Abstract
Cutaneous leishmaniasis treatment remains challenging due to the absence of a satisfactory treatment. The screening of natural compounds is a valuable strategy in the search of new drugs against leishmaniasis. The sesquiterpene (−)-α-bisabolol is effective in vivo against visceral leishmaniasis due to Leishmania infantum, but its mechanism of action remains elusive. The aim of this study is to validate this promising compound against the causative species of Old World cutaneous leishmaniasis and to get an insight into its antileishmanial mode of action. The compound was evaluated on L. tropica promastigotes and intracellular amastigotes using bone marrow-derived macrophages and its cytotoxicity was evaluated on L929 fibroblasts. The reactive oxygen species generation was evaluated using a sensitive probe. Mitochondrial depolarization was assessed evaluating the fluorescence due to rhodamine 123 in a flow cytometer. Apoptosis was investigated by measuring the fluorescence due to annexin V and propidium iodide in a flow cytometer. The ultrastructure of treated promastigotes and intracellular amastigotes was analysed through transmission electron microscopy. (−)-α-Bisabolol was active against L. tropica intracellular amastigotes displaying an inhibitory concentration 50 % of 25.2 µM and showing low cytotoxicity. This compound induced time and dose-dependent oxidative stress, mitochondrial depolarization and phosphatidilserine externalization (a marker of apoptosis). These effects were noticed at a low concentration and short exposure time. In the ultrastructural analyses, the treated parasites showed mitochondrial disruption, presence of electron-dense structures and chromatin condensation. These results suggest that this natural compound induces oxidative stress and mitochondrial-dependent apoptosis on Leishmania without disturbing the plasma membrane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cutaneous leishmaniasis (CL) is a parasitic disease caused by protozoa of the genus Leishmania. The disease is endemic in more than 70 countries across the world and its incidence is estimated in about 1.5 million new cases per year [1]. The spectrum of CL clinical manifestations is very wide and range from small cutaneous nodules to mucosal tissue destruction, some of them being seriously handicapping. In the Old World, CL is mainly due to parasites of the L. tropica complex, which extends from northwestern Africa to Middle-East with an extension to Europe (Greece) and L. major, with a geographical range from West Africa to the Middle East and India [2]. L. infantum is the only species that cause both cutaneous and visceral leishmaniasis although the cutaneous form is usually underdiagnosed [3, 4]. Furthermore, L. infantum is the most widely distributed species, being the only one present both in the New World (syn. L. chagasi) and in the Old World. In addition, the other viscerotropic species, L. donovani, can occasionally be responsible for CL and L. aethiopica is responsible for a rural CL mostly in the Ethiopian highlands and occasionally in Kenya [2]. There is currently no satisfactory treatment for CL [5], and the decision-making in clinical practice is utterly individualized, depending on the geographical area, the species responsible and the form of the disease. In addition, the effectiveness of current drugs against CL, mainly antimonials and miltefosine, varies from 55 to 98 % depending on those factors. Pentavalent antimonial drugs can have serious, although usually reversible, side-effects (e.g., musculoskeletal pain, renal failure, hepatotoxicity, and cardiotoxicity), and are of variable efficacy against mucosal leishmaniasis (Reithinger et al. 2007) in a scenario where drug-resistant parasite strains are increasing. Need for a new treatment against CL includes a safe, effective and affordable treatment that can be feasibly administered in the field.
The non-profit organisation Drugs for Neglected Tropical Diseases Initiative (DNDi) decided to focus on development of treatment for CL that is predominantly caused by L. tropica in the Old World and L. braziliensis species, given their severity and public health importance [6]. CL due to L. tropica is an anthroponotic disease, thereby being the treatment of cases essential for controlling transmission. In addition, a treatment effective against these species is likely to be active against other.
The evaluation of plant-derived compounds is a promising strategy among the different approaches used in the discovery of new active compounds against leishmaniasis. In this regard, the monocyclic sesquiterpene (−)-α-bisabolol has been proven effective against Leishmania spp. in vitro [7–10] and in vivo against L. infantum in a murine visceral leishmaniasis model through oral route without showing toxicity [10]. This compound is found in Matricaria chamomilla essential oil, as well as in other plants, at up to 50 % concentrations and it is widely used in cosmetic and dermatologic preparations due to its good organoleptic properties and its anti-inflammatory and microbicidal activities [11, 12]. The toxicity of (-)-α-bisabolol has been evaluated in several laboratory animals for its safety assessment, including dogs, in which it resulted non-toxic when administered orally [LOAEL (Lowest-observed-adverse-effect level) 2 mL/kg] [13]. This sesquiterpene is effectively absorbed through oral and topical route [14] and shows a good cytochrome P450 inhibitory profile, since it is a weak inhibitor of CYP1A2, CYP2C9 and CYP3A4 isoforms, and a moderate inhibitor of CYP2D6 [15].
