Abstract
Intestinal ischemia–reperfusion (I/R) is a serious clinical dilemma with high morbidity and mortality. Remote organ damage, especially acute lung injury and liver injury are common complications that contribute to the high mortality rate. We previously demonstrated that activation of PKCβII is specifically involved in the primary injury of intestinal I/R. Considering the tissue-specific features of PKC activation, we hypothesized that some kind of PKC isoform may play important roles in the progression of secondary injury in the remote organ. Mice were studied in in vivo model of intestinal I/R. The activation of PKC isoforms were screened in the lung and liver. Interestingly, we found that PKCβII was also activated exclusively in the lung and liver after intestinal I/R. PKCβII suppression by a specific inhibitor, LY333531, significantly attenuated I/R-induced histologic damage, inflammatory cell infiltration, oxidative stress, and apoptosis in these organs, and also alleviated systemic inflammation. In addition, LY333531 markedly restrained p66shc activation, mitochondrial translocation, and binding to cytochrome-c. These resulted in the decrease of cytochrome-c release and caspase-3 cleavage, and an increase in glutathione and glutathione peroxidase. These data indicated that activated PKC isoform in the remote organ, specifically PKCβII, is the same as that in the intestine after intestinal I/R. PKCβII suppression protects against remote organ injury, which may be partially attributed to the p66shc-cytochrome-c axis. Combined with our previous study, the development of a specific inhibitor for prophylaxis against intestinal I/R is promising, to prevent multiple organ injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal ischemia–reperfusion (I/R) is a lethal complication that occurs in severe burns, hemorrhagic shock, and trauma [1–3]. Critically ill patients who undergo these severe clinical situations have usually experienced occult intestinal ischemia, given the preferential shunting of blood flow to the heart [4] and brain [5]. After fluid resuscitation, intestinal reperfusion immediately resumes. Although close monitoring of these patients had improved outcomes, morbidity and mortality remains high [6]. Intestinal I/R results in gut barrier dysfunction and bacterial translocation, which leads to inflammatory cytokine release, reactive oxygen species (ROS) overproduction, and even apoptosis. Ultimately, systemic inflammation and multiple organ failure results [7–9]. Remote organs, especially the lung [7] and liver [8], are very susceptible to intestinal I/R injury. Increasingly reports demonstrated that the secondary remote organ injury (including acute respiratory distress syndrome and acute liver failure, etc.) is much more severe than the primary damage and is proved to be the leading cause of death in intestinal I/R patients [7–9]. However, multiple signaling pathways are involved in this pathogenesis and the specific mechanism is extremely complicated.
The protein kinase C (PKC) family comprises a group of multi-functional protein kinases that play important roles as signal transducers of cellular stress by phosphorylation of the serine and threonine residues [10]. Activation of PKC, indicated by translocation of the protein from the cytoplasm to the membrane with subsequent phosphorylation, occurs in response to many conditions, such as I/R injury [11] and hemorrhagic shock [12]. Of the various PKC isoforms, PKCβII is exclusively activated during the primary injury of intestinal I/R [13]. We have demonstrated that inhibition of PKCβII protects intestine from the I/R injury [13]. However, other researchers have reported that the activation of individual PKC isoforms in particular pathogenesis is tissue-specific [10]. Thus, it is essential to further determine which PKC isoform contributes to the remote organ injury induced by intestinal I/R and whether it has a critical pathological role during disease progression.
Adaptor protein p66shc (p66shc) is a member of the ShcA protein family, and is comprised of two other proteins, p46shc and p52shc. Phosphorylation of the tyrosine residues of p46shc and p52shc plays an important role in their interaction with the epidermal growth factor receptor. In contrast to p46shc and p52shc, p66shc has a unique N-terminal proline-rich domain with a serine phosphorylation site (serine 36). Endogenous or exogenous stress, such as free radicals attack, results in serine 36 phosphorylation of the p66shc, contributing to cell oxidative stress and apoptosis [14, 15]. Prior studies have demonstrated that p66shc knockout mice showed strong resistance to severe tissue injury after heart or hind limb ischemia and secondary ROS attack induced by reperfusion [16, 17]. Likewise, p66shc-deficient cells were protected against oxidative stress and apoptosis when exposed to hypoxia and reoxygenation [18]. Although our previous study has demonstrated that p66shc phosphorylation was associated with acute lung injury (ALI) after intestinal I/R [19], the detailed mechanism by which p66shc exerts its deleterious effect in I/R induced remote organ injury requires further investigation.
