Abstract
Autophagic (type II) cell death has been suggested to play pathogenetic roles in cerebral ischemia. Growth arrest and DNA damage response 45b (Gadd45b) has been shown to protect against rat brain ischemia injury through inhibiting apoptosis. However, the relationship between Gadd45b and autophagy in cerebral ischemia/reperfusion (I/R) injury remains uncertain. The aim of this study is to investigate the effect of Gadd45b on autophagy. We adopt the oxygen-glucose deprivation and reperfusion (OGD/R) model of rat primary cortex neurons, and lentivirus interference used to silence Gadd45b expression. Cell viability and injury assay were performed using CCK-8 and LDH kit. Autophagy activation was monitored by expression of ATG5, LC3, Beclin-1, ATG7 and ATG3. Neuron apoptosis was monitored by expression of Bcl-2, Bax, cleaved caspase3, p53 and TUNEL assay. Neuron neurites were assayed by double immunofluorescent labeling with Tuj1 and LC3B. Here, we demonstrated that the expression of Gadd45b was strongly up-regulated at 24 h after 3 h OGD treatment. ShRNA-Gadd45b increased the expression of autophagy related proteins, aggravated OGD/R-induced neuron cell apoptosis and neurites injury. ShRNA-Gadd45b co-treatment with autophagy inhibitor 3-methyladenine (3-MA) or Wortmannin partly inhibited the ratio of LC3II/LC3I, and slightly ameliorated neuron cell apoptosis under OGD/R. Furthermore, shRNA-Gadd45b inhibited the p-p38 level involved in autophagy, but increased the p-JNK level involved in apoptosis. ShRNA-Gadd45b co-treatment with p38 inhibitor obviously induced autophagy. ShRNA-Gadd45b co-treatment with JNK inhibitor alleviated neuron cell apoptosis. In conclusion, our data suggested that Gadd45b inhibited autophagy and apoptosis under OGD/R. Gadd45b may be a common regulatory protein to control autophagy and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia–reperfusion (I/R) injury is the main cause for the aggravation of cerebral injury and functional impairment. The prevention and treatment of cerebral I/R injury have been considered pivotal strategies for stroke intervention [1]. Multiple cell signaling pathways and biological processes are involved in cerebral I/R injury, including apoptosis and autophagy [2, 3].
Autophagy, a catabolic and conserved lysosomal degradation pathway, has received much attention in ischemic disease recently [4]. Mounting evidence indicates that cerebral I/R injury induces autophagy-like cell death (also called type II programmed cell death), and the regulation of autophagy affects the results of the cerebral I/R injury [5]. Autophagy has been considered a double-edged sword with pro-survival or pro-death potential in cerebral I/R injury [6]. The inhibition of autophagy by autophagic inhibitor 3-methyladenine (3-MA) could prevent ischemic neuronal death [7–9]. However, some investigators have shown that autophagy is involved in neuroprotection in cerebral I/R injury [10, 11]. Emerging evidence indicates that the interplay of apoptosis (also called type I programmed cell death) and autophagy may be more complex than previously thought. There are many evidences that autophagy and apoptosis may share common molecular inducers and regulatory mechanisms [12–15].
Growth arrest and DNA damage response 45 beta (Gadd45b), originally termed MyD118, is involved in growth arrest and DNA repair [16]. Previously, our study indicates that Gadd45b is a beneficial mediator of neuronal apoptosis by decreasing the expression of the pro-apoptotic protein Bax and active caspase-3, and increasing the expression of the anti-apoptotic protein Bcl-2 [17]. In many circumstances, autophagy and apoptosis can coexist or occur sequentially [18]. A previous study reported that Gadd45b negatively regulated the autophagic process [19, 20]. However, whether Gadd45b regulates autophagy in the context of cerebral I/R injury is uncertain. The aim of this study was to investigate the potential involvement of Gadd45b with autophagy in the pathological process of cerebral I/R injury. In the present study, the oxygen glucose deprivation and reperfusion (OGD/R) model of cortical neuronal cells was used to mimic cerebral ischemia/reperfusion conditions in vitro [21]. To determine the effect of Gadd45b on autophagy under cerebral OGD/R, lentivirus silencing Gadd45b expression was employed. In order to pursue further the mechanisms of Gadd45b on the cultured cortical neuronal cells exposed to OGD insult, we also investigated the potential role of p38 and autophay related molecules. Finally, we investigated the effects of Gadd45b on OGD-induced cell apoptosis and neuritis injury. In this study, we showed that Gadd45b inhibited autophagy in cortical neurons upon ischemia/reperfusion stress, and significantly decreased neuronal apoptosis. Furthermore, p38 signaling might be involved in the Gadd45b-inhibited autophagy pathway. This modulation on autophagic process contributes to the neuroprotective effect of Gadd45b, indicating that Gadd45b could protect against cerebral ischemia though multiple mechanisms.
Materials and methods
Primary cortical neuron culture
All procedures on animals followed guidelines established by the Institutional Animal Care Committee and China Council on Animal Care. Rat primary cortical neuron cultures were obtained from foetal Sprague–Dawley rats at embryonic Day 17 as described previously [22, 23]. The cerebral cortex was dissected from the brain, minced, and digested with 0.125 % trypsin solution (Sigma, Cat. No. T4549) and 0.05 % DNase I (Sigma, Cat. No. DN25) at 37 °C for 20 min. Neurons were suspended in DMEM with 10 % fetal bovine serum (FBS, Gibco, Cat. No. 10099-141) to inactivate the trypsin, and filtered through a cell strainer (70 μm, BD Falcon, Cat. No. 352350). Before seeding, culture plates and coverslips were pre-coated with poly-d-lysine (Sigma, Cat. No. P0899). Neurons cells were seeded into 6-well plates (1.5 × 106 per well for Western blot and real-time PCR experiments), or on 24 mm × 24 mm coverslips (4 × 105 per well for immunocytochemistry studies) respectively. The cells were maintained in Neurobasal medium (Gibco, Cat. No. 21103-049), supplemented with 2 % B27 (Gibco, Cat. No. 17504-044), 100 U/ml penicillin/streptomycin, and 25 mM GlutaMAX (Gibco, Cat. No. 35050-061). Neuronal cultures were maintained in an incubator (5 % CO2/95 % air) at 37 °C. Medium was half changed every three days. Experiments were performed after in vitro Days (DIV) 7–12. At DIV 5, neurons were identified by staining with neuron-specific marker rabbit anti-microtubule associated protein 2 antibody (MAP2, Proteintech, Cat. No. 17490-1-AP) and mouse anti-glial fibrillary acidic protein antibody (GFAP, Proteintech, Cat. No. 60190-1-Ig) simultaneously. The percentage of MAP2 immuno-positive neurons was over 90 % by immunocytochemistry.