The mechanism of action of (−)-α-bisabolol is not clear: whereas some authors have put forward a possible inhibition of ergosterol biosynthesis in fungi [16], others have suggested mitochondrial damage [8] a mechanism previously reported in cancer cells [17].
The aim of this study is to evaluate the activity of (-)-α-bisabolol against the main causative agents of Old World CL, L. tropica and L. major, and to provide an insight into the mode of action of this natural compound, disclosing the events that lead to parasite death, particularly regarding a possible mitochondrial impairment.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS), (-)-α-bisabolol (≥95 %, GC), 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), propidium iodide (PI), 2′,7′-Dichlorodihydrofluorescein diacetate (H2DCFDA), Rhodamine-123 (rh123), cyclosporine A (CsA) and Hank’s balanced salt solution (HBSS) were purchased from Sigma-Aldrich (St. Louis, United States); Annexin V-FITC (AV) was purchased from BioLegend (San Diego, United States); pentamidine isethionate (Pentacarinat®) and meglumine antimoniate (Glucantime®) were purchased from Sanofi (France).
Parasites
The L. tropica (MHOM/MA/1988/LEM1314) and L. major (MRHO/SU/1959/LEM129) strains were maintained in RPMI-1640 medium supplemented with 10 % FBS at 26 °C.
Animals
4–6 week-old female Swiss (ICR CD-1) mice were purchased from Harlan Iberica (Barcelona, Spain). They were properly housed under 12 h light-cycles and provided with water and food ad libitum.
Cell cultures and culture media
L929 fibroblasts (ECACC 85011425) were purchased from the Cell Bank of University of Granada. They were cultured in RPMI-1640 medium supplemented with 10 % FBS at 37 °C 5 % CO2. They were used for obtaining L929 Cell Conditioned Medium (LCCM) and in cytotoxicity assays. Bone marrow-derived macrophages (BMM) were obtained as undifferentiated bone marrow cells from bone marrows of healthy ICR (CD-1) mice. They were differentiated into macrophages by culturing them in RPMI-1640 supplemented with 20 % FBS, 30 % LCCM at 37 °C 5 % CO2, as previously described [18]. For in vitro experiments, macrophages were cultured in RPMI-1640 supplemented with 20 % FBS, 5 % LCCM at 37 °C 5 % CO2.
Assay on promastigotes
5 × 105 L. tropica log-phase promastigotes were incubated in culture medium with (−)-α-bisabolol at increasing concentrations (5, 10, 25, 50, 100 and 250 µM), vehicle (DMSO 0.5 %) or pentamidine isethionate (1 µM) for 48 h. Then, they were washed and resuspended in PBS at 5 mg/mL MTT for 3 h in the dark. The formazan yielded was resuspended in acid isopropanol (HCl 0.04 M) and the absorbance of converted dye is measured at a wavelength of 570 nm (using background subtraction at 690 nm). IC50 was calculated using linear regression. Three independent experiments were performed in triplicate.
Intracellular amastigote assay
BMM were distributed in 24-wells plates with round coverslips (4 × 105 cells/well) and infected with L. tropica or L. major stationary-phase promastigotes (parasite: BMM ratio, 10:1) for 2 h, then washed and incubated with (−)-α-bisabolol at increasing concentrations (5, 10, 25, 50, 100 and 250 µM), vehicle (DMSO 0.5 %), pentamidine isethionate (0.1, 0.2, 0.5, 1, 2 and 5 µM) or meglumine antimoniate (20, 40, 80 160 and 320 µM) for 48 h. The coverslips with macrophages were washed, fixated with methanol and stained with Giemsa. The percentage of infection was quantified with optical microscopy. Macrophages were considered infected with at least one amastigote inside. A linear regression analysis was performed in order to establish a dose-response relationship and estimate the IC50. Three independent experiments were performed in triplicate.