Considering the tissue-specific features of PKC activation, we generated a mouse model of intestinal I/R and screened the activated isoform of PKC in the remote organ after intestinal I/R. Further, we explored whether the inhibition of PKC activation shows protective effects and elucidated the potential p66shc-mediated mechanisms. Our work aims to highlight a new target for preventing intestinal I/R induced remote organ injury.
Materials and methods
Experimental model
Adult male ICR mice (18–22 grams, SPF Animal Centre of Dalian Medical University, Dalian, China) were housed at a temperature of 22 ± 2 °C, kept on a 12:12-h photoperiod, and provided with food and water ad libitum. Animals were premedicated by intragastric administration of the PKCβ inhibitor LY333531 (10 mg/kg daily; Enzo Life Sciences, NY, USA), or vehicle, 3 days prior to I/R surgery. The murine intestinal I/R model was used, according to previously established standards [20]. Briefly, a midline laparotomy was performed, and the superior mesenteric artery (SMA) was isolated at its origin, and occluded with an atraumatic microvascular clamp. After 45 min of ischemia, the clamp was removed and mice were administered 0.5 ml of warm sterile saline intraperitoneally to improve hydration. Reperfusion was then performed at 45, 90, or 180 min intervals. Sham animals underwent the same protocol without occlusion of the SMA. After reperfusion, animals were sacrificed by exsanguination via the abdominal aorta. Intestinal, lung and liver tissues and blood samples were collected for analysis.
All experiments were performed in accordance with the institutional guidelines for the care and use of laboratory animals and were approved by the Committee on the Ethics of Animal Experiments of the Dalian Medical University (Dalian, China).
Histopathologic examination
Intestinal, lung, and liver tissues were fixed in 10 % buffered formalin phosphate and paraffin-embedded. Tissue blocks were sectioned at a thickness of 4 μm and hematoxylin-eosin stained. Histopathologic examination of these samples was performed under light microscopy in a blinded manner. The intestinal injury score was graded on a six-tiered scale modified from Chiu [21]. The severity of lung injury was scored based on Mikawa’s report [22]. Liver pathologic scores were evaluated according to Eckhoff’s report [23].
Measurement of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), TNF-α, and interleukin (IL)-6
Serum ALT and AST levels were determined with a commercial assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and presented as international units per liter (IU/L). Serum IL-6 and TNF-α levels were measured by a enzyme-linked immunosorbent assay kit (BOSTER, Wuhan, China) according to the manufacturer’s instructions.
Lung and liver H2O2, malondialdehyde (MDA), reduced glutathione (GSH), glutathione peroxidase (GSH-PX), and myeloperoxidase (MPO) assay
H2O2, MDA and GSH levels, and GSH-PX and MPO activity were analyzed using commercial assay kits, according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and were expressed as mmol/g protein, nmol/mg protein, mg/g protein, U/mg protein, and U/g, respectively.
Western blot
Membranous, cytosolic, and mitochondrial protein fractions or total protein were prepared using a commercial protein isolation kit (KeyGEN Biotech, Nanjing, China). Equal amounts of total protein (50 μg/sample) were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis (10–15 %, Bio-Rad, Hercules, USA) and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, USA). Western blot analysis was performed with antibodies specific for PKCβII, PKCγ, PKCδ, PKCε, cleaved-caspase-3, and ATPase (Bioworld Technology, Inc., MN, USA), PKCβI (Santa Cruz Biotechnology, CA, USA), phospho-PKCβII (Cell Signaling Technology, MA, USA), manganese superoxide dismutase (MnSOD), phospho-p66shc, total-p66shc (Abcam Ltd., Cambridge, UK), cytochrome-c (Beyotime Institute of Biotechnology, Shanghai, China), voltage-dependent anion channels (VDAC, Proteintech Group, Wuhan, China), and β-actin (ZSGB-BIO, Beijing, China). Nonspecific binding was blocked by incubation of membranes with 5 % skim milk for 2 h at 37 °C. Membranes were exposed to enhanced chemiluminescence plus reagents (Beyotime Institute of Biotechnology, Shanghai, China). Spectrophotometric analysis was performed with a BioSpectrum-410 multispectral imaging system and analyzed with a Gel-Pro Analyzer Version 4.0 (Media Cybernetics, MD, USA).
