Abstract
Multidrug resistance (MDR) remains a major clinical obstacle in the treatment of gastric cancer (GC) since it causes tumor recurrence and metastasis. The transcription factor activator protein-2α (AP-2α) has been implicated in drug-resistance in breast cancer; however, its effects on MDR of gastric cancer are far from understood. In this study, we aimed to explore the effects of AP-2α on the MDR in gastric cancer cells selected by vincristine (VCR). Decreased AP-2α levels were markedly detected by RT-PCR and Western blot in gastric cancer cell lines (BGC-823, SGC-7901, AGS, MKN-45) compared with that in the gastric epithelial cell line (GES-1). Furthermore, we found that the expression of AP-2α in SGC7901/VCR or SGC7901/adriamycin (ADR) cells was lower than in SGC7901 cells. Thus, a vector overexpressing AP-2α was constructed and used to perform AP-2α gain-of-function studies in SGC7901/VCR cells. The decreased IC50 values of the anti-cancer drugs in sensitive and resistant cells after transfect with pcDNA3.1/AP-2α were determined in SGC7901/VCR cells by MTT assay. Moreover, flow cytometry analysis indicated that overexpressed AP-2α induced cell cycle arrest in the G0/G1 phase and promoted cell apoptosis of VCR-selected SGC7901/VCR cells. RT-PCR and Western blot demonstrated that overexpressed AP-2α can significantly induce the down-regulation of Notch1, Hes-1, P-gp and MRP1 in SGC7901/VCR cells. Similar effects can be observed when Numb (Notch inhibitor) was introduced. In addition, the intracellular ADR accumulation was markedly detected in AP-2α overexpressed or Numb cells. In conclusion, our results indicate that AP-2α can reverse the MDR of gastric cancer cells, which may be realized by inhibiting the Notch signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers are the second leading causes of death worldwide, with gastric cancer (GC) remaining one of the most common malignancies [1]. Although the treatment methods for gastric carcinoma have made great progress over the past 2 decades, a large number of patients still fail to achieve complete remission or suffer relapse due to the phenomenon of multidrug resistance (MDR). The complicated mechanisms of MDR in cancer cells include increased drug efflux, redistribution of intracellular drug accumulation, alterations in the drug’s target molecules, increased DNA damage repair, and suppression of drug-induced cell apoptosis [2,3,4]. Studies suggest that mechanisms of MDR in gastric cancer are likely to be multifaceted and associated with the overexpression of ATP-binding cassette transporters P-gp and MRP1, as well as the activation of growth promoter pathways [5,6,7].
Transcription factors that have been implicated in the pathogenesis of malignancy can serve as novel therapeutic targets [8]. The transcription factor activator proteins-2 (AP-2) family is comprised of five highly conserved DNA-binding transcription factors, which are referred to as AP-2α, AP-2β, AP-2γ, AP-2δ and AP-2ε [9]. AP-2 proteins are involved in diversified cellular functions such as proliferation, differentiation and apoptosis, and play key roles in embryonic development and carcinogenesis [10]. A previous study has demonstrated that high nuclear AP-2α expression suppresses the tumor progression in breast cancer [11]. Interference with AP-2α leads to the induction of apoptosis and an increase in chemo- and radiation-sensitivity in breast cancer cells. In studies of gastric cancer, reduced AP-2α expression independently predicted an unfavorable prognosis for gastric adenocarcinoma patients, thus functioning as a tumor suppressor [12]. However, whether can AP-2α work in gastric cancer cells is still unknown and poorly investigated.
Notch is one of the most important pathways in several tumor microenvironments [13]. The binding of Notch receptor to its ligand leads to proteolytic cleavage and release of the activated intracellular domain of Notch1, which commonly translocates into the nucleus and drives the expression of multiple target proteins such as Hes/Hey families and c-Myc [14]. In human cancers, a previous study showed that Notch signaling exhibits both tumor promoting [15] and inhibiting functions [16], depending on the cell type [17]. Importantly, Notch signaling has also been reported to be involved in the resistance of different cancer cells to chemotherapeutic reagents [18,19,20]. Meanwhile, a regulatory effect of AP-2α on Notch signaling has been demonstrated in other cell types such as neurons [21]. However, the role of Notch signaling in gastric cancer cell chemoresistance has not been thoroughly reported. Besides, whether there is any relationships between AP-2α and Notch signaling during MDR of gastric cancer cells needs to be explored and revealed.
