeled streptavidin. The detection limit of this method is ca. 0.2–500 ng/mL of HA [28]. The samples were assayed in triplicate and repeated three times. The plates were read in a Victor 2-Wallac Ou fluorimeter (Turku, Finland).
Statistical analysis
All data are expressed as the mean ± standard error of the mean. Statistical significance was assessed using analysis of ANOVA. P values < 0.05 were considered statistically significant.
Results
Analysis of Ras/ERK signaling pathway in anoikis-resistant endothelial cells
To investigate the gene and protein expression levels of H-Ras and ERK by parental, Adh-EC and EJ-ras EC cells, we used qPCR and Western Blotting analyses, respectively.
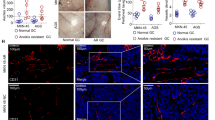
Figure 1a shows an increase in the expression of H-Ras gene in Adh-EC and EJ-ras EC cells when compared with EC cells. In addition, Fig. 1b shows an increase in the proteic expression of H-Ras in Adh-EC and EJ-ras EC cells when compared to the parental line. Similar data were observed using immunofluorescence assay (Fig. 1c).
Gene and protein expression of H-ras in EC-derived cell lines. a Expression of H-Ras detected by qPCR. GAPDH was used as a loading control. b Protein expression of H-Ras by western blot. GAPDH was used as a loading control. Histogram depicting H-Ras protein levels normalized to GAPDH. c Immunofluorescent analysis of H-Ras. H-Ras was stained with anti-H-Ras antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (100 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
The ERK gene expression analysis by qPCR revealed an increase of ERK mRNA levels in the Adh-EC and EJ-ras EC cells, when compared to EC, as shown in Fig. 2a. The level of phosphorylated ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) is significantly higher in Adh-EC and EJ-ras EC cells compared to parental lines (Fig. 2b). Similar data were observed using immunofluorescence assay (Fig. 2c).
Gene and protein expression of ERK in EC-derived cell lines. a Expression of ERK detected by qPCR. GAPDH was used as a loading control. b Protein expression of phospho-ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) by western blot. GAPDH was used as a loading control. Histogram depicting ERK protein levels normalized to GAPDH. c Immunofluorescent analysis of phospho-ERK 1/2 (Thr202/Tyr204, Thr185/Tyr187) ERK was stained with anti-Phospho-ERK antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (100 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC: EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
Analysis of PI3K/Akt signaling pathway in anoikis-resistant endothelial cells
In order to also investigate the gene and protein expression levels of PI3K and Akt by parental, Adh-EC and EJ-ras EC cells, we used qPCR and Western Blotting analyses, respectively.
Figure 3a shows an increase in the expression of PI3K (p110α) gene in endothelial cells resistant to anoikis and EJ-ras transfected cells in relation to parental cells. Consistent with these findings, western blot (Fig. 3b) and immunofluorescence (Fig. 3c) analysis shows an increase in the PI3K (p110α) protein levels.
Gene and protein expression of PI3K (p110α) in EC-derived cell lines. a Expression of PI3K (p110α) detected by qPCR. GAPDH was used as a loading control. b Protein expression of PI3K (p110α) by western blot. β-Actin was used as a loading control. Histogram depicting PI3K protein levels normalized to β-Actin. c Immunofluorescent analysis of PI3K (p110α). PI3K was stained with anti- PI3K (p110α) antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (100 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
Furthermore, we analysed the Akt expression in endothelial cells resistant to anoikis. qPCR analysis showed that Akt gene expression levels were similar in Adh-EC and EC cells (Fig. 4a). For protein expression, we observed an increase in the Akt (phosphorylated at Thr 308) expression in anoikis-resistant endothelial cells and EJ-ras EC in relation to EC cells (Fig. 4b, c).
Gene and protein expression of Akt in EC-derived cell lines. a Expression of Akt detected by qPCR. GAPDH was used as a loading control. b Protein expression of phospho-Akt (Thr308) by western blot. β-Actin was used as a loading control. Histogram depicting Akt protein levels normalized to β-Actin. c Immunofluorescent analysis of phospho-Akt (Thr308). Akt was stained with anti-phospho-Akt (Thr308) antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (100 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
ERK and PI3K inhibition affects growth, apoptosis and invasiveness in anoikis-resistant endothelial cells
To test the role of the Ras/ERK and PI3K/Akt pathways in cell growth, apoptosis and invasiveness of endothelial cells resistant to anoikis, selectives inhibitors of MEK 1/2, 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126) and PI3K, 2-(4-morpholinyl)-8-phenyl-4H-l-benzopyran-4 (LY294002) were used [29, 30].
To analyze the cell growth, EC, EJ-ras EC, Adh1-EC and Adh2-EC cells were treated with U0126 10 µM for 2 h or LY294002 3 μM for 1 h and added into the different cell culture dishes and maintained in F12 medium supplemented with 10% FCS. Every 2 days, cells were removed from the dishes and counted using a Neubauer chamber. As shown in Figs. 5a and 6a, respectively, the cell growth rate decreased in all cell lines after treatment with U0126 or LY294002, especially in cells resistant to anoikis.