Cytotoxicity assay
L929 fibroblasts were distributed in 24-wells plates (5 × 104 cells/well) and incubated with diluent (DMSO 0.5 %) increasing concentrations of (−)-α-bisabolol (100, 250, 500, 1000, 1250 and 1500 µM), pentamidine isethionate (0.5, 1, 2, 5, 10 and 20 µM) or meglumine antimoniate (50, 100, 150, 200 and 500 µM). After 72 h cells were collected and incubated with Trypan Blue® (0.4 %) in culture medium (1:1). After a few minutes, alive and dead cells were counted in haemocytometer and a survival ratio was established for every concentration by comparing them with control groups. Cytotoxic concentration 50 % (CC50), the concentration that inhibits cell growth by 50 %, was calculated for (−)-α-bisabolol and reference drugs. Selectivity index (SI) was calculated upon the following formula: SI = CC50 × IC −150 . Three independent experiments were performed in triplicate.
Detection of reactive oxygen species (ROS) production
The generation of ROS was quantified using the cell-permeant dye H2DCFDA, which is fluorogenic after its oxidation to dichlorofluorescein. L. tropica promastigotes (1 × 107 parasites/mL) were incubated in HBSS with (−)-α-bisabolol 10, 25 and 100 µM or DMSO for 30 or 60 min. As a positive control, promastigotes were incubated in H2O2 (50 µM) for 15 min. Then, they were washed and incubated in H2DCFDA at 10 µM for 15 min and the fluorescence was measured in a fluorometer at an excitation wavelength of 485 nm and an emission wavelength of 520 nm. Three independent experiments were performed in triplicate.
Flow cytometry analyses
Flow cytometry analyses were performed in a FACS Canto II (Becton–Dickinson, San José, United States), equipped with a blue laser (488 nm). Data were collected using channels FL1 (FITC) with a BP filter (530 ± 30 nm) for fluorescein and rhodamine 123; and FL2 (IP) with a BP filter (570 ± 20 nm) for propidium iodide. Samples were analysed at 600 events/s and at least 10,000 events were taken. Data were analysed using the software Diva 8.0 (Becton–Dickinson). Three experiments were performed.
Analysis of the mitochondrial membrane potential
Mitochondrial damage was evaluated through the analysis of the mitochondrial membrane potential (ΔΨm). L. tropica promastigotes (1 × 107 parasites/mL) were incubated in HBSS with (−)-α-bisabolol at 25 and 100 µM or DMSO for 15 min, 1 h or 24 h. As a positive control, promastigotes were incubated in an uncoupler of the electron transport chain, CCCP (100 µM) for 10 min. The influence of the pre-treatment with CsA (10 µM for 1 h), a mitochondrial transition pore (MTP) blocker was also assessed. Then, they were washed and incubated in rh-123 at 1 µM for 5 min, washed twice, resuspended in PBS and analysed by flow cytometry as described above (“Flow cytometry analyses” section).
Determination of phosphatidilserine externalization
Phosphatidilserine (PS) translocation in the plasma membrane is a characteristic event of apoptosis. PS exposure was analysed by measuring FITC-conjugated Annexin V binding to the cells. L. tropica promastigotes (1 × 107 parasites/mL) were incubated in HBSS with (−)-α-bisabolol at 25 and 100 µM or vehicle for 15 min, 1 h or 24 h. The influence of the pre-treatment with CsA (10 µM for 1 h) was also assessed. Then, they were washed and incubated in 100 µL Annexin V binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4) containing 0.5 µg/mL propidium iodide and 5 µg/mL AV for 15 min, as previously described [19]. Afterwards, 400 µL annexin binding buffer were added and the fluorescence was measured by flow cytometry as described above (“Flow cytometry analyses” section). Three experiments were carried out.