In the present study, we first detected abnormally decreased AP-2α expression in gastric cancer cell lines (BGC-823, SGC-7901, AGS, MKN-45), which is more obvious in SGC7901 cells. Then, SGC7901 was chosen for the following research. To explore the effects of AP-2α on the MDR of gastric cells, we established the vincristine (VCR)-resistant human gastric cancer cell line SGC7901/VCR. The SGC7901/VCR cells could resist several anti-cancer drugs such as adriamycin (ADR) and 5-Fu except for VCR. After the selection of SGC7901 cells by VCR, the effects of exogenous AP-2α on MDR, cell apoptosis and intracellular ADR accumulation and its possible mechanism in SGC7901/VCR cells were further explored. AP-2α reverses the multidrug resistance of gastric cancer cells by inhibiting the Notch pathway.
Materials and methods
Cell culture
The human non-malignant gastric epithelial cell line GES-1 and gastric cancer cell lines (BGC-823, SGC-7901, AGS, MKN-45) were cultured in RPMI1640 medium (Invitrogen, Gaithersburg, MD) containing 10% fetal bovine serum (FBS, Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Rockville, MD, USA). The vincristine-resistant human gastric cancer cell line SGC7901/VCR was developed by treating the parental SGC7901 cells to VCR (Wanle, Shenzhen, China), as previously described [22]. The SGC7901 cells were cultured in the presence of 1 μg/ml VCR to maintain the SGC7901/VCR drug-resistance phenotype, and then SGC7901/VCR were grown in drug-free medium 2 weeks before the experiments. Then the logarithmic phase cells of SGC7901/VCR were used in experiments.
Western blot
Cells were harvested, lysed, and centrifuged routinely [23]. Protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA). Samples containing 50 µg of proteins were subjected to 10% SDS-PAGE and electro-transferred to nitrocellulose membranes. After blocking with 5% skim milk, membranes were incubated with primary antibodies including anti-AP-2α (sc-70361, 1:2000 dilution), anti-P-gp (sc-71557, 1:1000 dilution), anti-MRP1 (sc-136447, 1:2000 dilution), anti-p53 (sc-47698, 1:1000 dilution), anti-Bcl-2 (sc-56015, 1:1000 dilution), anti-Bax (sc-20067, 1:1000 dilution), anti-cleaved Notch1 (sc-376403, 1:500 dilution), anti-Numb (sc-136554, 1:1000) and anti-Hes-1 (sc-166378, 1:500 dilution) as well as β-actin (sc-130300, 1:2000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature followed by horseradish-peroxidase-conjugated secondary antibody. Protein bands were visualized with ECL (Santa Cruz Biotechnology).
Real-time PCR
Total cell RNA was isolated using TRIzol reagent (Invitrogen). The cDNA was synthesized with PrimeScript reverse transcriptase (Biostar, Shanghai, China) according to the manufacturer’s instructions. RT-PCR was performed using a SYBR-Green supermix (Invitrogen) on the ABI PRISM 7700 Sequence Detection system (PE Applied Biosystems, Waltham, MA, USA). The primers for RT-PCR are listed in Table 1. β-actin was used as an internal control. The relative mRNA levels of the target genes were calculated using the 2−ΔΔCT method [24].
Construction of plasmids
To prepare the plasmid constructs of AP-2α and Numb, the cDNA was synthesized by reverse-transcription of total RNA, using a PrimeScript RT reagent kit (Takara, Dalian, China) with oligo-dT primers, according to the manufacturer’s protocol. AP-2α or Numb cDNA amplified by reverse-transcription (RT)-PCR was cloned into a pcDNA3.1 Directional TOPO Expression kit (Invitrogen) to produce pcDNA3.1/AP-2α and pcDNA3.1/Numb.