Cell growth analysis, percentage of apoptosis and invasion capacity of EC-derived cell lines after ERK inhibition. a Growth curves of EC, EJ-ras transfected cells (EJ-ras EC) and EC anoikis resistant (Adh1-EC and Adh2-EC) after U0126 treatment (10 μM). b Percentage of apoptotic cells detected by Annexin V-FITIC/PI double staining method in EC, EJ-ras transfected cells (EJ-ras EC) and EC anoikis resistant (Adh1-EC and Adh2-EC) cells after U0126 treatment (10 μM). c Invasion rate in EC, EJ-ras EC, Adh1-EC and Adh2-EC cells after U0126 treatment (10 μM). Invasion activity of EC-derived cell lines was analyzed by using a Transwell coated with ECL cell attachment matrix as described in “Methodology”. All experiments were performed in triplicate and repeated three times. The bars represent the standard error. *P < 0.05
Cell growth analysis, percentage of apoptosis and invasion capacity of EC-derived cell lines after PI3K inhibition. a Growth curves of EC, EJ-ras transfected cells (EJ-ras EC) and EC anoikis resistant (Adh1-EC and Adh2-EC) after LY294002 treatment (3 μM). b Percentage of apoptotic cells detected by Annexin V-FITIC/PI double staining method in EC, EJ-ras transfected cells (EJ-ras EC) and EC anoikis resistant (Adh1-EC and Adh2-EC) cells after LY294002 treatment (3 μM). c Invasion rate in EC, EJ-ras EC, Adh1-EC and Adh2-EC cells after LY294002 treatment (3 μM). Invasion activity of EC-derived cell lines was analyzed by using a Transwell coated with ECL cell attachment matrix as described in “Methodology”. All experiments were performed in triplicate and repeated three times. The bars represent the standard error. *P < 0.05
To determine the percentage of apoptotic cells, EC, EJ-ras EC, Adh1-EC and Adh2-EC cells were treated with U0126 10 µM for 2 h or LY294002 3 μM for 1 h, harvested and stained with Annexin V-FITC and propidium iodide (PI). Stained cells were analyzed by flow cytometry. The results obtained with flow cytometry revealed a significant increase in apoptosis rate in all EC-derived cell lines treated with U0126 or LY294002 inhibitors (Figs. 5b, 6b, respectively).
To analyze the invasiveness, EC, EJ-ras EC, Adh1-EC and Adh2-EC cells were treated with U0126 10 µM for 2 h or LY294002 and added into the plates using polycarbonate transwell coated with ECL cell attachment matrix (Merck Millipore). As shown in Figs. 5c and 6c, respectively, the invasiveness decreased in all cell lines after treatment with U0126 or LY294002, mainly in Adh1-EC, Adh2-EC and EJ-ras EC cells.
Expression of ECM molecules and receptors in anoikis-resistant endothelial cells
In order to investigate the role of ECM in anoikis resistance, we analyzed the expression of some molecules present in the extracellular matrix, such as: fibronectin, collagen IV, laminin, vitronectin, perlecan, hyaluronic acid, hyaluronidases 1, 2 and 3, MMPs 2 and 9, αvβ3 and α5β1 integrins. The gene and protein expression levels were determined using qPCR and Western blot analysis, respectively. The hyaluronic acid synthesis was determined by “ELISA-like” fluorimetric method [28].
Figure 7a shows a decrease in the expression of fibronectin gene in Adh-EC and EJ-ras EC cells when compared with EC cells. In addition, Fig. 7b shows a decrease in the proteic expression of fibronectin in Adh-EC and EJ-ras EC cells when compared to the parental line. Similar data were observed using immunofluorescence assay (Fig. 7c).
Gene and protein expression of fibronectin in EC-derived cell lines. a Expression of fibronectin detected by qPCR. GAPDH was used as a loading control. b Protein expression of fibronectin by western blot. β-Actin was used as a loading control. Histogram depicting fibronectin protein levels normalized to β-Actin. c Immunofluorescent analysis of fibronectin. Fibronectin was stained with anti-fibronectin antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (20 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
Figure 8a shows an increase in the expression of collagen IV gene in Adh-EC and EJ-ras EC cells when compared with EC cells. Furthermore, Fig. 8b shows a decrease in the proteic expression of collagen IV in Adh-EC and EJ-ras EC cells when compared to the parental line. Similar data were observed using immunofluorescence assay (Fig. 8c).
Gene and protein expression of collagen IV in EC-derived cell lines. a Expression of collagen IV detected by qPCR. GAPDH was used as a loading control. b Protein expression of collagen IV by western blot. β-Actin was used as a loading control. Histogram depicting collagen IV protein levels normalized to β-Actin. c Immunofluorescent analysis of collagen IV. Collagen IV was stained with anti- collagen IV antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (20 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
The perlecan gene expression analysis by qPCR revealed an increase of perlecan mRNA levels in Adh-EC and EJ-ras EC cells when compared to EC, as shown in Fig. 10a. In addition, western blot analysis showed that protein expression levels of laminin and perlecan are increased in endothelial cells resistant to anoikis and EJ-ras transfected cells in relation to parental cells (Figs. 9a, 10b, respectively). Similar data were observed using immunofluorescence assay (Figs. 9b, 10c).