Ultrastructural analysis
The structural alterations of (−)-α-bisabolol treatment were evaluated through transmission electron microscopy (TEM). L. tropica promastigotes (1 × 107 parasites/mL) were incubated in culture medium with (−)-α-bisabolol at 25 and 100 µM or vehicle for 24 h. For intracellular amastigotes, L. tropica-infected BMMs (“Intracellular amastigote assay” section) were incubated with the compound at 25 and 100 µM or vehicle in culture medium for 24 h. Then, they were washed with PBS and incubated in phosphate-buffered 2 % paraformaldehyde and 2.5 % glutaraldehyde in 0.05 M cacodilate buffer (pH 7.2) for 24 h at 4 °C. After fixing, the cell suspensions were washed in 0.1 M cacodilate buffer (pH 7.2), embedded in molten 2 % agar (Merck, Darmstadt, Germany) and post-fixed in a mixture of 1 % phosphate-buffered osmium tetroxide and 1 % potassium ferrocyanide for 1 h prior and infiltration and embedding in a propylene oxide-Epon sequence (PolyBed 812, Polysciences, Warrington, PA, USA). Thin sections were cut using an ultramicrotome (Ultracut R, Leica) and mounted on 300-mesh copper grids before staining with uranyl acetate and lead citrate. The samples were viewed using a TEM (Libra 120 Plus, Zeiss, Germany).
Results
In vitro activity and cytotoxicity of (-)-α-bisabolol
The sesquiterpene (−)-α-bisabolol was active against L. tropica promastigotes (IC50 22.6 ± 1.6 µM). Table 1 shows the activity of this natural compound against Leishmania intracellular amastigotes, which was similar to that found for the promastigote stage. This natural compound was more active against L. tropica amastigotes than against L. major amastigotes. (−)-α-Bisabolol displayed very low cytotoxicity on L929 cells, leading to high selectivity indices against both species, much higher than that found for the reference drugs (Table 1).
(−)-α-bisabolol induces oxidative stress in L. tropica promastigotes
Oxidative stress is a marker of mitochondrial dysfunction. In order to determine whether the generation of ROS is incremented in (−)-α-bisabolol treated promastigotes, these were submitted to short treatments of natural compounds. A time- and dose-dependent increase of fluorescence due to the probe H2DCFDA was noticed even at the shortest exposition time (30 min; p < 0.01, ANOVA test) and the lowest concentration used (Fig. 1). After the incubation at 100 µM for 30 min, ROS level was similar to the positive control, treated with H2O2, while after 60 min exposition the ROS level was higher (p < 0.01, ANOVA test).
(−)-α-bisabolol induces depolarization of the mitochondrial membrane
The loss of mitochondrial transmembrane potential is the hallmark of mitochondrial damage, usually brought about by its own ROS generation. In order mitochondrial damage in the parasite, promastigotes treated for 1 h with a high (−)-α-bisabolol concentration were evaluated using the rh123 dye. Figure 2 shows the effect of this sesquiterpene on the mitochondrial potential: treatment of parasites with 25 µM led to a mean decrease in the rh123 fluorescence after 15 min (Fig. 2b), a decline that was stressed after 1 h (Fig. 2c) with the appearance of two populations of cells, a pattern that remained after 24 h of treatment (Fig. 2d). Conversely, treatment with the sesquiterpene at 100 µM induced a marked decreased in the mitochondrial potential after only 15 min of exposure (Fig. 2e). Mean fluorescence at this concentration decreased steadily after 1 h (Fig. 2f) and 24 h (Fig. 2g). Pre-incubation with CsA (10 µM, 1 h; Fig. 2h) slightly preserved mitochondrial transmembrane potential from the treatment of (−)-α-bisabolol for 1 h (mean fluorescences 340 and 520, respectively).
Mitochondrial Membrane Potential. a control cells, b parasites treated with (−)-α-bisabolol at 25 µM for 15 min, c parasites treated with (−)-α-bisabolol at 25 µM for 1 h, d parasites treated with (−)-α-bisabolol at 25 µM for 24 h, e parasites treated with (−)-α-bisabolol at 100 µM for 15 min, f parasites treated with (−)-α-bisabolol at 100 µM for 1 h, g parasites treated with (−)-α-bisabolol at 100 µM for 24 h, h parasites pre-incubated with CsA for 1 h and treated with (−)-α-bisabolol at 100 µM for 1 h, i parasites treated with CCCP at 100 µM for 15 min
(−)-α-bisabolol induces PS translocation, a hallmark of apoptosis
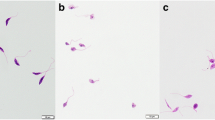
PS translocation is a characteristic event of apoptosis in protozoa, irrespective of the triggering mechanism. Figure 3 shows the flow cytometry results of L. tropica promastigotes treated with (−)-α-bisabolol: treatment with 25 µM induced the externalization of PS in 45.3 % of the cell population after 15 min (Fig. 3b), increasing to 87.4 % of cells after 1 h (Fig. 3c), while after 24 h 13.3 % of cells progressed to a late-apoptotic stage, showing high fluorescence due to PI (Fig. 3d). When treated with the highest concentration, most cells showed an apoptotic or late-apoptotic pattern after 15 min (84.3 and 11.5 %, respectively; Fig. 3e) and 1 h (65.2 and 33.8 %, respectively; Fig. 3f). After 24 h at 100 µM, 18.9 % of cells displayed necrosis and 63.6 % showed a late apoptotic pattern (Fig. 3g). Pre-incubation with CsA prevented the progression to a late-apoptotic stage after treatment with (−)-α-bisabolol for 1 h (Fig. 3h), as only 5.2 % of cells were in that stage, but most cells displayed and apoptotic pattern (90.2 %).