SGC7901 and SGC7901/VCR cells were plated into 12-well plates and allowed to adhere for 24 h. Then, all plasmids were transfected into these cells according to the manufacturer’s instructions.
Small interfering RNA
An AccuTargetTM siRNA Set targeting AP-2α was purchased from Bioneer Corporation. The coding sequence of plant chlorophyll a/b-binding protein mRNA of siRNA duplex was synthesized as a negative control by Bioneer Corporation. The cells were transfected using OligofectAMINE (Invitrogen, Inc.) at 100 nmol/l concentration of siRNA.
Detection of drug sensitivity
SGC7901/VCR or SGC7901 cells were inoculated into 6-well culture plates, and co-cultured with adriamycin (ADR, 20 μg/ml), vincristine (VCR, 20 μg/ml), etoposide (VP-16, 100 μg/ml), 5-Fluorouracil (5-FU, 50 μg/ml), and cisplatin (CDDP, 100 μg/ml) for 72 h. Then, drug sensitivity of these cells was determined using MTT assay as previously described [25]. The degree of resistance was detected by dividing the IC50 for the MDR cells by that of the parental sensitive cells; and the reverse effect of MDR was evaluated by dividing the IC50 of the anticancer drug in the existence of AP-2α by that transfected with the pcDNA3.1/AP-2α. Results were represented as the mean of three separate experiments each performed in quadruplicate.
Flow cytometric analysis of cell apoptosis
VCR (2 μg/ml) was added to SGC7901/VCR, SGC7901/VCR+pcDNA3.1, SGC7901/VCR+AP-2α, SGC7901/VCR+si-Ctrl or SGC7901/VCR+si-AP-2α cells. Then, 48 h later, cells were harvested and re-suspended in 2.5 ml phosphate buffered solution (PBS). After incubation with a mixture containing Annexin V-FITC and propidium iodide (Roche Diagnostics, PI, USA) in binding buffer for 15 min in the dark, the stained cells were counted using flow cytometry (Mountain View, BD, USA). Annexin V-FITC binds to cells that express phosphatidylserine on the outer layer of the cell membrane, and (PI) stains the cellular DNA of cells with a compromised cell membrane.
Flow cytometry analysis of accumulation of ADR
Fluorescence intensity of intracellular ADR was determined by flow cytometry as described previously [26]. Briefly, SGC7901/VCR, SGC7901/VCR + pcDNA3.1, SGC7901/VCR+AP-2α or SGC7901/VCR+AP-2α+Numb cells were seeded into 6-well plates (1 × 106 cells/well) and cultured overnight at 37 °C. After adding ADR at a final concentration of 5 μg/ml for 1 h, cells were then either harvested to detect ADR accumulation or continued being cultured in a drug-free medium for another 2 h to detect ADR retention. After washing with cold PBS three times, the mean fluorescence intensity of intracellular ADR was detected using flow cytometry under an excitation wavelength of 488 nm and an emission wavelength of 575 nm.
Statistical analyses
Each experiment was repeated three times. The data are expressed as means ± SD. The difference between means was analyzed by Student’s t test. All statistical analyses were performed using SPSS 19.0 software. A value of p < 0.05 was considered to be statistically significant.
Results
AP-2α expression is markedly decreased in the drug-resistant human GC SGC7901/VCR cells
To determine whether AP-2α is involved in the development of MDR in GC cells, AP-2α mRNA and protein expression levels were detected by RT-PCR and Western blot, respectively, in the non-malignant gastric epithelial cell line GES-1, gastric cancer cell lines (BGC-823, SGC-7901, AGS, MKN-45) and their MDR variants (SGC7901/VCR and SGC7901/ADR). Compared with that in GES-1 cells, the levels of AP-2α were markedly decreased in gastric cancer cell lines (Fig. 1a), most significantly in SGC-7901 cells. Furthermore, we found that the level of AP-2α in SGC7901/VCR and SGC7901/ADR cells was lower than that in SGC7901 cells (Fig. 1b). As predicted, the level of AP-2α in SGC7901/VCR cells was increased after the transfection of pcDNA3.1/AP-2α, while that was decreased after siRNA-AP-2α transfection (Fig. 1c). Moreover, the level of AP-2α in SGC7901 cells was also increased after the transfection of pcDNA3.1/AP-2α (Fig. 1d). Thus, the abnormal expressed AP-2α in SGC7901/VCR cells indicates that AP-2α may be involved in the MDR of gastric cancer cells.