Protein expression of laminin and vitronectin in EC-derived cell lines. a Protein expression of laminin by western blot. GAPDH was used as a loading control. Histogram depicting laminin protein levels normalized to GAPDH. b Immunofluorescent analysis of laminin. Laminin was stained with anti-laminin antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (20 μM Scale bar). c Protein expression of vitronectin by western blot. β-actin was used as a loading control. Histogram depicting vitronectin protein levels normalized to β-Actin. All experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
Gene and protein expression of perlecan in EC-derived cell lines. a Expression of perlecan detected by qPCR. GAPDH was used as a loading control. b Protein expression of perlecan by western blot. β-Actin was used as a loading control. Histogram depicting perlecan protein levels normalized to β-Actin. c Immunofluorescent analysis of perlecan. Perlecan was stained with anti-perlecan antibody followed by secondary Hilyte Fluor™-594-labelled antibody (red). Nucleus stained with DAPI (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8, Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (20 μM Scale bar). a Experiment was performed in triplicate and b and c experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05. (Color figure online)
Figure 9c shows that the protein expression levels of vitronectin were similar in all cell lines.
The hyaluronic acid synthesis was determined by fluorimetric assay [28]. In Fig. 11a, it is possible to observe a decrease in the synthesis of this glycosaminoglycan in cells resistant to anoikis (Adh1-EC and Adh2-EC) and cells transfected with EJ-ras oncogene when compared to parental cells.
Synthesis of hyaluronic acid and protein expression of hyaluronidases 1, 2 and 3 in EC-derived cell lines. a Synthesis of hyaluronic acid. The samples were subjected to non-competitive fluorometric ELISA-like assay to detect HA. b Protein expression of hyaluronidase 1 (HYAL-1) by western blot. β-Actin was used as a loading control. Histogram depicting HYAL-1 protein levels normalized to β-Actin. c Protein expression of hyaluronidase 2 (HYAL-2) by western blot. β-Actin was used as a loading control. Histogram depicting HYAL-2 protein levels normalized to β-Actin. d Protein expression of hyaluronidase 3 (HYAL-3) by western blot. β-Actin was used as a loading control. Histogram depicting HYAL-3 protein levels normalized to β-Actin. a Experiment was performed in triplicate and b, c and d experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05
As the hyaluronic acid synthesis is reduced, we decided to analyze the expression of enzymes that degrade this molecule, the hyaluronidases (HYALs), in anoikis-resistant endothelial cells. The protein expression levels of HYALs 1, 2 and 3 are significantly higher in Adh-EC and EJ-ras EC cells compared to parental cells (Fig. 11b–d, respectively).
As changes were observed in the expression of syndecan-4 [6], fibronectin and collagen IV in anoikis-resistant endothelial cells, we decided to investigate the expression of enzymes involved in the degradation of these molecules, the metalloproteinases (MMPs) 2 and 9, in these cells. Figure 12a, b show an increase in the proteic expression of MMP 2 and 9, respectively, in Adh-EC and EJ-ras EC cells when compared to the parental cells.
Protein expression of MMP-2 and 9 in EC-derived cell lines. a Protein expression of MMP-2 (active enzyme/64 kDa) by western blot. β-Actin was used as a loading control. Histogram depicting MMP-2 protein levels normalized to β-Actin. b Protein expression of MMP-9 (active enzyme/84 kDa) by western blot. β-Actin was used as a loading control. Histogram depicting MMP-9 protein levels normalized to β-Actin. All experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05
Based on these results, we decided to evaluate the expression levels of αv, β3, α5 and β1 integrins subunits in all cell lines using western blot analysis. Figure 13a–d show an increase in the expression levels of all subunits in endothelial cells resistant to anoikis and EJ-ras transfected cells in relation to parental cells.
Protein expression of αv, β3, α5 and β1 integrins subunits in EC-derived cell lines. a Protein expression of αv integrin subunit by western blot. β-Actin was used as a loading control. Histogram depicting αv integrin subunit protein levels normalized to β-Actin. b Protein expression of β3 integrin subunit by western blot. β-Actin was used as a loading control. Histogram depicting β3 integrin subunit protein levels normalized to β-Actin. c Protein expression of α5 integrin subunit by western blot. β-Actin was used as a loading control. Histogram depicting α5 integrin subunit protein levels normalized to β-Actin. d Protein expression of β1 integrin subunit by western blot. β-Actin was used as a loading control. Histogram depicting β1 integrin subunit protein levels normalized to β-Actin. All experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05
Also, as changes were observed in the expression of ECM molecules and H-Ras in cells resistant to anoikis, we decided to investigate the expression these molecules after treatment with PI3K inhibitor. Figure 14 shows that inhibition of the PI3K pathway leads to reversal of protein expression changes of perlecan, collagen IV and laminin in anoikis-resistant endothelial cells. In contrast, vitronectin and H-Ras expression levels were not significantly altered.
Protein expression of H-Ras and ECM molecules in EC-derived cell lines after PI3K inhibition. a Protein expression of H-Ras by western blot. β-Actin was used as a loading control. Histogram depicting H-Ras protein levels normalized to β-Actin. b Protein expression of vitronectin by western blot. β-Actin was used as a loading control. Histogram depicting vitronectin protein levels normalized to β-Actin. c Protein expression of perlecan by western blot. β-Actin was used as a loading control. Histogram depicting perlecan protein levels normalized to β-Actin. d Protein expression of collagen IV by western blot. β-Actin was used as a loading control. Histogram depicting collagen IV protein levels normalized to β-Actin. e Protein expression of laminin by western blot. β-Actin was used as a loading control. Histogram depicting laminin protein levels normalized to β-Actin. All experiments were performed in duplicate. EC parental endothelial cells, EJ-ras EC EJ-ras transfected endothelial cells, Adh1-EC and Adh2-EC anoikis-resistant endothelial cells. The bars represent the standard error. *P < 0.05
Discussion
Recently, we reported that anoikis-resistant endothelial cells display morphological alterations, high rate of proliferation, poor adhesion to fibronectin, laminin and collagen IV and deregulation of the cell cycle, becoming less serum-dependent. Furthermore, anoikis-resistant cell lines display a high invasive potential and a low rate of apoptosis. This is accompanied by an increase in the levels of heparan sulfate and chondroitin sulfate as well as by changes in the expression of syndecan-4 and heparanase [6].