Analysis of PS externalization and PI internalization. X-axis shows fluorescence due to FITC-Annexin V and Y-axis shows fluorescence due to propidium iodide. Q1 necrotic cells, Q2 late apoptotic cells; Q3 normal cells; Q4 early apoptotic cells. a control cells, b parasites treated with (−)-α-bisabolol at 25 µM for 15 min, c parasites treated with (−)-α-bisabolol at 25 µM for 1 h, d parasites treated with (−)-α-bisabolol at 25 µM for 24 h, e parasites treated with (−)-α-bisabolol at 100 µM for 15 min, f parasites treated with (−)-α-bisabolol at 100 µM for 1 h, g parasites treated with (−)-α-bisabolol at 100 µM for 24 h, h parasites pre-incubated with CsA for 1 h and treated with (−)-α-bisabolol at 100 µM for 1 h
Ultrastructural alterations driven by (−)-α-bisabolol treatment
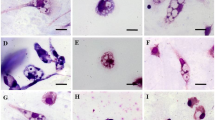
Apoptosis in protozoa is often accompanied by a variety of structural damage (cell shrinkage, mitochondrial disruption and chromatin condensation in the nucleus). Morphological changes induced by treatment with this natural compound for 24 h were investigated using transmission electron microscopy. Figure 4 shows the ultrastructural features of control promastigotes (A) and promastigotes treated with 25 µM (B and C) or 100 µM (D). Most parasites treated with the natural compound at 25 µM showed mitochondrial swelling accompanied by a disruption in the cristae. Chromatin condensation was noticed as well in these treated parasites. Other morphological changes that were not reported in the control cells were cell shrinkage, presence of vesicles inside the flagellar pocket and the existence of electron-dense structures that might correspond to lysosomes or peroxisomes. Other cell structures, such as the endoplasmic reticulum or Golgi apparatus, were not affected by treatment, including the cell membrane. Promastigotes treated with the highest concentration showed a drastic damage, what led to an “empty” cytoplasm appearance in most of them and only a swelled mitochondria and electron-dense structures were reported inside a damaged plasma membrane. These cells displayed vacuolization in the cytoplasm, membrane blebbing and chromatin condensation as well.
Ultrastructure of L. tropica promastigotes either untreated (a) or treated with 25 μM (−)-α-bisabolol (b, c) or 100 μM (−)-α-bisabolol (d). After the 25 μM treatment, the parasites show mitochondrial swelling and cristae disruption, presence of vesicles in the flagellar pocket, electron-dense structures (white asterisk) and vacuolization (white arrows). At a high dose of (−)-α-bisabolol (D), most cells display an “empty cytoplasm”, mitochondrial disruption, chromatin condensation, electron-dense structures, membrane blebbing (black arrows) and intense vacuolization (white arrows). M mitochondrion, n nucleus, fp flagelar pocket, cp cytoplasm
Figure 5 shows the ultrastructure of intracellular amastigotes. Parasites treated with 25 µM (−)-α-bisabolol displayed chromatin condensation (Fig. 5b), a swelled and disrupted mitochondrion (Fig. 5c) and intense vacuolization (Fig. 5c). Intracellular amastigotes treated with 100 µM of the compound (Fig. 5d) showed cell disruption and many of them contained large amounts of electron-dense structures.