Induction of oxygen-glucose deprivation and reperfusion (OGD/R)
Primary cortical neurons oxygen-glucose deprivation and reperfusion is used as a model of hypoxic-ischemic insult in vitro, essentially performed as described previously [21, 24]. Briefly, at DIV 7, primary cortical neurons cultures were subjected to oxygen-glucose deprivation (OGD) in a glucose-free DMEM (Gibco, Cat. No. 11966-025) within an anaerobic chamber (0.3 % O2; 5 % CO2) at 37 °C for 3 h to mimic ischemic insult. After 3 h, OGD was terminated by incubating cells with normal culture medium and returning cells to normal conditions (95 % air; 5 % CO2) for 6, 24, 48, and 72 h at 37 °C (reperfusion, R). This resupply of glucose and oxygen is similar to the reperfusion period of ischemic injury in vivo. Cells in the control group were treated identically except that they were not exposed to OGD/R.
Neuronal viability assay and LDH secretion
To evaluate the OGD/R model, primary cortical neuron cells were seeded in 96-well plates. The cell viability was quantified by the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Cat. No. CK04). Cell injury was assessed by phase contrast microscopy and by measuring release of lactate dehydrogenase (LDH) into culture media, which corresponds to the number of damaged neurons. The LDH assay was using a LDH quantification kit (Sigma, Cat. No. 11644793001). At the indicated time, cell viability and injury were determined critically following the manufacturer’s instructions.
Lentiviral vector construction and Lentivirus infection
Silencing Gadd45b Lentiviral vector (TRC- pLKO.1 system) was ordered from Open Biosystem (Oligo Design for Target Sequence ‘TGAAGAGAGCAGAGGCAATAA’) produced with a three-plasmid system by transient transfection of 293FT cells, as previously described [25, 26]. Lentivirus scramble vector was also produced by three-plasmid transient transfection and served as a control in this study. Lentiviral vectors were produced, concentrated and titrated according to standard protocols. Briefly,for lentivirus production, 293FT cells plated to 70 % confluency were cotransfected with pLKO.1 shRNA plasmid, pCMVdr-8.91 packaging plasmid and pMD2.G envelope plasmid using Lipofectamine®3000 Transfection Reagent (Invitrogen, Cat. No. L3000). Medium was collected at 48 h and 72 h after transfection, and lentivirus was concentrated from medium by ultracentrifugation at 27,000 r.p.m. for 2.5 h. The resulting pellets were resuspended in phosphatebuffered saline (PBS, pH 7.2) and stored at −80 °C. Knockdown efficiency of shRNA-Gadd45b lentivirus was assessed on primary cortical neurons. At DIV 3, three in four of the culture medium was removed from per well of 6-well plates, and add 100 μl lentivirus, 500 μl fresh normal culture medium and 5 μg/ml polybrene (Sigma, Cat. No. AL-118). The cells were incubated for 24 h at 37 °C, and then medium was changed followed by continuous incubation for 4 days. After 4 days, neurons were harvested from each well. Successful transduction was confirmed by examining neuronal cultures for Gadd45b protein level using Western blot and mRNA expression using quantitative real time PCR on ABI Prism 7500.
Drug treatment
3-Methyladenine (3-MA, Cat. No. M9281), Wortmannin (Cat. No. W1628), JNK inhibitor (SP600125, Cat. No. S5567) and p38 MAPK inhibitor (SB203580, Cat. No. S8307) were purchased from Sigma-Aldrich and dissolved in DMSO respectively. Autophagy inhibitor 3-MA (final concentration of 10 mM) or Wortmannin (final concentration of 1 μM) was administered to the medium at the onset of reperfusion at DIV 7. SP600125 (final concentration of 10 mM) or SB203508 (final concentration of 20 mM) was administered to the medium for incubation 30 min before and through OGD/R. The cells were harvested for subsequent experiments. All cells were compared with a control group (received the same amount of DMSO).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
Cell apoptosis was assessed using TUNEL staining. TUNEL staining was performed using the In Situ Cell Death Detection kit (Roche, Cat. No. 11684817910), in accordance with the manufacturer’s instructions. Briefly, cells fixed on coverlips were treated with 0.1 % Triton X-100 for 10 min. Cells were washed in PBS and incubated with TUNEL reaction mixture at 37 °C for 1 h. Cells were then counterstained with 4,6-diamidino-2-phenylindole (DAPI). Images were taken using a fluorescent inverted microscope (Nikon) at ×400 magnification. The percentage of TUNEL-positive nuclei was quantified using Image J software. The apoptotic index (AI) was calculated as follows: AI = (number of apoptotic cells/total number counted) × 100 %.
Immunofluorescence
For double immunofluorescence, cells were fixed in 4 % paraformaldehyde for 10 min, and washed three times in PBS (5 min for each). Cells were simultaneously incubated with rabbit anti-LC3B antibody (1:50, Proteintech, Cat. No. 18725-1-AP) and mouse anti-Tuj1 antibody (1:250, Chemicon, Cat. No. MAB1637) at 4 °C overnight, treated with Alexa 594-labeled goat anti-mouse IgG and Alexa 488-labeled goat anti-rabbit IgG secondary antibody (1:500, Invitrogen) at room temperature for 2 h, washed with PBS, incubated with DAPI for nuclear stain, and then mounted with ProLong Antifade Reagents (Invitrogen). Images were obtained by a fluorescence inversion microscope (Nikon). Digital images were recorded and analyzed using Image-Pro Plus software and Adobe Photoshop software.