Anoikis resistance is a vital step during cancer progression and metastatic colonization [3]. Unregulated expression of growth factor receptors or components of their signaling pathways are associated with tumor malignancy due to their inhibition of cell death pathways and activation of cell survival pathways [5, 8]. Numerous kinase/phosphatase signaling molecules have been implicated in anoikis as central regulators. Because ras activation prevents anoikis and integrins can stimulate various aspects of the ras pathway, anoikis research has focused on the two major ras effectors, the kinases PI3K (phosphoinositide-3 kinase) and raf [31].
Our findings show an increase in the levels of H-Ras and Phospho-ERK (Thr202/Tyr204, Thr185/Tyr187) in anoikis resistant endothelial cells when compared with parental cells. After treatment with U0126, a selective inhibitor of MEK1/2, all EC-derived cell lines displayed lower growth, a decrease in invasive potential and a higher rate of apoptosis. Lopes et al. [27] showed that endothelial cells transfected with the EJ-ras oncogene display morphological alterations and deregulation of the cell cycle, becoming less serum-dependent, in contrast with the parental endothelial cells. Activation of the Ras/ERK signaling pathway can inhibit apoptosis in response to growth factor withdrawal, death receptor activation, and, as recently described for fibroblasts and MDCK cells, detachment from extracellular matrix [32, 33]. Activation of the Raf-1/MAP kinase pathway provides a potent antiapoptotic signal in the absence of extracellular matrix support [33]. Treatment of Ras-transformed cells with the U0126 ERK inhibitor caused partial reversion to an anoikis-sensitive state, indicating that extracellular signal-regulated kinase (ERK) activation contributes to inhibition of anoikis [34]. U0126 and PD98059, ERK inhibitors, also selectively repressed anchorage-independent growth of human breast cancer cell line MDA-MB231 [35].
ERK is a mitogen-activated protein kinase (MAPK) that functions as the major effector of the Ras oncoprotein [9]. Several reports have pointed that Raf-ERK activation providing one route that bypasses adhesion signals and elicits resistance to anoikis [36]. Enhanced ERK activity, via activation of the EGF receptor, in suspended keratinocytes provides protection against anoikis [37]. Further studies showed that reactive oxygen species (ROS) leads to the SRC-mediated phosphorylation of EGF receptor at Tyr845, a site that is well known to promote the activation of EGF receptor [38]. Indeed, this ROS-mediated activation of SRC (and EGFR) was shown to be necessary for anoikis inhibition through ERK-mediated modulation of pro-apoptotic protein BIM-EL [39,40,41]. Another study shows that raf-1 can in turn be activated by EGFRs, this result implicates a positive feedback loop that promotes cell survival involving raf and autocrine growth stimulation. It has been found that hepatocyte growth factor (HGF) inhibited anoikis of human head and neck squamous cell carcinoma (HNSCC) cells. The inhibition of anoikis mediated by HGF was dependent on activator protein-1 activity by activating the ERK-signalling pathway [42].
PI3K/Akt pathway also plays a major role in various cellular functions such as proliferation, invasion, and angiogenesis [43]. The role of PI3K in cancer was highlighted by the observation that the gene coding the catalytic α subunit of p110 is frequently mutated in the most common human cancers [44, 45]. Akt is viewed as a major downstream effector of PI3K [45], as this protein kinase is recruited to the plasma membrane and is activated by phosphorylation at residues T308 and S473 by PDK1 [46]. Activated Akt is a powerful promoter of cell survival, as it antagonizes apoptosis by phosphorylating and inactivating various components of the apoptotic machinery such as Bad [47], caspase-9 [48], and forkhead transcription factor family members [49, 50]. Here we show an up-regulation of PI3K/Akt pathway by high expression of PI3K (p110α) and the high activation of Akt by phosphorylation of Thr 308 in anoikis-resistant endothelial cells. After treatment with LY294002, a selective inhibitor of PI3K, all cell lineages displayed lower growth, a decrease in invasive potential and a higher rate of apoptosis. In contrast, PI3K inhibition does not alter the H-Ras protein expression. Inhibition of PI3K-AKT pathway by LY294002 led to a decrease in growth and increase in apoptosis rates in medullary thyroid cancer cell line (TT), suggesting that PI3K-AKT pathway may play a critical role in the survival of medullary thyroid cancer cells by protecting cells from apoptosis [51]. Another study showed that the introduction of activated forms of the PI3K p110 subunit, or of Akt, rescued the MDCK cells from anoikis. Attachment-mediated activation of PI3K and protein kinase B/Akt protects cells from anoikis. In contrast, inhibition of PI3K and protein kinase B/Akt results in an induction of anoikis [52].