Co-immunoprecipitation
Mitochondrial fractions were extracted as described above. An equal amount of cytochrome-c antibody was added into 500 μg proteins and gently rotated at 4 °C overnight. Immunocomplexes were captured by adding 40 μl protein A + G agarose beads (Beyotime Institute of Biotechnology, Shanghai, China) and gently rotating at 4 °C for 4 h. The mixture was then centrifuged at 1,000g for 5 min at 4 °C and the supernatant was discarded. The precipitate was washed for 5 times with ice-cold phosphate buffer saline. After washing, the immunocomplex was boiled in sodium dodecyl sulfate sample buffer for 5 min to dissociate the immunocomplex from the beads. Samples were then subjected to western blot with anti-p66shc and anti-cytochrome-c antibody, according to the manufacturer’s instructions.
Terminal deoxynucleotidyl transferase dUTP nick end-labeling assay
Lung or liver histopathologic slides were dewaxed and treated with 20 μg/ml of proteinase K. Slides were stained using a terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) kit (Roche Diagnostics, Indianapolis, IN). All images were captured under fluorescent microscopy. Apoptotic cells appeared fluorescent green and were counted per 10 visual fields.
Caspase-3 activity assay
The caspase-3 activity was determined using a commercial assay kit (Beyotime Institute of Biotechnology, Shanghai, China), according to the manufacturer’s protocols.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The statistical analysis was carried out using the SPSS16.0 statistical software package (SPSS Inc., Chicago, IL, USA). Statistical comparisons were analyzed by Kruskal–Wallis test for non-normal distributions followed by Wilcoxon Rank Sum test with Bonferroni adjustments for multiple comparisons, or a one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls (SNK) test for normal distributions. A p value less than 0.05 was considered statistically significant.
Results
PKCβ is activated in response to intestinal I/R in the lung and liver
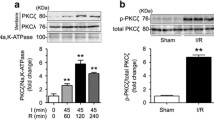
When mice were subjected to intestinal I/R, a significant translocation of PKCβII to the cell membrane in the lung and liver was observed, implying activation of PKCβII. The increase of PKCβII in the membrane fraction reached an apparent maximum after 90 min reperfusion (Fig. 1a). In contrast, the PKCβI isoform showed no significant change (Fig. 1b). Meanwhile, membranous PKCγ, PKCδ, and PKCε showed no significant change after any period of reperfusion (Fig. 1b). Additionally, we detected phosphorylated PKCβII, recognized to be a crucial element in the catalytic function of PKCβII. As shown in Fig. 1c, 90 min of reperfusion of the intestine resulted in significantly increased level of Thr641-phosphorylated PKCβII without affecting the total level of PKCβII. Thus, we confirmed that the activated principal isoform of PKC relevant to intestinal I/R in the lung and liver was the PKCβII isoform, and not the βI, γ, δ or ε isoforms.
PKCβ is activated in response to intestinal I/R in the lung and liver. a Protein expression of membranous PKCβII in the lung and liver after intestinal I/R. ATPase was used to normalize membranous protein levels. b Protein expression of membranous PKCβI, PKCγ, PKCδ, and PKCε in the lung and liver after intestinal I/R. ATPase was used to normalize membranous protein levels. c Relative expression of phosphorylated PKCβII and total-PKCβII in the lung and liver after intestinal I/R. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham. I indicates ischemia, R reperfusion, Thr Threonine
LY333531 attenuates lung and liver injury induced by intestinal I/R
To determine the role of PKCβ in intestinal I/R induced remote organ injury, we used LY333531, a specific inhibitor of PKCβ, to suppress PKCβII activation. As shown in Fig. 2a, LY333531 suppressed the membranous translocation of PKCβII after intestinal I/R, but without impact on PKCβI translocation. These findings were consistent with previous reports [24]. Furthermore, intestinal I/R led to marked phosphorylation of PKCβII, which was abolished by administration of LY333531 (Fig. 2b).