Western blotting
Proteins were extracted from cells in cold RIPA lysis buffer. Western blotting was performed as standard protocols. Total protein was quantified and separated on a 10 or 15 % SDS-PAGE and blotted onto PVDF membranes (Millipore). Antibodies included rabbit anti-Gadd45b (1:10000, Abcam, Cat. No. ab128920), rabbit anti-Beclin-1 (1:1000, Cell Signaling Technology, Cat. No. 3495), rabbit anti-ATG7 (1:10000, Abcam. Cat. No. ab133528), rabbit anti-ATG3 (1:10000, Abcam, Cat. No. ab108282), rabbit anti-APG5L/ATG5 (1:1000, Abcam, Cat. No. ab109490), rabbit anti-LC3 A/B (1:1000, Cell Signaling Technology, Cat. No. 4108), rabbit anti-cleaved caspase3 (1:1000, Cell Signaling Technology, Cat. No. 9664P), rabbit anti-Bcl-2 (1:500, Proteintech, Cat. No. 12789-1-AP), rabbit anti-Bax (1:2000, Abcam, Cat. No. ab32503), rabbit anti-p38 (1:1000, Cell Signaling Technology, Cat. No. 8690), rabbit anti-phospho Thr180/Tyr182-p38 (p-38, 1:1000, Cell Signaling Technology, Cat. No. 4511), rabbit anti-JNK (1:1000, Cell Signaling Technology, Cat. No. 9252), rabbit anti-phospho Thr183/Tyr185-JNK (p-JNK, 1:1000, Cell Signaling Technology, Cat. No. 4671), rabbit anti-p53 (1:1000, Abcam, Cat. No. ab183544), and mouse anti-β Tubulin (1:10000, Zhengneng Biotechnology) at 4 °C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The protein levels were normalized to β Tubulin. Immunoreactivity was detected with a Chemiluminescent reagent (Millipore) on films. Optical density of the bands was quantitatively analyzed using Image J software.
Quantitative real-time polymerase chain reaction (PCR)
SYBR green-based quantitative real time RT-PCR was used to examine changes in mRNA level of Gadd45b. RNAs prepared from primary rat cortex neuron treated as described above. Total RNAs were isolated using Trizol reagent (Invitrogen). For real-time PCR analysis, the reaction solution contained cDNA template, forward and reverse primers, and SYBR Green I Master Mix (Invitrogen) according to the manufacturer’s instructions. Reactions were conducted in the cycler (ABI Prism 7500, Life Sciences). GADPH gene was used as internal control. The primers used for PCR were as follows:
-
Gadd45b: forward: 5′-GTCACCTCCGTCTTCTTG-3′,
-
reverse: 5′-GATTCAGTCACACTTCACAG-3′
-
GADPH: forward: 5′-GCCAAAAGGGTCATCATCTC-3′
-
reverse: 5′-GTAGAGGCAGGGATGATGTTC-3′
Statistical analysis
All data were expressed as mean ± S.D. and analyzed using Graph Pad Prism 5 Software. The one way ANOVA or Student t test was used to assess differences among groups. P < 0.05 was defined as statistically significant.
Results
Oxygen glucose deprivation and reperfusion (OGD/R) injury induced Gadd45b expression
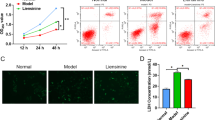
OGD/R-induced cortex neuron cell injury acts as a model to mimic cerebral I/R injury in vitro and results in neuronal insult. In our previous study, we had found that neuronal cell viability declined progressively as the reperfusion time prolonged [27]. Only 40.8 % of cells remained viable at 24 h. Meanwhile, LDH leakage was markedly increased from 3 h OGD to 72 h after reperfusion (data not shown). We also found that the expression of Gadd45b was increased after OGD/R and reached the peak level at reperfusion 24 h (data not shown) [27].
Inhibition of Gadd45b increased the expression of autophagy-related proteins after OGD/R injury
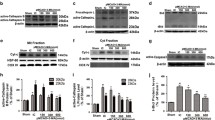
To identify the influence of Gadd45b on autophagy, we firstly examined the expression of autophagy-related molecules during OGD/R. Two biochemical markers unique to autophagy are the covalent conjugates of ATG12-ATG5 and microtubule-associated protein light chain 3 (LC3) [28]. ATG5, a key molecule involved in autophagic vacuole formation, is required for the autophagic cell death. The conversion of LC3 I (16 kDa) to LC3 II (14 kDa), indicated as “LC3 puncta processing”, yielding LC3 II used for the detection of autophagic activity [4]. As shown in Fig. 1a, a time course analysis of ATG5 and LC3 were conducted in primary cultured neurons at OGD 3 h and reperfusion 6, 24, 48, and 72 h. ATG5 antibody labeled primarily the ATG12-ATG5 conjugated form (~53 kDa) in all subcellular fractions, whereas the unconjugated (free form) ATG5 (~32 kDa) was barely detected [29]. Western blot results showed the expression of ATG5 (~53 kDa) increased significantly from 24 to 72 h after reperfusion (Fig. 1a). The ratio of LC3 II/LC3 I was increased at OGD 3 h, and lasted up to 72 h after reperfusion (Fig. 1a). These findings suggested an enhancement of neuronal autophagy in this OGD/R model. The p38 pathway plays an important role in transducing signals involved in autophagy [30]. As shown in Fig. 1a, OGD/R affected the rate of p-p38/p38.