Ras/ERK and PI3K/Akt were originally modeled as linear signaling conduits activated by different stimuli, yet even early experiments hinted that they might intersect to regulate each other and co-regulate downstream functions. During recent years, many mechanisms and modes of cross-talk have been uncovered. These include cross-inhibition, crossactivation, and pathway convergence on substrates [9]. The Ras/ERK pathway cross-activates PI3K/Akt signaling. Ras-GTP can directly bind and allosterically activate PI3K, similarly, components of the Ras-ERK pathway (Ras, Raf, ERK, and RSK) also positively regulate the PI3K/mTORC1 pathway [9, 53,54,55]. Once activated, ERK and Akt often act on the same substrate, sometimes in concert, to promote cell survival, proliferation, metabolism, and motility. Examples include FOXO (forkhead box O) and GSK3 (glycogen synthase kinase 3), which can be inhibited by ERK and Akt [9, 56, 57]. FOXOs regulates the expression of apoptotic proteins and cell cycle regulators to suppress cell survival and proliferation. GSK3 inhibits pro-survival, proliferation and motility proteins, such as adhesion proteins and the b-catenin transcription factor that drives the expression of cyclin D and MYC [9]. This leads us to believe that pharmacological inhibition of PI3K activity with LY294002 in endotelial cells resistant to anoikis attenuates the negative regulation of FOXOs and GSK3 by Akt, consequently inhibits the cell proliferation and increases the apoptosis rate.
The signaling pathways that drive cell survival, proliferation and motility also are controlled by microenvironmental cues, including cell–cell interactions and ECM-integrin engagement [58]. Tumor cells actively remodel their local extracellular microenvironment either by directly releasing ECM remodeling enzymes such as MMPs, serine and cysteine proteases or hyaluronidases [59,60,61]. Here we show that anoikis-resistant endothelial cells display a decrease in the expression of fibronectin, collagen IV and hyaluronic acid and an increase in the expression of laminin, perlecan, αv, β3, α5 and β1 integrins subunits, HYALs 1, 2 and 3 and MMPs 2 and 9. Inhibition of the PI3K-AKT pathway by LY294002 leads to reversal of protein expression changes of perlecan, collagen IV and laminin in anoikis-resistant endothelial cells.
Different extracellular matrix components can transduce distinct survival signals through preferential activation of subsets of multiple integrin-mediated signaling pathways. Survival signals from laminin-10/11 are mainly through the PI3 kinase/Akt pathway, whereas survival signals from fibronectin are conveyed by MEK1/ERK through FAK. Laminin-10/11 has more survival potential than fibronectin [62]. The overexpression of laminin-5 γ2 chain increases cell proliferation, and thereby promotes tumor growth, indicating the possibility that this molecule could be used as a marker for disease progression and malignancy [63,64,65]. In addition, vitronectin induces Akt activity and rescue detached HEK293 cells from anoikis [66] and loss of type IV collagen during tumor development can potentially provide a permissive microenvironment for invasiveness phenotype of cancer [67, 68]. Zeng et al. [69] showed that loss of basal membrane type IV collagen occurs during colorectal tumorigenesis and is associated with elevations in the active forms of MMP-2 and MMP-9. This consequently leads to an increase in the survival and proliferation of tumor cells.
Integrins mediate anchorage and migration of cells via recognition of variable extracellular matrix molecules. Only specific ligated integrins suppress anoikis in certain cell types. For example, serum-starved HT29 carcinoma cells readily underwent apoptosis, which was partially suppressed by transfection of the α integrin subunit [70]. The ectopic expression of α5ß1 integrin, but not αvß1 integrin, suppressed anoikis of fibronectin-bound CHO cells under serum-free conditions [71]. α5β1 integrin controls invasion of breast cancer cells by modulation of MMP-1 [72] and MMP-2 collagenase activity [73]. Fibronectin secreted by peritoneal tissue activates α5β1 integrin on ovarian cancer cells to stimulate their invasiveness through an increase of MMP-9 activity [74]. In addition, αvβ3 integrin mediates endothelial cell binding to the extracellular matrix and transduces an intracellular signal promoting survival of endothelial cells and various tumor cells [75]. αvβ3 integrin is important for adhesion of melanoma cells to dermal collagen and the suppression of anoikis [76], most likely by altering the Bcl2/Bax ratio [77].
Other ECM molecules are also involved in anoikis resistance. Perlecan, a heparan sulfate (HS) proteoglycan, supports various biological functions, including cell adhesion, growth factor binding, and modulation of apoptosis. A C-terminal fragment released from endothelial cell-derived perlecan protects fibroblasts from apoptosis [78, 79]. Anoikis resistant melanocytes show an increase in perlecan expression [80]. Increased perlecan levels are detected in breast carcinomas and in metastatic melanomas that correlate with a more aggressive phenotype [81].
Hyaluronic acid (HA) is a nonsulfated glycosaminoglycan that interacts with cell surface receptors, including cluster determinant 44 (CD44). Binding of HA with CD44 has been implicated in a variety of physiological events including cell–cell and cell-substrate adhesion, cell migration, cell proliferation and HA uptake and degradation [82,83,84]. Degradation of HA is achieved through the enzymatic action of hyaluronidases (Hyal-1, Hyal-2 and Hyal-3), whose expression is increased or suppressed in some malignant conditions compared with normal tissues [85]. It is believed that the various HA fragments have distinct effects in cancer progression, angiogenesis and metastasis [61, 85] and may also activate matrix metalloproteinases (MMPs), thus enhancing tumor invasiveness [86].