Ultrastructure of L. tropica intracellular amastigotes either untreated (a) or treated with 25 μM (−)-α-bisabolol (b, c) or 100 μM (−)-α-bisabolol (d). After the 25 μM treatment, the parasites show mitochondrial swelling and disruption, chromatin condensation, and vacuolization (white arrows). At a high dose of (−)-α-bisabolol (d), many cells show intense cell disruption and large numbers of electron-dense structures (white asterisk). M mitochondrion, n nucleus
Discussion
(−)-α-Bisabolol has been proved as a stage-independent antileishmanial compound against L. amazonensis, L. infantum and L. donovani in vitro, and in the present study this activity was confirmed against the main causative agents of Old World CL. This sesquiterpene showed more active against these species than to the promastigote stage of L. infantum (IC50 49 µM) reported in the past [7]. Regarding the amastigote stage, its activity was similar to that displayed on promastigotes and some differences were found between the two species evaluated: (−)-α-bisabolol was more active against L. tropica than to L. major. Furthermore, these activities were higher than that found against L. infantum amastigotes and more similar to the activity displayed against L. donovani amastigotes [10]. These differences among species have been reported previously [20]. In addition, the selectivity indices obtained in this study were higher than those previously obtained for the viscerotropic species, reason why this sesquiterpene could be considered a suitable candidate for in vivo evaluation. The present results also suggest that the activity of this natural compound is stage-independent against L. tropica, reaching the amastigotes carried by the macrophages without losing activity and interacting with its target.
Kinetoplastid parasites have a unique and large mitochondrion that has been suggested as an appealing drug target given the key role mitochondria play in apoptosis. The present results indicate that (−)-α-bisabolol is an effective inducer of apoptosis in L. tropica that is confirmed by a time and dose-dependent PS translocation. This event is preceded by oxidative stress and depolarization of the mitochondrial membrane. Many drugs for the treatment of leishmaniasis, such as amphotericin B and miltefosine, induce apoptosis as a mode of action, as well as many natural compounds used for the treatment of parasitic diseases, such as artemisin [21] and other terpenes such as clerodane [22], a compound that induces mitochondrial-dependent apoptosis.
An abrupt, time and dose-dependent generation of ROS was described in the present study, even at low concentrations and short exposition times: this fact suggests that (−)-α-bisabolol induces oxidative stress, which might be due to mitochondrial damage, triggering ROS generation by the mitochondrion itself or by the endoplasmic reticulum, as reported previously [23].
(−)-α-Bisabolol induced the loss of the mitochondrial membrane potential, an event that was time and dose-dependent as well which led to the collapse of the mitochondria in all parasites treated with the highest concentration for only 15 min. Other authors have achieved 69 % of depolarised mitochondria by treating L. amazonensis promastigotes with (−)-α-bisabolol IC50 for 24 h [8], differences that might be explained by the lowest activity of this sesquiterpene on this species (IC50 36 µM). Reportedly, mitochondrial depolarisation triggers programmed cell death in mammalian cells and it is the pathway suggested for the anticancer activity of this natural compound as well [24].
In cancer cells, this terpene induces ROS generation and mitochondrial-dependent apoptosis and some authors have pointed out the opening of the mitochondrial permeability transition pore as a possible target for this compound [25]. These authors confirmed the influence of CsA (a blocker of the mitochondrial transition pore) on the anticancer activity of this sesquiterpene. However, in the present study the effect of CsA was poor, preserving slightly the mitochondrial potential in pre-treated cells and impeding the advance to a late-apoptotic stage, but without preventing apoptosis. This minor influence of CsA might be due to the differences in the mitochondrial transition pore of mammals and protozoa, implying that the target of either the sesquiterpene or CsA is not the Leishmania mitochondrial transition pore.
Variations in the mitochondrial membrane potential can be a consequence of a variety of events, such as the inhibition of the electron transport chain that leads to its decrease and to an increase of ROS formation as well. Both events were observed after treatment with this sesquiterpene, suggesting a possible inhibition of the complex II of the electron transport chain: this is supported by the fact that known complex II inhibitors drive a decrease in MMP and that the inhibition of this complex is the main source of H2DCFDA oxidation [21, 26].