Oxygen glucose deprivation and Reperfusion affected the expression of autophagy-related proteins and the rate of p-p38/p38 at 24 h after reperfusion. a Western blot results of autophagy-related proteins ATG5 and LC3 and p-p38. ATG5 antibody labeled mainly ATG12-ATG5 conjugates (~53 kD). LC3 antibody labeled LC3 I (16 kD) and LC3 II (14 kD). b, c, d Quantitative forms of (a). β Tubulin was used as an internal control. *p < 0.05 versus Control
Because of the expression of Gadd45b was on peak at 24 h after 3 h OGD, so we choose this point time for next study. Cells were pretreated with lentivirus shRNA-Gadd45b at DIV 3, and then followed by OGD 3 h and reperfusion 24 h at DIV 7. We prepared whole cell extracts from neuron cells, and then performed western blot and qPCR. Treatment with shRNA-Gadd45b, obviously decreased the protein expression of Gadd45b at 24 h after 3 h OGD, compared to lentivirus scramble vector (Fig. 2a). QPCR result showed that knockdown efficiency of lentivirus shRNA-Gadd45b was 92.33 % (Fig. 2c). As shown in Fig. 2d, shRNA-Gadd45b increased the expression of ATG5 and the LC3 II/LC3 I ratio at 24 h after reperfusion. We also examined other autophagy-related molecules, such as Beclin-1, ATG7 and ATG3. A significant increase in the expression of Beclin-1, ATG7 and ATG3 was observed at 24 h after reperfusion in cultured neurons treated with shRNA-Gadd45b. These results indicated that inhibition of Gadd45b increased the expression of autophagy-related proteins after OGD/R Injury.
Inhibition of Gadd45b increased the expression of autophagy-related protiens at reperfusion 24 h. Lentivirus shRNA-Gadd45b was added to the cortex neuron culture medium at DIV 3 as described in the methods. Cultured cortical neurons were exposed to OGD/R injury at DIV 7. a Note that lentivirus shRNA-Gadd45b decreased the protein level of Gadd45b at 24 h after reperfusion, as seen by western blot. b Quantitative forms of (a). c Note that lentivirus shRNA-Gadd45b decreased the mRNA expression of Gadd45b at 24 h after reperfusion, as seen by qPCR. d Western blot results showed that treatment with shRNA-Gadd45b obviously increased the expression of autophagy-related proteins (ATG5, LC3, Beclin-1, ATG7, ATG3) and decreased the expression of p-p38, compared to the lentivirus scramble vector. e Quantitative forms of (d). β Tubulin was used as an internal control for western blot; GADPH was used as an internal control for qPCR. #p < 0.05 versus vector + OGD/R
We next explored the potential mechanisms underlying the regulation of Gadd45b on autophagy in neurons under OGD/R. Gadd45b has been described as regulators of MAPK signaling, activated MEKK4 and the downstream activator of the p38 MAPK pathway to inhibit autophagy [19]. Treatment with shRNA-Gadd45b significantly decreased the p-p38 at 24 h after 3 h OGD, compared to treatment with scramble vector (Fig. 2d), whereas total p38 content was unchanged.
Inhibition of Gadd45b increased the expression of apoptosis-related proteins after OGD/R injury
Treatment with shRNA-Gadd45b significantly decreased the expression of anti-apoptotic protein Bcl-2, but increased the expression of pro-apoptotic protein Bax at 24 h post 3 h OGD (Fig. 3a). Similarly, we found that the level of cleaved caspase3 was significantly up-regulated after shRNA-Gadd45b treatment at 24 h (Fig. 3a). However, the expression of p53 was significantly down-regulated after shRNA-Gadd45b treatment at 24 h (Fig. 3a).
Treatment of shRNA-Gadd45b and JNK inhibitor modulated the expression of apoptotic key proteins. a Cortex neuron cells were treated with lentivirus shRNA-Gadd45b at DIV 3. Cells were harvested for western blot analysis at 24 h after OGD/R. Note that shRNA-Gadd45b significantly increased the expression of Bax and cleaved caspase3, but decreased the expression of Bcl-2. b Quantitative forms of (a). c The expression of p-JNK and JNK at different time points under OGD/R. d Quantitative forms of (c). e JNK inhibitor (SP) was induced as described in the methods. Inhibition of JNK decreased the expression of Bax and cleaved caspase3. ShRNA-Gadd45b co-treatment with JNK inhibitor also decreased the expression of Bax and cleaved caspase3. f Quantitative forms of (e). β Tubulin was used as an internal control. *p < 0.05 versus control, #p < 0.05 versus DMSO or vector or vector + DMSO
We next explored the potential mechanisms underlying the regulation of Gadd45b on apoptosis in neurons under OGD/R. Gadd45b had been demonstrated that protecting hematopoietic cells from UV-induced apoptosis via targeting MKK4 to mediate inhibition of JNK activation [31]. As shown in Fig. 3c, there was an increased trend of p-JNK at 24 h after 3 h OGD. ShRNA-Gadd45b significantly increased the ratio of p-JNK/JNK at 24 h (Fig. 3e). SP600125, a common JNK pathway inhibitor, obviously decreased the ratio of p-JNK/JNK at 24 h. Inhibition of JNK also decreased the expression of Bax, cleaved caspase3 and increased the expression of Bcl-2 (Fig. 3e). ShRNA-Gadd45b co-treatment with SP600125 decreased the ratio of p-JNK/JNK, and decreased the expression of Bax and cleaved caspse3, compared to vector co-treatment with DMSO (Fig. 3e). Thus, Gadd45b may through JNK signaling pathway to inhibit apoptosis in the content of OGD/R injury.
Inhibition of Gadd45b and autophagy affect apoptosis after OGD/R injury
Autophagy develops as a primary response to stress stimuli and then triggers either apoptosis or necrotic cell death that kills the cell [32]. Autophagy and apoptosis are controlled in cooperation with certain common regulatory proteins [15]. It seems that the same stimuli can induce either autophagy or apoptosis depending on the threshold [33]. To conclude that whether Gadd45b plays a role in the anti-apoptosis effects against OGD/R injury through inhibiting autophagy, we used autophagy inhibitors 3-MA and Wortmannin to treat with cortex neuron cells at the onset of reperfusion.