Down-regulated levels of AP-2α in gastric cancer cells. a Relative levels of AP-2α in the gastric cells (BGC-823, SGC-7901, AGS, MKN-45) and non-malignant gastric epithelial cells (GES-1) were detected by RT-PCR and Western blot. b Relative levels of AP-2α in the SGC7901, SGC7901/VCR, and SGC7901/ADR cells were detected by RT-PCR and Western blot. c RT-PCR and Western blot analysis of AP-2α levels in SGC7901/VCR cells transfected with an AP-2α expression vector or an AP-2α silence vector. d RT-PCR and Western blot analysis of AP-2α levels in SGC7901 cells transfected with an AP-2α expression vector (n = 3/group, * P < 0.05 versus GES group in A; * P < 0.05 versus SGC-7901 group in B; * P < 0.05 vs. pcDNA3.1 group, ^P < 0.05 versus si-Ctrl group in C; * P < 0.05 versus pcDNA3.1 group in d)
Effects of AP-2α on the MDR in SGC7901/VCR cells
The effect of AP-2α on the resistant phenotype of gastric cancer cells was investigated using the MTT assay. The IC50 values of the anti-cancer drugs in sensitive and resistant cells after transfection with pcDNA3.1/AP-2α were determined and analyzed. It could be seen that the overexpression of AP-2α decreased the IC50 values of 5-FU, VCR, ADR and CDDP in SGC7901/VCR cells, while the silence of AP-2α enhanced the IC50 values of these drugs in SGC7901/VCR cells. However, AP-2α had no significant effect on the cytotoxicity of the anti-cancer drugs in SGC7901 cells (Table 2).
Effects of AP-2α on the cell cycle and apoptosis of VCR-induced SGC7901/VCR
The development of drug resistance in various cancer cells has been linked to a reduced susceptibility for drug-induced apoptosis, at least in some cases [27, 28]. We therefore investigated the capacity of SGC7901/VCR cells to undergo VCR-induced cell cycle profile and apoptosis by flow cytometry. The population of cells in G0/G1 phase in pcDNA3.1/AP-2α transfected cells was statistically increased when compared to cells in the VCR group, While there was no obvious change in the cell cycle of siRNA-AP-2α transfected cells compared with siRNA-Ctrl cells (Fig. 2a, b). Moreover, the flow cytometry showed that the percentage of apoptosis cells was increased in pcDNA3.1/AP-2α transfected and reduced in siRNA-AP-2α transfected SGC7901/VCR cells when compared with that in solely VCR-induced SGC7901/VCR cells (Fig. 3a). The increased expression of p53, Bax and the decreased expression of Bcl-2 were detected in pcDNA3.1/AP-2α transfected SGC7901/VCR cells after treatment with VCR, indicating that AP-2α promoted VCR-induced apoptosis of SGC7901/VCR cells. On the contrary, siRNA-AP-2α transfection reduced the expression of p53, Bax and increased the expression of Bcl-2 in SGC7901/VCR cells (Fig. 3b). These results demonstrated that AP-2α could reverse the MDR phenotype to GC cells by blocking the cell cycle and inhibiting cell apoptosis.