Electron microscopy analyses showed a dose-dependent activity as well, progressively inducing damage in the mitochondria that led to the complete disruption of the cells at the highest concentration tested, 100 µM. Many of the damage signs noticed in treated promastigotes were observed in intracellular amastigotes as well, supporting the stage-independent activity of this compound. This damage was also characterized by the presence of electron-dense structures that might correspond to lysosomes: lysosomal damage has been recently reported as a mechanism of action of this sesquiterpene in cancer cells [27]. Membrane blebbing was found only in some of these latter cells but not in those treated with 25 µM for 24 h. These results suggest that treatment with (−)-α-bisabolol leads to a mitochondrial-dependent apoptosis without causing major damage in the plasma membrane of the parasite, in contrast to other authors who have found damage in cell membrane at 36 µM after 24 h [8].
There is no defined target for (−)-α-bisabolol in Leishmania: ergosterol biosynthesis has been suggested in the past due to the structural similarity between this natural compound and one precursor of ergosterol, zymosterol, thus inhibiting sterol C-24 methyltransferase, a reported drug target [28]. The transformation of zymosterol to fecosterol takes place in the mitochondria of S. cerevisae [29], therefore its inhibition might lead to the accumulation of zymosterol in this organelle causing stress. However, treatment with (−)-α-bisabolol did not lead to a major cell membrane disruption at any concentration tested, as shown in the apoptosis and ultrastructural analyses, what might be expected of an inhibitor of ergosterol biosynthesis, as reported for inhibitors of enzymes of the sterol biosynthesis pathway.
In conclusion, (−)-α-bisabolol is active against L. tropica and L. major amastigotes without showing cytotoxicity, what along with its pharmacological properties and topical bioavailability, makes it a promising compound for the treatment of cutaneous leishmaniasis. In addition, these findings reveal that the orally active sesquiterpene (−)-α-bisabolol induces a ROS-associated, mitochondrial-dependent apoptosis in Leishmania, what sheds light on the activity of this promising compound, facilitating further research on its molecular target.
References
Reithinger R, Dujardin J-C, Louzir H et al (2007) Cutaneous leishmaniasis. Lancet Infect Dis 7:581–596. doi:10.1016/S1473-3099(07)70209-8
Pratlong F, Dereure J, Ravel C et al (2009) Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Heal 14:1071–1085. doi:10.1111/j.1365-3156.2009.02336.x
Aliaga L, Cobo F, Mediavilla JD et al (2003) Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine (Baltimore) 82:147–158. doi:10.1097/01.md.0000076009.64510.b8
Faucher B, Pomares C, Fourcade S et al (2011) Mucosal Leishmania infantum leishmaniasis: specific pattern in a multicentre survey and historical cases. J Infect 63:76–82. doi:10.1016/j.jinf.2011.03.012
Monge-Maillo B, López-Vélez R (2013) Therapeutic options for old world cutaneous leishmaniasis and new world cutaneous and mucocutaneous leishmaniasis. Drugs 73:1889–1920. doi:10.1007/s40265-013-0132-1
Mears ER, Modabber F, Don R, Johnson GE (2015) A review: the current in vivo models for the discovery and utility of new anti-leishmanial drugs targeting cutaneous leishmaniasis. PLoS Negl Trop Dis 9:e0003889. doi:10.1371/journal.pntd.0003889
Morales-Yuste M, Morillas-Márquez F, Martín-Sánchez J et al (2010) Activity of (−)alpha-bisabolol against Leishmania infantum promastigotes. Phytomedicine 17:279–281. doi:10.1016/j.phymed.2009.05.019
Rottini MM, Amaral ACF, Ferreira JLP et al (2015) In vitro evaluation of (−)α-bisabolol as a promising agent against Leishmania amazonensis. Exp Parasitol 148:66–72. doi:10.1016/j.exppara.2014.10.001
Colares AV, Almeida-Souza F, Taniwaki NN et al (2013) In vitro antileishmanial activity of essential oil of Vanillosmopsis arborea (Asteraceae) baker. Evid Based Complement Alternat Med 2013:1–7. doi:10.1155/2013/727042
Corpas-López V, Morillas-Márquez F, Navarro-Moll MC et al (2015) (−)-α-Bisabolol, a promising oral compound for the treatment of visceral leishmaniasis. J Nat Prod 78:1202–1207. doi:10.1021/np5008697