Firstly, we examined the effect of autophagy inhibitor on the expression of ATG5 and LC3. Inhibition of Gadd45b increased the rate of LC3 II/LC3 I and the expression of ATG5. As shown in Fig. 4a, 10 mM 3-MA and 1 µM Wortmannin significantly decreased the ratio of LC3 II/LC3 I at 24 h after OGD. 3-MA co-treatment with shRNA-Gadd45b slightly increased the ratio of LC3 II/LC3 I at 24 h. Wortmannin co-treatment with shRNA-Gadd45b decreased the ratio of LC3 II/LC3 I. It indicated that inhibition of Gadd45b also induced autophagy even after co-treatment with 3-MA. Wortmannin slightly decreased the expression of ATG5 at 24 h. However, Wortmannin co-treatment with shRNA-Gadd45b had not affected the expression of ATG5 at 24 h. Inhibition of Gadd45b decreased the expression of p-p38. Treatment with 3-MA or Wortmannin did not affect phosphorylation of p38 at 24 h after OGD (Fig. 4a). Similarly, we found that shRNA-Gadd45b co-treatment with 3-MA or Wortmannin led to inactivation of p38 as measured at 24 h.
Treatment with autophagy inhibitor or p38 inhibitor modulated the autophagic and apoptotic key proteins. Cortex neuron cells were treated with lentivirus shRNA-Gadd45b at DIV 3. Autophagy inhibiotor 3-MA or Wortmannin (Wort) and p38 inhibitor (SB) were used as described in the methods. Cells were harvested for western blot analysis at 24 h after OGD/R. a Western blot results of LC3, ATG5, p-p38,p38, Bcl-2, Bax, cleaved caspase3. Note that 3-MA and Wortmannin significantly suppressed the rate of LC3 II/LC3 I, and had no effect on p-p38. 3-MA or Wortmannin increased the Bcl-2 level and decreased the Bax level. Wortmannin also decreased the cleaved caspase3 level. 3-MA or Wortmannin co-treatment with shRNA-Gadd45b affected the expression of Bcl-2, Bax and cleaved caspase3. b Quantitative results from (a). β Tubulin was used as an internal control. #p < 0.05 versus DMSO or vector or DMSO + vector
Inhibition of Gadd45b decreased the expression of Bcl-2 and increased the expression of Bax and cleaved caspase3. Western blot results showed that 3-MA or Wortmannin increased the expression of Bcl-2 and decreased the expression of Bax under OGD/R injury (Fig. 4a). 3-MA co-treatment with shRNA-Gadd45b decreased the Bcl-2 level, and increased the cleaved caspase3 level, compared to scramble vector co-treatment with DMSO. However, there was no significant difference in the amount of Bax between the shRNA-Gadd45b + 3-MA group and Vector + DMSO group at 24 h, the levels of Bax and cleaved caspase3 exhibited a decrease trend in the shRNA-Gadd45b + 3-MA group (Fig. 4a). Wortmannin also increased the expression of Bcl-2 and decreased the expression of Bax and cleaved caspase3 under OGD/R injury. Wortmannin co-treatment with shRNA-Gadd45b did not affect the level of Bcl-2 at 24 h (Fig. 4a). However, Wortmannin co-treatment with shRNA-Gadd45b also decreased the level of Bax and cleaved caspase3.
SB203580, as a common p38 inhibitor, obviously decreased the expression of p-p38, and increased the expression of ATG5 and the ratio of LC3 II/LC3 I. SB203580 also decreased the expression of Bcl-2 and increased the expression of Bax and cleaved caspase3. SB203580 co-treatment with shRNA-Gadd45b obviously decreased the expression of Bcl-2 and increased the expression of Bax and cleaved caspase3 (Fig. 4a).
Neurons were used to detect apoptosis using TUNEL at 24 h after 3 h OGD. We found that TUNEL-positive cells were increased at 24 h (AI: 47.09 ± 8.64) (Fig. 5b), compared to normal controls (AI: 10.89 ± 0.69) (Fig. 5a). Treatment with shRNA-Gadd45b obviously aggravated the neuronal apoptosis at 24 h after OGD (80.18 ± 7.43) (Fig. 5d). Treatment with 3-MA or Wortmannin reduced apoptosis at 24 h (Fig. 5f, g). As shown in Fig. 5i, 3-MA co-treatment with shRNA-Gadd45b slightly increased the apoptosis at 24 h after OGD. However, there were no differences between 3-MA + shRNA-Gadd45b and DMSO + vector (58.28 ± 8.73). Wortmannin co-treatment with shRNA-Gadd45b decreased the apoptosis at 24 h (22.29 ± 7.14) (Fig. 5j).
Inhibition of Gadd45b and autophagy affected neuronal apoptosis in vitro at 24 h by TUNEL assay. a The TUNEL-positive cells were hardly detected in the normal cortex neurons controls. b The TUNEL-positive cells were increased at 24 h after OGD. d ShRNA-Gadd45b obviously induced the neuron cellular apoptosis at 24 h after OGD, compared to the scramble vector lentivirus (c). Treatment with 3-MA (f) or Wortmannin (g) reduced cellular apoptosis at 24 h after OGD, compared to the DMSO treatment (e). ShRNA-Gadd45b co-treatment with 3-MA (i) slight increased the neuron cellular apoptosis at 24 h after OGD, compared to the scramble vector lentivirus co-treatment with DMSO (h). ShRNA-Gadd45b co-treatment with Wortmannin (j) slight decreased the neuron cellular apoptosis at 24 h. k Quantitative forms of TUNEL results. *p < 0.05 versus control, #p < 0.05 versus DMSO or vector or DMSO + vector. Scale bar 10 µm
The immunofluorescence results of Tuj1 and LC3B
It seems that autophagy in brain ischemia might contribute to neurites degeneration [34]. These were confirmed by the recent research, where rapid and massive degeneration of cortical neuronal axons after 24 h of ischemia/hypoxia was observed [18]. Because inhibition of Gadd45b induced neuronal autophagy and apoptosis at 24 h after OGD, we further investigated whether Gadd45b could affect the integrity of neuronal structure. In addition to detect the changes of axon morphology, neurons were stained with specific antibodies Tuj1. Tuj1 is a somatodendritic marker for dendrites and axons. LC3B has been used to monitor autophagy, measured as an increase in punctate LC3 [35]. As shown in Fig. 6a, normal neurons developed normal polarity, having a single axon and multiple dendrites (Fig. 6a). The cell body and proximal end of neurites became shorten and degraded at 24 h after OGD (Fig. 6b). Treatment with shRNA-Gadd45b increased the LC3B at 24 h (Fig. 6g), and the neuron cells lost the cell polarity (Fig. 6d). 10 mM 3-MA decreased the LC3B at 24 h, and slightly improved neurites injury, showing relatively intact structure and polarity of part of neurons after OGD (Fig. 6e). 3-MA co-treatment with shRNA-Gadd45b partly inhibited autophagy induced by shRNA-Gadd45b, but had not obviously improved neurites injury (Fig. 6f).