Overexpressed AP-2α induces cell cycle arrests in G0/G1 phases in VCR-induced SGC7901/VCR cells. SGC7901/VCR cells were transfected with pcDNA3.1/AP-2α or AP-2α siRNA after treatment with VCR. a Cell circle distributions were detected by flow cytometry analysis. b Quantification of the cell cycle phase in SGC7901/VCR cells. (* P < 0.05 vs. VCR+pcDNA3.1 group)
Overexpressed AP-2α promotes VCR-induced SGC7901/VCR cell apoptosis. SGC7901/VCR cells were transfected with pcDNA3.1/AP-2α or AP-2α siRNA after treatment with VCR. a Cell apoptosis was measured by flow cytometry. b The levels of p53, Bax and Bcl-2 were measured by western blot and RT-PCR. (n = 3/group, * P < 0.05 vs. control group, # P < 0.05 versus VCR + pcDNA3.1 group, ^P < 0.05 versus VCR + si-Ctrl group)
Overexpressed AP-2α modulates the expression of P-gp by inhibiting the Notch pathway
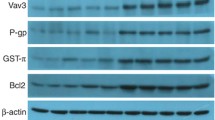
In order to explore the underlying molecular mechanism of the MDR reversion mediated by AP-2α, we analyzed the endogenous expression of marker proteins of the Notch pathway (Cleaved Notch1 and Hes-1) and multidrug resistance associated proteins (P-gp and MRP1). Cells stably expressing AP-2α (SGC7901/AP-2α) showed a clear decrease in Cleaved Notch1, Hes-1, P-gp and MRP1 mRNA and protein expression levels, while the stable expression of Numb (Notch inhibitor) in SGC7901/VCR cells caused a similar attenuation in the mRNA and protein expression levels of those molecules (Fig. 4a). Moreover, since MDR in cancer cells is mainly due to alterations in drug influx and efflux, we explored the intracellular accumulation of ADR. The fluorescence intensity of ADR was markedly increased in pcDNA3.1/AP-2α transfected SGC7901/VCR cells when compared to cells in the control or VCR group (Fig. 4b). Similar results can be observed in Numb-transfected SGC7901/VCR cells (Fig. 4b). In addition, flow cytometry analysis showed that the effects of Numb on cell cycle and apoptosis were similar with that of AP-2α (Fig. 4c, d). These results indicate that AP-2α can reverse the MDR of gastric cancer cells (SGC7901) through inhibiting the Notch pathway.
Overexpressed AP-2α modulates the expression of P-gp by inhibiting the Notch pathway. SGC7901/VCR cells were transfected with pcDNA3.1, pcDNA3.1/AP-2α or pcDNA3.1/Numb after treatment with VCR. a Relative levels of AP-2α, Numb, Cleaved Notch1, Hes-1, P-gp and MRP1 were measured by western blot and RT-PCR. b The fluorescence intensity of intracellular ADR was measured with flow cytometry. c Cell cycle and d cell apoptosis were detected by flow cytometry analysis. (n = 3/group, * P < 0.05 versus VCR + pcDNA3.1 group)
Discussion
At present, chemotherapy still remains an important treatment option in the multi-disciplinary treatment strategy against carcinoma. Multidrug resistance (MDR) is a cross-resistance developed by tumor cells to a series of structurally and functionally uncorrelated drugs, which has been reported to be a major cause of chemotherapy failure in carcinoma [29]. Thus, a therapeutic strategy that can conquer MDR will be a great progress of clinical treatment of gastric carcinoma. A previous study reported that Sunitinib reversed MDR in gastric cancer cell lines though modulating STAT3 and inhibiting P-gp function [29]. In addition, in transgenic mouse studies, overexpression of AP-2α in the mammary epithelium led to impaired mammary gland growth caused by a reduction in proliferation and a simultaneous increase in apoptosis [30]. This fits the data where silencing AP-2α resulted in a decrease in apoptosis and an increased resistance towards chemotherapeutic drugs in vitro [31]. Furthermore, AP-2α is involved in chemo-resistance in colorectal cancer [32]. Although single AP-2α has been implicated in chemo-sensitivity in various types of cancers, its affect in MDR of gastric cancer is far from being understood. Wei Wang et al. [12] found that AP-2α level was down-regulated in gastric adenocarcinoma patients compared with the corresponding normal tissues. In this study, we focused on investigating the role of AP-2α in drug resistance and the potential molecular mechanisms at play in GC. We found that AP-2α mRNA and protein expression was significantly reduced in gastric cancer cell lines (BGC-823, SGC-7901, AGS, MKN-45) compared to that in the gastric epithelia cell line GES-1. Interestingly, the levels of AP-2α in SGC7901/VCR and SGC7901/ADR cells were decreased compared with their parental cells. These results suggest a vital role for AP-2α in the MDR of GC cells in culture. Then the vector overexpressing AP-2α (pcDNA3.1/AP-2α) and silencing AP-2α (siRNA AP-2α) were used to perform AP-2α gain-of-function studies in SGC7901/VCR cells.