Maurya AK, Singh M, Dubey V et al (2014) α-(−)-bisabolol reduces pro-inflammatory cytokine production and ameliorates skin inflammation. Curr Pharm Biotechnol 15:173–181. doi:10.2174/1389201015666140528152946
Forrer M, Kulik EM, Filippi A, Waltimo T (2013) The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch Oral Biol 58:10–16. doi:10.1016/j.archoralbio.2012.08.001
Bhatia SP, McGinty D, Letizia CS, Api AM (2008) Fragrance material review on alpha-bisabolol. Food Chem Toxicol 46(Suppl 1):S72–S76. doi:10.1016/j.fct.2008.06.025
Andersen FA (1999) Final report on the safety assessment of bisabolol. Int J Toxicol 18:33–40. doi:10.1177/109158189901800305
Ganzera M, Schneider P, Stuppner H (2006) Inhibitory effects of the essential oil of chamomile (Matricaria recutita L.) and its major constituents on human cytochrome P450 enzymes. Life Sci 78:856–861. doi:10.1016/j.lfs.2005.05.095
Pauli A (2006) α-Bisabolol from Chamomile—A specific ergosterol biosynthesis inhibitor? Int J Aromather 16:21–25. doi:10.1016/j.ijat.2006.01.002
Chen W, Hou J, Yin Y et al (2010) Alpha-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFkappaB. Biochem Pharmacol 80:247–254. doi:10.1016/j.bcp.2010.03.021
Zamboni DS, Rabinovitch M (2003) Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect Immun 71:1225–1233
Carvalho L, Luque-Ortega JR, López-Martín C et al (2011) The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob Agents Chemother 55:4204–4210. doi:10.1128/AAC.00520-11
Escobar P, Matu S, Marques C, Croft SL (2002) Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop 81:151–157
Fidalgo LM, Gille L (2011) Mitochondria and trypanosomatids: targets and drugs. Pharm Res 28:2758–2770. doi:10.1007/s11095-011-0586-3
Kathuria M, Bhattacharjee A, Sashidhara KV et al (2014) Induction of mitochondrial dysfunction and oxidative stress in Leishmania donovani by orally active clerodane diterpene. Antimicrob Agents Chemother 58:5916–5928. doi:10.1128/AAC.02459-14
Murphy MP (2013) Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab 18:145–146. doi:10.1016/j.cmet.2013.07.006
Cavalieri E, Mariotto S, Fabrizi C et al (2004) Alpha-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem Biophys Res Commun 315:589–594. doi:10.1016/j.bbrc.2004.01.088
Cavalieri E, Bergamini C, Mariotto S et al (2009) Involvement of mitochondrial permeability transition pore opening in alpha-bisabolol induced apoptosis. FEBS J 276:3990–4000. doi:10.1111/j.1742-4658.2009.07108.x
Cui L, Su X (2009) Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther 7:999–1013. doi:10.1586/eri.09.68
Rigo A, Vinante F (2016) The antineoplastic agent α-bisabolol promotes cell death by inducing pores in mitochondria and lysosomes. Apoptosis 21:917–927. doi:10.1007/s10495-016-1257-y
Lorente SO, Rodrigues JCF, Jiménez Jiménez C et al (2004) Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob Agents Chemother 48:2937–2950. doi:10.1128/AAC.48.8.2937-2950.2004
Thompson ED, Bailey RB, Parks LW (1974) Subcellular location of S-adenosylmethionine: Δ24 sterol methyltransferase in Saccharomyces cerevisiae. Biochim Biophys Acta Enzymol 334:116–126. doi:10.1016/0005-2744(74)90155-7
Acknowledgments
The authors wish to thank Dr. Montserrat Gállego (University of Barcelona) and Dr. Pratlong (University of Montpellier) for kindly donating the Leishmania strains used in this work and the CIC (University of Granada) for facilitating the use of his transmission electron microscope and flow cytometer.
Funding
This work was supported by the Project PI14-01024, Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Madrid and Feder Funds for Regional Development from the European Union, “One way to make Europe”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable European guidelines for the care and use of animals were followed (Directive 2010/63/EU on the protection of animals used for scientific purposes). All experiments were approved by the Ethics Committee of Animal Experimentation of the University of Granada (CEEA 455-2013).
Rights and permissions
About this article
Cite this article
Corpas-López, V., Merino-Espinosa, G., Díaz-Sáez, V. et al. The sesquiterpene (−)-α-bisabolol is active against the causative agents of Old World cutaneous leishmaniasis through the induction of mitochondrial-dependent apoptosis. Apoptosis 21, 1071–1081 (2016). https://doi.org/10.1007/s10495-016-1282-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1282-x