The immunofluorescent staining results of Tuj1 and LC3B. Cultured cortical neurons were treated with shRNA-Gadd45b with/without 3-MA followed by OGD/R 24 h. After treatment, the cells were performed double immunofluorescence with anti-Tuj1 and anti-LC3B antibodies. LC3B is an autophagy marker (green), and individual axons are brightly stained with Tuj1 (red). Blue is DAPI for neuron nucleus. Green arrow showed that LC3B positive neuron cells. a LC3B was in basal level in normal neurons (green arrowheads). Normal neurons developed normal polarity, with most of them having a single axon and multiple dendrites. b, c OGD/R 24 h induced LC3B expression. The cell body and proximal end of neurite became shorten and degrade (white arrowheads) at 24 h after OGD. d ShRNA-Gadd45b obviously increased the expression of LC3B at 24 h after OGD. The neuron lost polarity after treated with shRNA-Gadd45b lentivirus. e 3-MA at 10 mM decreased the autophagy at 24 h. 3-MA slightly reduced neurite degradation, showing relatively intact structure and polarity of some neurons at 24 h. f shRNA-Gadd45b lentivirus co-treatment with 3-MA could not significant reduced the neurite injury. g Average optical density of LC3B. *p < 0.05 versus control, #p < 0.05 versus DMSO or scramble vector lentivirus. Scale bar 10 µm
Discussion
In the present study, we examined the effect of Gadd45b on autophagy and apoptosis against cerebral oxygen-glucose deprivation and reperfusion injury in vitro. The present study reveals that silencing Gadd45b lentivirus preconditioning significantly increases the expression of autophagy related proteins in the cortex neuron cells followed by oxygen-glucose deprivation and reperfusion. Gadd45b inhibited autophagy through p38 pathway. Inhibition of Gadd45b increased the pro-apoptosis protein Bax and cleaved caspase 3, and decreased the anti-apoptosis Bcl-2. 3-MA or Wortmannin treatment relieved the cerebral neuron apoptosis under OGD/R. The effect of shRNA-Gadd45b on neuron apoptosis was just the opposite to that of 3-MA or Wortmannin treatment. In addition, shRNA-Gadd45b obviously aggravated the neurites injury by morphology assay. Inhibition of autophagy mildly alleviated neurites injury at 24 h after OGD. These results suggest that the inhibition of autophagy underlies, at least partly, the neuroprotection mechanism of Gadd45b against cerebral ischemia and reperfusion. Gadd45b may be a common regulatory protein to control autophagy and apoptosis.
Cell death plays an essential role in ischemic stroke pathogenesis. Recent studies have shown autophagy activation in cerebral ischemia in vitro and in vivo [2]. Although moderate autophagy has been thought to have neuroprotection generally, accumulating evidences have documented that ischemia and reperfusion-induced autophagy leads to neuronal death following ischemic stroke [18]. Autophagy may play dual roles during ischemia and reperfusion: protective during ischemia while detrimental during reperfusion [6, 29]. Suppression of autophagy by 3-MA can block neuronal death in response to various stress conditions in the CNS such as rodent traumatic or ischemic brain injury [36]. Substantial reports have documented that ischemia-induced autophagy leads to neuronal death following ischemic stroke [2, 6, 29, 34]. In the present study, our data provided the first evidence that Gadd45b inhibited autophagy in the content of OGD/R of primary rat cortical neurons, as judged by an increase in the protein expression of ATG5, Beclin-1, ATG7, ATG3 and the ratio of LC3 II/LC3I. ATG5 plays an important role in initiating autophagosome generation. In this study, 3-MA, at the early stage of autophagosome biogenesis, partly prevented shRNA-Gadd45b-induced autophagy. Our study suggested that Gadd45b may activate signaling cascades that inhibit autophagosome generation. In addition, our results indicated that shRNA-Gadd45b increased the ratio of LC3 II/LC3 I. It could be a result of either LC3 I to LC3 II conversion or defects in LC3 II degradation. If the LC3 II level was up in response to experimental conditions compared to controls, it can be due to increased conversion of LC3-I to LC3-II as in the case of inhibition by Gadd45b. Autophagy process is mainly regulated by the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway [12]. The action of 3-MA, a phosphoratidylinositol-3-kinase (PI3K) inhibitor, has been proposed to prevent PIP3, which is important in the recruitment of Atg proteins on the autophagosome membrane [37]. Gadd45b can interact with the upstream kinase MTK1/MEKK4, and subsequently activate downstream kinase p38 [20]. The stress-responsive p38 MAPK pathways regulate cell cycle, apoptosis and mediate autophagy in response to stress. On the basis of these findings, it appears that p38 is involved in the Gadd45b signalling pathways. One study demonstrated that the spatial regulation of p38 by Gadd45b/MEKK4 negatively regulates the autophagic process [20]. In our study, we also have shown that inhibition of Gadd45b leading to p38 inactivation. We may conclude that Gadd45 inhibited autophagy through the p38 pathway under OGD/R. However, the mechanism underlying Gadd45b-mediated p38 signaling and its inhibition to autophagy required further investigation. We need to further study by special inhibitor targeting signal transduction pathway.