Having shown that AP-2α level was down-regulated in SGC7901/VCR cells, its effect on the resistant phenotype of gastric cancer cells was investigated using the MTT assay. Overexpression of AP-2α induced a resensitization of SGC7901/VCR cells to VCR, ADR, 5-FU, and CDDP compared with control cells, while silence of AP-2α reduced the sensitization of SGC7901/VCR cells to those drugs, as indicated by an in vitro drug sensitivity assay.
To explain our observation, we investigated the effects of AP-2α on the cell cycle and apoptosis. We found that AP-2α prevented cell progression from G0 into G1 phase and blocked cell proliferation, which is consistent with previous studies conducted in several human cancer cell lines [33]. Moreover, our results showed an increased percentage of apoptosis cells and levels of p53 and Bax/Bcl-2 ratio after AP-2α was transfected in VCR-induced SGC7901/VCR cells, while the percentage of apoptosis, levels of p53 and Bax/Bcl-2 ratio were decreased after siRNA-AP-2α transfection, indicating that AP-2α promoted VCR-induced apoptosis of SGC7901/VCR cells.
In the past, ATP-binding cassette (ABC) transporters have been associated with the MDR of various cancer cells. These transporters can pump cytotoxic drugs out of cells in an ATP-dependent manner [34]. At present, many ABC transporters have been identified [35], including P-glycoprotein (P-gp, ABCB1) and the multidrug resistance-associated protein 1 (MRP1, ABCC1) [36]. Elevated expression of P-gp and MRP1 has been involved in the MDR of breast, prostate and bladder cancers, resulting in low survival rates and poor patient prognoses [37, 38]. The expression of MRP1 might be correlated with the MDR reversion of Tetramethylpyrazine in human bladder cancer [39].
A previous study indicated that the MRP1 protein was down-regulated in osteosarcoma cells by Notch protein repression [13]. The Notch signaling pathway is closely related and participate in drug resistance of tumors. Interestingly, it has showed that AP-2α could affect cellular function by mediating Notch signaling [21]. Therefore, to explore the underlying molecular mechanism of the MDR reversion mediated by AP-2α, we analyzed the endogenous expression of P-gp and MRP1 and marker proteins of the Notch pathway. We found that AP-2α decreased the expression of P-gp, MRP1, Hes-1 and Notch1 mRNA and protein expression, while exogenous Numb (Notch inhibitor) caused an attenuation in those protein mRNA and protein expression. Then we used ADR as a probe to evaluate drug accumulation in GC cells in order to investigate the mechanisms of MDR mediated by AP-2α. Besides, the fluorescence intensity of ADR markedly increased in SGC7901/VCR cells transfected with AP-2α compared to cells in the control group. Thus, our results indicate that AP-2α may reverse the MDR of gastric cancer cells through inhibiting the Notch pathway.