During cancer progression, tumor-associated ECM is continuingly remodeled to create a more suitable microenvironment for malignant cellular growth, survival, adhesion and invasive migration. This remodeling process includes the ECM degradation process mainly mediated by matrix metalloproteinases (MMPs) [87]. MMP-2 and MMP-9 have been shown to play critical roles in the “angiogenic switch” and tumor cells could synthesise and secrete large amounts of MMP-2 and MMP-9 in a paracrine and/or autocrine manner to stimulate angiogenesis and increase VEGF release [88]. Signaling pathways are involved in the regulation of MMPs. Kim et al. [89] found that both the PI3K and MAP kinase pathways are involved in the induction of MMP-9. Both pathways seem to contribute to MMP-9 production in a synergistic manner and the inhibition of these pathways resulted in total inhibition of MMP-9 production. These data are consistent with our findings using PI3K inhibitor that decrease the invasiveness in anoikis resistant cell lines.
In conclusion, this study revealed that the acquisition of anoikis resistance induced remodeling of the extracellular matrix and overexpression of the PI3K/Akt and Ras/ERK pathways components, as this overexpression is crucial to keep the tumorigenic characteristics as higher proliferation, lower apoptosis and higher invasiveness. Acquisition of resistance to anoikis is a potentially crucial step in endothelial cell transformation.
References
Malagobadan S, Nagoor NH (2015) Evaluation of MicroRNAs regulating anoikis pathways and its therapeutic potential. Biomed Res Int 2015:716816. doi:10.1155/2015/716816
Chiarugi P, Giannoni E (2008) Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol 76(11):1352–1364. doi:10.1016/j.bcp.2008.07.023
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 1833(12):3481–3498. doi:10.1016/j.bbamcr.2013.06.026
Simpson CD, Anyiwe K, Schimmer AD (2008) Anoikis resistance and tumor metastasis. Cancer Lett 272(2):177–185. doi:10.1016/j.canlet.2008.05.029
Sharma SV, Bell DW, Settleman J, Haber DA (2007) Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 7(3):169–181. doi:10.1038/nrc2088
Carneiro BR, Pernambuco Filho PC, Mesquita AP, da Silva DS, Pinhal MA, Nader HB, Lopes CC (2014) Acquisition of anoikis resistance up-regulates syndecan-4 expression in endothelial cells. PLoS ONE 9(12):e116001. doi:10.1371/journal.pone.0116001
Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 22:287–309. doi:10.1146/annurev.cellbio.22.010305.104315
Kim YN, Koo KH, Sung JY, Yun UJ, Kim H (2012) Anoikis resistance: an essential prerequisite for tumor metastasis. Int. J Cell Biol 2012:306879. doi:10.1155/2012/306879
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36(6):320–328. doi:10.1016/j.tibs.2011.03.006
McKay MM, Morrison DK (2007) Integrating signals from RTKs to ERK/MAPK. Oncogene 26(22):3113–3121. doi:10.1038/sj.onc.1210394
Rozengurt E (2007) Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213(3):589–602. doi:10.1002/jcp.21246
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40(2):310–322. doi:10.1016/j.molcel.2010.09.026
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5 (5):402–418. doi:10.1016/j.apsb.2015.07.005
Sakamoto S, Kyprianou N (2010) Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med 31(2):205–214. doi:10.1016/j.mam.2010.02.001
Stivarou T, Patsavoudi E (2015) Extracellular molecules involved in cancer cell invasion. Cancers 7 (1):238–265. doi:10.3390/cancers7010238
Hynes RO, Naba A (2012) Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4(1):a004903. doi:10.1101/cshperspect.a004903
Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO (2006) The echinoderm adhesome. Dev Biol 300(1):252–266. doi:10.1016/j.ydbio.2006.07.044
Yue B (2014) Biology of the extracellular matrix: an overview. J Glaucoma 23(8 Suppl 1):S20-s23. doi:10.1097/IJG.0000000000000108
Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23(1):47–55. doi:10.1038/nbt1055
Geiger B, Bershadsky A, Pankov R, Yamada KM (2001) Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol 2(11):793–805. doi:10.1038/35099066
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22. doi:10.1038/nrc2748
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a005058
Cawston TE, Young DA (2010) Proteinases involved in matrix turnover during cartilage and bone breakdown. Cell Tissue Res 339(1):221–235. doi:10.1007/s00441-009-0887-6
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516. doi:10.1146/annurev.cellbio.17.1.463
McAtee CO, Barycki JJ, Simpson MA (2014) Emerging roles for hyaluronidase in cancer metastasis and therapy. Adv Cancer Res 123:1–34. doi:10.1016/B978-0-12-800092-2.00001-0
Buonassisi V, Venter JC (1976) Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci USA 73(5):1612–1616
Lopes CC, Toma L, Pinhal MA, Porcionatto MA, Sogayar MC, Dietrich CP, Nader HB (2006) EJ-ras oncogene transfection of endothelial cells upregulates the expression of syndecan-4 and downregulates heparan sulfate sulfotransferases and epimerase. Biochimie 88(10):1493–1504. doi:10.1016/j.biochi.2006.04.009