Excessive or dys-regulated autophagy results in neuron damage or death, known as “autophagic cell death”, also referred as type II programmed cell death in cerebral ischemia, with associated increased levels of light chain 3 (LC3)-II and Beclin-1 [38]. Apoptosis is referred as type I cell death. Autophagic pathways overlap with the apoptosis through the involvement of both autophagic and apoptotic proteins [15]. There is extensive cross-talk between autophagy and apoptosis [15]. Autophagy is carried out through interaction of various molecules such as Bcl-2, Beclin-1, Atg5 and Atg12, resulting in the activation of the intrinsic apoptosis pathway [2]. Moreover, depending on cell types, environment and stimulation manners, autophagy can precede, inhibit or enhance apoptotic cell death. However, 3-MA or suppression of autophagy regulatory pathways may provoke apoptosis, some studies have suggested that autophagy might also mediate cell death [28]. The pro-death hypothesis suggests that activation of autophagy mediates or induces cell death via over digestion of cellular contents or by activation of apoptotic enzymes. The discrepancies may be duo to the complex and diverse interactions among autophagy, apoptosis and cell death. In addition, previous study also confirmed that cells have co-localized apoptosis and autophagy [6, 37]. It seems that autophagy-apoptosis relation varies according to the cerebral area. In the cortex those processes are closely linked to each other, while in the hippocampus they rather act independently [37]. Neurons that presented both enhanced level of autophagy and apoptotic features were also observed in a rat model of severe perinatal asphyxia [28]. Previously, our study indicated that Gadd45b is a beneficial mediator of neuronal apoptosis, by affect levels of Bax and active caspase-3, and Bcl-2 [17]. In this study, we have found shRNA-Gadd45b in cultured rat cortical neurons resulted in autophagy induction, concomitant with the neuronal apoptosis in early stage of cerebral cortex neuron cells ischemia and reperfusion in vitro as mentioned above. Based on these studies, we then asked whether the regulation of Gadd45b on autophagy contributed to the anti-apoptosis effect of Gadd45b. The autophagy inhibitor 3-MA or Wortmannin protected neurons against OGD/R-induced neuronal apoptosis and neurite injury, suggesting the involvement of autophagy-mediated apoptosis. However, co-treatment with shRNA-Gadd45b and 3-MA partly protected neurons from neuronal apoptosis and neurite injury. However, we cannot exclude the possibility for 3-MA direct inhibition on apoptosis completely. Whether other factors in Gadd45b-inhibited autophagy, such as oxidative stress, or mitochondrial dysfunction, could contribute to autophagic cell death or require further investigation. In addition, ATG5 seems to be directly involved in the induction of apoptosis, as a calpain-generated fragment of ATG5 associates with Bcl-xL at mitochondria, resulting in the activation of the intrinsic apoptosis pathway [34]. Thus, ATG5 could have a key role in controlling cell fate. More specific siRNA knockdown of the Atg protein (ex: ATG5) will be performed for further study to elucidate the interaction between autophagy and apoptosis. Based on our study, Gadd45b may be a common regulatory protein to control autophagy and apoptosis.
Although inhibition of Gadd45b affected the autophagy and apoptosis under OGD/R, we found that shRNA-Gadd45b decreased the transcription factor p53 expression. One study showed that Gadd45b was a novel downstream target gene of p53 [39]. Gadd45b mRNA and protein expression were significantly inhibited by p53 siRNA in a rat ischemic heart model [39]. In addition, p38α-mediated phosphorylation of p53 at both Ser15 and Ser20 was shown to be essential for the expression of Gadd45b mRNA and protein during anoxia [39]. These results reveal the p38α-p53-Gadd45b axis as a novel signaling module in the anoxia-induced apoptotic death pathway. Whether there exists a feedback mechanism between Gadd45b and p53. The correlation of Gadd45b and p53 need further researches.
Conclusion
In summary, induced autophagy by silencing Gadd45b expression contributes to the neuronal cell apoptosis process. In other words, Gadd45b as pro-survival gene(s), is a critical mediator of ischemia/hypoxia-induced autophay death in cortex neuron cells. Our findings indicated that Gadd45b could protect cortex neuron cells against cerebral ischemia though multiple mechanisms including autophagy and apoptosis. The integrated regulation of autophagy and apoptosis determines cell fate, requires to be established in the future.
References
Su J, Zhang T, Wang K, Zhu T, Li X (2014) Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochem Res 39(11):2068–2077. doi:10.1007/s11064-014-1396-x
Xu F, Gu JH, Qin ZH (2012) Neuronal autophagy in cerebral ischemia. Neurosci Bull 28(5):658–666. doi:10.1007/s12264-012-1268-9
Kost A, Kasprowska D, Labuzek K, Wiaderkiewicz R, Gabryel B (2011) Autofagia w niedokrwieniu mózgu [Autophagy in brain ischemia]. Postepy higieny i medycyny doswiadczalnej 65:524–533
Zhang J (2015) Teaching the basics of autophagy and mitophagy to redox biologists–mechanisms and experimental approaches. Redox Biol 4:242–259. doi:10.1016/j.redox.2015.01.003
Luo T, Park Y, Sun X, Liu C, Hu B (2013) Protein misfolding, aggregation, and autophagy after brain ischemia. Transl Stroke Res 4(6):581–588. doi:10.1007/s12975-013-0299-5
Wei K, Wang P, Miao CY (2012) A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther 18(11):879–886. doi:10.1111/cns.12005
Zheng C, Han J, Xia W, Shi S, Liu J, Ying W (2012) NAD(+) administration decreases ischemic brain damage partially by blocking autophagy in a mouse model of brain ischemia. Neurosci Lett 512(2):67–71. doi:10.1016/j.neulet.2012.01.007
Zhao G, Zhang W, Li L, Wu S, Du G (2014) Pinocembrin protects the brain against ischemia-reperfusion injury and reverses the autophagy dysfunction in the penumbra area. Molecules 19(10):15786–15798. doi:10.3390/molecules191015786