In conclusion, our results suggest that AP-2α might reverse MDR in gastric cancer cells by directly inhibiting the Notch pathway, leading to an increased intracellular level of anti-cancer drugs and multidrug resistance proteins (P-gp and MRP1). The recent advantages in AP-2 research suggest that expanded knowledge of different AP-2 proteins may offer new strategies for cancer treatment and prognosis in the future.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F (2006) The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr Drug Targets 7:893–909
Zhang D, Fan D (2010) New insights into the mechanisms of gastric cancer multidrug resistance and future perspectives. Future Oncol 6:527–537. doi:10.2217/fon.10.21
Noguchi K, Katayama K, Sugimoto Y. (2014) Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenom Pers Med 7:53–64. doi:10.2147/PGPM.S38295
Hong L, Piao Y, Han Y et al (2005) Zinc ribbon domain-containing 1 (ZNRD1) mediates multidrug resistance of leukemia cells through regulation of P-glycoprotein and Bcl-2. Mol Cancer Ther 4:1936–1942. doi:10.1158/1535-7163.MCT-05-0182
Huang S, Chen M, Shen Y et al (2012) Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett 315:198–205. doi:10.1016/j.canlet.2011.10.011
Zhu W, Xu H, Zhu D et al (2012) miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol 69:723–731. doi:10.1007/s00280-011-1752-3
Karjalainen JM, Kellokoski JK, Eskelinen MJ, Alhava EM, Kosma VM (1998) Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J Clin Oncol 16:3584–3591
Motley AM, Berg N, Taylor MJ et al (2006) Functional analysis of AP-2 alpha and mu2 subunits. Mol Biol Cell 17:5298–5308. doi:10.1091/mbc.E06-05-0452
Eckert D, Buhl S, Weber S, Jager R, Schorle H (2005) The AP-2 family of transcription factors. Genome Biol 6:246. doi:10.1186/gb-2005-6-13-246
Pellikainen JM, Kosma VM (2007) Activator protein-2 in carcinogenesis with a special reference to breast cancer—a mini review. Int J Cancer 120:2061–2067. doi:10.1002/ijc.22648
Wang W, Lv L, Pan K et al (2011) Reduced expression of transcription factor AP-2alpha is associated with gastric adenocarcinoma prognosis. PLoS ONE 6:e24897. doi:10.1371/journal.pone.0024897
Li C, Guo D, Tang B, Zhang Y, Zhang K, Nie L. (2016) Notch1 is associated with the multidrug resistance of hypoxic osteosarcoma by regulating MRP1 gene expression. Neoplasma. doi:10.4149/neo_2016_510
Radtke F, Raj K (2003) The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer 3:756–767. doi:10.1038/nrc1186
Stoyanova T, Riedinger M, Lin S et al (2016) Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc Natl Acad Sci USA 113:E6457–E6466. doi:10.1073/pnas.1614529113
Sun L, Liu M, Sun GC et al (2016) Notch signaling activation in cervical cancer cells induces cell growth arrest with the involvement of the nuclear receptor NR4A2. J Cancer 7:1388–1395. doi:10.7150/jca.15274
Wael H, Yoshida R, Kudoh S, Hasegawa K, Niimori-Kita K, Ito T (2014) Notch1 signaling controls cell proliferation, apoptosis and differentiation in lung carcinoma. Lung Cancer 85:131–140. doi:10.1016/j.lungcan.2014.05.001
Osipo C, Patel P, Rizzo P et al (2008) ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene 27:5019–5032. doi:10.1038/onc.2008]
Zou W, Ma X, Hua W, Chen B, Cai G (2015) Caveolin-1 mediates chemoresistance in cisplatin-resistant ovarian cancer cells by targeting apoptosis through the Notch-1/Akt/NF-kappaB pathway. Oncol Rep 34:3256–3263. doi:10.3892/or.2015.4320
Kim B, Stephen SL, Hanby AM et al (2015) Chemotherapy induces Notch1-dependent MRP1 up-regulation, inhibition of which sensitizes breast cancer cells to chemotherapy. BMC Cancer 15:634. doi:10.1186/s12885-015-1625-y