Martins JR, Passerotti CC, Maciel RM, Sampaio LO, Dietrich CP, Nader HB (2003) Practical determination of hyaluronan by a new noncompetitive fluorescence-based assay on serum of normal and cirrhotic patients. Anal Biochem 319(1):65–72
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269(7):5241–5248
Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM (1998) Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273(29):18623–18632
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124(4):619–626
Holmstrom TH, Chow SC, Elo I, Coffey ET, Orrenius S, Sistonen L, Eriksson JE (1998) Suppression of Fas/APO-1-mediated apoptosis by mitogen-activated kinase signaling. J Immunol 160(6):2626–2636
Le Gall M, Chambard JC, Breittmayer JP, Grall D, Pouyssegur J, Van Obberghen-Schilling E (2000) The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell 11(3):1103–1112
McFall A, Ulku A, Lambert QT, Kusa A, Rogers-Graham K, Der CJ (2001) Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol Cell Biol 21(16):5488–5499. doi:10.1128/MCB.21.16.5488-5499.2001
Fukazawa H, Noguchi K, Murakami Y, Uehara Y (2002) Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol Cancer Ther 1(5):303–309
Zhan M, Zhao H, Han ZC (2004) Signalling mechanisms of anoikis. Histol Histopathol 19(3):973–983
Jost M, Huggett TM, Kari C, Rodeck U (2001) Matrix-independent survival of human keratinocytes through an EGF receptor/MAPK-kinase-dependent pathway. Mol Biol Cell 12(5):1519–1527
Boerner JL, Demory ML, Silva C, Parsons SJ (2004) Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 24(16):7059–7071. doi:10.1128/MCB.24.16.7059-7071.2004
Giannoni E, Buricchi F, Grimaldi G, Parri M, Cialdai F, Taddei ML, Raugei G, Ramponi G, Chiarugi P (2008) Redox regulation of anoikis: reactive oxygen species as essential mediators of cell survival. Cell Death Differ 15(5):867–878. doi:10.1038/cdd.2008.3
Giannoni E, Fiaschi T, Ramponi G, Chiarugi P (2009) Redox regulation of anoikis resistance of metastatic prostate cancer cells: key role for Src and EGFR-mediated pro-survival signals. Oncogene 28(20):2074–2086. doi:10.1038/onc.2009.77
Buchheit CL, Weigel KJ, Schafer ZT (2014) Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer 14(9):632–641. doi:10.1038/nrc3789
Zeng Q, Chen S, You Z, Yang F, Carey TE, Saims D, Wang CY (2002) Hepatocyte growth factor inhibits anoikis in head and neck squamous cell carcinoma cells by activation of ERK and Akt signaling independent of NFkappa B. J Biol Chem 277(28):25203–25208. doi:10.1074/jbc.M201598200
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068. doi:10.1038/nature07385
Courtney KD, Corcoran RB, Engelman JA (2010) The PI3K pathway as drug target in human cancer. J Clin Oncol 28(6):1075–1083. doi:10.1200/JCO.2009.25.3641
Faes S, Dormond O (2015) PI3K and AKT: unfaithful partners in cancer. Int J Mol Sci 16(9):21138–21152. doi:10.3390/ijms160921138
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773(8):1263–1284. doi:10.1016/j.bbamcr.2006.10.001
Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91(2):231–241
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282(5392):1318–1321
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401(6748):82–85. doi:10.1038/43466
Kunnimalaiyaan M, Ndiaye M, Chen H (2006) Apoptosis-mediated medullary thyroid cancer growth suppression by the PI3K inhibitor LY294002. Surgery 140 (6):1009–1014. doi:10.1016/j.surg.2006.06.040. (discussion 1014–1005)
Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J 16(10):2783–2793. doi:10.1093/emboj/16.10.2783
Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ (1994) The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol 4(9):798–806
Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J (1994) Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370(6490):527–532. doi:10.1038/370527a0
Suire S, Hawkins P, Stephens L (2002) Activation of phosphoinositide 3-kinase gamma by Ras. Curr Biol 12(13):1068–1075
Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC (2008) ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol 10(2):138–148. doi:10.1038/ncb1676
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274. doi:10.1016/j.cell.2007.06.009
Abrahamsen I, Lorens JB (2013) Evaluating extracellular matrix influence on adherent cell signaling by cold trypsin phosphorylation-specific flow cytometry. BMC Cell Biol 14:36. doi:10.1186/1471-2121-14-36
Lopez-Otin C, Matrisian LM (2007) Emerging roles of proteases in tumour suppression. Nat Rev Cancer 7(10):800–808. doi:10.1038/nrc2228
Lokeshwar VB, Gomez P, Kramer M, Knapp J, McCornack MA, Lopez LE, Fregien N, Dhir N, Scherer S, Klumpp DJ, Manoharan M, Soloway MS, Lokeshwar BL (2008) Epigenetic regulation of HYAL-1 hyaluronidase expression. identification of HYAL-1 promoter. J Biol Chem 283(43):29215–29227. doi:10.1074/jbc.M801101200
Stern R (2008) Hyaluronidases in cancer biology. Semin Cancer Biol 18(4):275–280. doi:10.1016/j.semcancer.2008.03.017
Gu J, Fujibayashi A, Yamada KM, Sekiguchi K (2002) Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J Biol Chem 277(22):19922–19928. doi:10.1074/jbc.M200383200
Skyldberg B, Salo S, Eriksson E, Aspenblad U, Moberger B, Tryggvason K, Auer G (1999) Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst 91(21):1882–1887