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 18(3):250–260. doi:10.1111/j.1755-5949.2012.00295.x
Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z (2013) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9(9):1321–1333. doi:10.4161/auto.25132
Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L (2011) Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res 1402:109–121. doi:10.1016/j.brainres.2011.05.049
Balduini W, Carloni S, Buonocore G (2009) Autophagy in hypoxia-ischemia induced brain injury: evidence and speculations. Autophagy 5(2):221–223
Balduini W, Carloni S, Buonocore G (2012) Autophagy in hypoxia-ischemia induced brain injury. J Matern Fetal Neonatal Med 25(Suppl 1):30–34. doi:10.3109/14767058.2012.663176
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172(2):454–469. doi:10.2353/ajpath.2008.070876
Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG (2015) The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol 39:63–69. doi:10.1016/j.semcdb.2015.02.003
Parent JM (2009) Waiting for Gadd45b. Epilepsy Curr 9(6):170–172. doi:10.1111/j.1535-7511.2009.01332.x
Liu B, Zhang YH, Jiang Y, Li LL, Chen Q, He GQ, Tan XD, Li CQ (2015) Gadd45b is a novel mediator of neuronal apoptosis in ischemic stroke. Int J Biol Sci 11(3):353–360. doi:10.7150/ijbs.9813
Gabryel B, Kost A, Kasprowska D (2012) Neuronal autophagy in cerebral ischemia–a potential target for neuroprotective strategies? Pharmacol Rep 64(1):1–15
Hocker R, Walker A, Schmitz I (2013) Inhibition of autophagy through MAPK14-mediated phosphorylation of ATG5. Autophagy 9(3):426–428. doi:10.4161/auto.22924
Keil E, Hocker R, Schuster M, Essmann F, Ueffing N, Hoffman B, Liebermann DA, Pfeffer K, Schulze-Osthoff K, Schmitz I (2013) Phosphorylation of Atg5 by the Gadd45beta-MEKK4-p38 pathway inhibits autophagy. Cell Death Differ 20(2):321–332. doi:10.1038/cdd.2012.129
Gertz K, Kronenberg G, Kalin RE, Baldinger T, Werner C, Balkaya M, Eom GD, Hellmann-Regen J, Krober J, Miller KR, Lindauer U, Laufs U, Dirnagl U, Heppner FL, Endres M (2012) Essential role of interleukin-6 in post-stroke angiogenesis. Brain 135(Pt 6):1964–1980. doi:10.1093/brain/aws075
Xu SY, Wu YM, Ji Z, Gao XY, Pan SY (2012) A modified technique for culturing primary fetal rat cortical neurons. J Biomed Biotechnol 2012:803930. doi:10.1155/2012/803930
Fath T, Ke YD, Gunning P, Gotz J, Ittner LM (2009) Primary support cultures of hippocampal and substantia nigra neurons. Nat Protoc 4(1):78–85. doi:10.1038/nprot.2008.199
Jones SM, Novak AE, Elliott JP (2011) Primary culture of cellular subtypes from postnatal mouse for in vitro studies of oxygen glucose deprivation. J Neurosci Methods 199(2):241–248. doi:10.1016/j.jneumeth.2011.05.015
Ding B, Kilpatrick DL (2013) Lentiviral vector production, titration, and transduction of primary neurons. Methods Mol Biol 1018:119–131. doi:10.1007/978-1-62703-444-9_12
Reich A, Spering C, Gertz K, Harms C, Gerhardt E, Kronenberg G, Nave KA, Schwab M, Tauber SC, Drinkut A, Harms K, Beier CP, Voigt A, Gobbels S, Endres M, Schulz JB (2011) Fas/CD95 regulatory protein Faim2 is neuroprotective after transient brain ischemia. J Neurosci 31(1):225–233. doi:10.1523/JNEUROSCI.2188-10.2011
He GQ, Xu WM, Li JF, Li SS, Liu B, Tan XD, Li CQ (2015) Huwe1 interacts with Gadd45b under oxygen-glucose deprivation and reperfusion injury in primary Rat cortical neuronal cells. Mol Brain 8:88. doi:10.1186/s13041-015-0178-y
Hu Z, Yang B, Mo X, Xiao H (2014) Mechanism and Regulation of Autophagy and Its Role in Neuronal Diseases. Mol Neurobiol 52(3):1190–1209. doi:10.1007/s12035-014-8921-4
Liu C, Gao Y, Barrett J, Hu B (2010) Autophagy and protein aggregation after brain ischemia. J Neurochem 115(1):68–78. doi:10.1111/j.1471-4159.2010.06905.x
Nozaki K, Nishimura M, Hashimoto N (2001) Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol 23(1):1–19. doi:10.1385/MN:23:1:01
Gupta M, Gupta SK, Hoffman B, Liebermann DA (2006) Gadd45a and Gadd45b protect hematopoietic cells from UV-induced apoptosis via distinct signaling pathways, including p38 activation and JNK inhibition. J Biol Chem 281(26):17552–17558. doi:10.1074/jbc.M600950200
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752. doi:10.1038/nrm2239
Cui D, Wang L, Qi A, Zhou Q, Zhang X, Jiang W (2012) Propofol prevents autophagic cell death following oxygen and glucose deprivation in PC12 cells and cerebral ischemia-reperfusion injury in rats. PLoS One 7(4):e35324. doi:10.1371/journal.pone.0035324
Chu CT, Plowey ED, Dagda RK, Hickey RW, Cherra SJ 3rd, Clark RS (2009) Autophagy in neurite injury and neurodegeneration: in vitro and in vivo models. Methods Enzymol 453:217–249. doi:10.1016/S0076-6879(08)04011-1
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4(2):151–175
Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q, Wang K, Luo BY (2011) Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol 70(4):314–322. doi:10.1097/NEN.0b013e31821352bd
Ge P, Zhang F, Zhao J, Liu C, Sun L, Hu B (2012) Protein degradation pathways after brain ischemia. Curr Drug Targets 13(2):159–165
Thorburn A (2008) Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis Int J Program Cell Death 13(1):1–9. doi:10.1007/s10495-007-0154-9
Kim YA, Kim MY, Yu HY, Mishra SK, Lee JH, Choi KS, Kim JH, Xiang YK, Jung YS (2013) Gadd45beta is transcriptionally activated by p53 via p38alpha-mediated phosphorylation during myocardial ischemic injury. J Mol Med 91(11):1303–1313. doi:10.1007/s00109-013-1070-9
Acknowledgments
This work was supported by the National Natural Science Foundation (Grant No. 81271306).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no any competing financial interests in relation to the work described.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
He, G., Xu, W., Tong, L. et al. Gadd45b prevents autophagy and apoptosis against rat cerebral neuron oxygen-glucose deprivation/reperfusion injury. Apoptosis 21, 390–403 (2016). https://doi.org/10.1007/s10495-016-1213-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1213-x