Kantarci H, Edlund RK, Groves AK, Riley BB (2015) Tfap2a promotes specification and maturation of neurons in the inner ear through modulation of Bmp, Fgf and notch signaling. PLoS Genet 11:e1005037. doi:10.1371/journal.pgen.1005037
Chen Z, Zhang L, Xia L et al (2014) Genomic analysis of drug resistant gastric cancer cell lines by combining mRNA and microRNA expression profiling. Cancer Lett 350:43–51. doi:10.1016/j.canlet.2014.04.010
Zhang XF, Pan QZ, Pan K et al (2016) Expression and prognostic role of ubiquitination factor E4B in primary hepatocellular carcinoma. Mol Carcinog 55:64–76. doi:10.1002/mc.22259
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Wang Y, Liu L, Liu X et al (2013) Shugoshin1 enhances multidrug resistance of gastric cancer cells by regulating MRP1, Bcl-2, and Bax genes. Tumour Biol 34:2205–2214. doi:10.1007/s13277-013-0758-3
Hong L, Wang J, Han Y et al (2007) Reversal of multidrug resistance of vincristine-resistant gastric adenocarcinoma cells through up-regulation of DARPP-32. Cell Biol Int 31:1010–1015. doi:10.1016/j.cellbi.2007.03.020
Yuan CH, Horng CT, Lee CF et al (2016) Epigallocatechin gallate sensitizes cisplatin-resistant oral cancer CAR cell apoptosis and autophagy through stimulating AKT/STAT3 pathway and suppressing multidrug resistance 1 signaling. Environ Toxicol. doi:10.1002/tox.22284
Li D, Zhou L, Huang J, Xiao X. (2016) Effect of multidrug resistance 1/P-glycoprotein on the hypoxia-induced multidrug resistance of human laryngeal cancer cells. Oncol Lett 12:1569–1574. doi:10.3892/ol.2016.4749
Zhang Y, Wang Q (2013) Sunitinib reverse multidrug resistance in gastric cancer cells by modulating Stat3 and inhibiting P-gp function. Cell Biochem Biophys 67:575–581. doi:10.1007/s12013-013-9544-5
Zhang J, Brewer S, Huang J, Williams T (2003) Overexpression of transcription factor AP-2alpha suppresses mammary gland growth and morphogenesis. Dev Biol 256:127–145
Wajapeyee N, Raut CG, Somasundaram K (2005) Activator protein 2alpha status determines the chemosensitivity of cancer cells: implications in cancer chemotherapy. Cancer Res 65:8628–8634. doi:10.1158/0008-5472.CAN-05-1059
Ebert MP, Tanzer M, Balluff B et al (2012) TFAP2E-DKK4 and chemoresistance in colorectal cancer. N Engl J Med 366:44–53. doi:10.1056/NEJMoa1009473
Wajapeyee N, Somasundaram K (2003) Cell cycle arrest and apoptosis induction by activator protein 2alpha (AP-2alpha) and the role of p53 and p21WAF1/CIP1 in AP-2alpha-mediated growth inhibition. J Biol Chem 278:52093–52101. doi:10.1074/jbc.M305624200
Choudhuri S, Klaassen CD (2006) Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int J Toxicol 25:231–259. doi:10.1080/10915810600746023
Choi CH. (2005) ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int 5:30. doi:10.1186/1475-2867-5-30
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234. doi:10.1038/nrd1984
Louisa M, Soediro TM, Suyatna FD (2014) In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac J Cancer Prev 15:1639–1642
Chen YL, Yang TY, Chen KC, Wu CL, Hsu SL, Hsueh CM. (2016) Hypoxia can impair doxorubicin resistance of non-small cell lung cancer cells by inhibiting MRP1 and P-gp expression and boosting the chemosensitizing effects of MRP1 and P-gp blockers. Cell Oncol (Dordr). doi:10.1007/s13402-016-0285-5
Wang S, Lei T, Zhang M (2016) the reversal effect and its mechanisms of tetramethylpyrazine on multidrug resistance in human bladder cancer. PLoS ONE 11:e0157759. doi:10.1371/journal.pone.0157759
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lian, W., Zhang, L., Yang, L. et al. AP-2α reverses vincristine-induced multidrug resistance of SGC7901 gastric cancer cells by inhibiting the Notch pathway. Apoptosis 22, 933–941 (2017). https://doi.org/10.1007/s10495-017-1379-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1379-x