Xue LY, Zou SM, Zheng S, Liu XY, Wen P, Yuan YL, Lin DM, Lu N (2011) Expressions of the gamma2 chain of laminin-5 and secreted protein acidic and rich in cysteine in esophageal squamous cell carcinoma and their relation to prognosis. Chin J Cancer 30(1):69–78
Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K (2001) Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin Cancer Res 7(4):896–900
Miyazaki T, Shen M, Fujikura D, Tosa N, Kim HR, Kon S, Uede T, Reed JC (2004) Functional role of death-associated protein 3 (DAP3) in anoikis. J Biol Chem 279(43):44667–44672. doi:10.1074/jbc.M408101200
Ikeda K, Iyama K, Ishikawa N, Egami H, Nakao M, Sado Y, Ninomiya Y, Baba H (2006) Loss of expression of type IV collagen alpha5 and alpha6 chains in colorectal cancer associated with the hypermethylation of their promoter region. Am J Pathol 168(3):856–865
Tanjore H, Kalluri R (2006) The role of type IV collagen and basement membranes in cancer progression and metastasis. Am J Pathol 168(3):715–717. doi:10.2353/ajpath.2006.051321
Zeng ZS, Cohen AM, Guillem JG (1999) Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis 20(5):749–755
O’Brien V, Frisch SM, Juliano RL (1996) Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res 224(1):208–213. doi:10.1006/excr.1996.0130
Zhang Z, Morla A, Vuori K, Bauer J, Juliano R, Ruoslahti E (1993) The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 122:235–242
Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KM, Ethier SP, Livant DL (2004) Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res 64(23):8674–8681. doi:10.1158/0008-5472.CAN-04-0069
Morozevich G, Kozlova N, Cheglakov I, Ushakova N, Berman A (2009) Integrin alpha5beta1 controls invasion of human breast carcinoma cells by direct and indirect modulation of MMP-2 collagenase activity. Cell cycle 8(14):2219–2225. doi:10.4161/cc.8.14.8980
Shibata K, Kikkawa F, Nawa A, Suganuma N, Hamaguchi M (1997) Fibronectin secretion from human peritoneal tissue induces Mr 92,000 type IV collagenase expression and invasion in ovarian cancer cell lines. Cancer Res 57(23):5416–5420
Brassard DL, Maxwell E, Malkowski M, Nagabhushan TL, Kumar CC, Armstrong L (1999) Integrin alpha(v)beta(3)-mediated activation of apoptosis. Exp Cell Res 251(1):33–45. doi:10.1006/excr.1999.4559
Montgomery AM, Reisfeld RA, Cheresh DA (1994) Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA 91(19):8856–8860
Petitclerc E, Stromblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, Cheresh DA, Brooks PC (1999) Integrin alpha(v)beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res 59(11):2724–2730
Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hebert MJ (2006) Perlecan proteolysis induces an alpha2beta1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem 281(41):30383–30392. doi:10.1074/jbc.M606412200
Farach-Carson MC, Carson DD (2007) Perlecan—a multifunctional extracellular proteoglycan scaffold. Glycobiology 17(9):897–905. doi:10.1093/glycob/cwm043
Oba-Shinjo SM, Correa M, Ricca TI, Molognoni F, Pinhal MA, Neves IA, Marie SK, Sampaio LO, Nader HB, Chammas R, Jasiulionis MG (2006) Melanocyte transformation associated with substrate adhesion impediment. Neoplasia 8(3):231–241. doi:10.1593/neo.05781
Segev A, Nili N, Strauss BH (2004) The role of perlecan in arterial injury and angiogenesis. Cardiovasc Res 63(4):603–610. doi:10.1016/j.cardiores.2004.03.028
Chen WY, Abatangelo G (1999) Functions of hyaluronan in wound repair. Wound Repair Regen 7(2):79–89
Solis MA, Chen YH, Wong TY, Bittencourt VZ, Lin YC, Huang LL (2012) Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int 2012:346972. doi:10.1155/2012/346972
Park JB, Kwak HJ, Lee SH (2008) Role of hyaluronan in glioma invasion. Cell Adh Migr 2(3):202–207
Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavao MS, Tzanakakis GN, Karamanos NK (2012) Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J 279(7):1177–1197. doi:10.1111/j.1742-4658.2012.08529.x
Sa VK, Rocha TP, Moreira A, Soares FA, Takagaki T, Carvalho L, Nicholson AG, Capelozzi VL (2015) Hyaluronidases and hyaluronan synthases expression is inversely correlated with malignancy in lung/bronchial pre-neoplastic and neoplastic lesions, affecting prognosis. Braz J Med Biol Res 48(11):1039–1047. doi:10.1590/1414-431X20154693
Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang XA, Zhang F, Jiang L, Zhang Y, Hu Y, Xiang S, Shu Y, Bao R, Li H, Wu W, Weng H, Yen Y, Liu Y (2015) Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett 360(2):141–150. doi:10.1016/j.canlet.2015.01.041
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9(2):267–285
Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J 15(11):1953–1962. doi:10.1096/fj.01-0198com
Acknowledgements
Supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP); Equipamentos Multiusuário (EMU—FAPESP); Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP), Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa Mesquita, A.P., de Araújo Lopes, S., Pernambuco Filho, P.C. et al. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis 22, 1116–1137 (2017). https://doi.org/10.1007/s10495-017-1392-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1392-0