LY333531 inhibits PKCβII activation induced by intestinal I/R in the lung and liver. Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. a Membranous expression of PKCβI and PKCβII in the lung and liver. ATPase was used to normalize membranous protein levels. b Relative expression of phosphorylated PKCβII and total-PKCβII in the lung and liver from each group. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham; # p < 0.05 versus I/R; ## p < 0.01 versus I/R
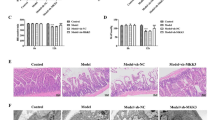
Next, we assessed whether PKCβ inhibition protected against intestinal I/R injury and secondary remote organ injury. As shown in Fig. 3, after intestinal I/R, small intestinal tissues were apparently damaged with severe irregularities of the villi, disintegrated lamina propria, and ulceration. Lung tissue was also markedly damaged, and was characterized by infiltration of inflammatory cells, thickened alveolar walls, and hemorrhage. Liver tissue was damaged with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and disintegration of hepatic cords. By contrast, in the LY333531 treated group, significantly decreased injury was shown on histopathology, in all three organ systems. Serum ALT and AST levels also showed the same trend, accordingly (Table 1).
PKCβ suppression improves intestinal I/R-induced intestine, lung and liver histopathologic injury. Mice were subjected to 45 min of intestinal ischemia followed by 90 min of reperfusion. Tissues were harvested at the end of reperfusion and were stained with hematoxylin-eosin. Slides were examined under light microscopy at ×100 (intestine), ×200 (lung), or ×400 (liver) magnification. Histologic injury scores in groups were quantified. Results are presented as the mean ± SD, n = 10. **p < 0.01 versus sham; ## p < 0.01 versus I/R group. LY indicates LY333531
To verify the effect of PKCβ suppression on neutrophil infiltration in the lung and liver, tissue MPO activity was measured. As shown in Table 1, intestinal I/R caused a significant increase of MPO activity compared to sham animals, which was remarkably reduced with administration of LY333531.
To determine whether intestinal I/R affected systemic inflammation, we measured serum TNF-α and IL-6 concentrations. Compared with the sham group, TNF-α and IL-6 concentrations were significantly elevated in the I/R group. In contrast, PKCβ inhibition by LY333531 administration prevented TNF-α and IL-6 overproduction (Table 1).
LY333531 ameliorates oxidative stress and apoptosis in the lung and liver after intestinal I/R
Oxidative stress is certainly involved in the lung and liver injury after intestinal I/R [19, 25]. As a major component of ROS, the content of H2O2 were significantly increased after intestinal I/R, while LY333531 administration reduced the content of H2O2 markedly (Fig. 4a). MDA is an end-product of lipid peroxidation, which is regarded as a major marker of oxidative stress [26]. As shown in Fig. 4b, MDA content was markedly increased after intestinal I/R. However, LY333531 administration decreased MDA levels in comparison with the I/R group. Anti-oxidant enzyme MnSOD (a downstream molecule of p66shc-foxo3a axis) expression was also assayed, and found to be significantly suppressed after intestinal I/R. LY333531 administration prevented suppression of MnSOD expression, implying a potential antioxidative effect of PKCβ suppression (Fig. 4c).
PKCβ suppression ameliorates lung and liver oxidative stress after intestinal I/R. Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. a Lung and liver H2O2 levels. Results are presented as the mean ± SD, n = 10. b Lung and liver MDA levels. Results are presented as the mean ± SD, n = 10. c Protein expression of MnSOD in the lung and liver; β-actin was used to normalized protein levels. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham; # p < 0.05 versus I/R; ## p < 0.01 versus I/R. Prot. indicates protein
To determine the effect of PKCβ suppression on apoptosis, TUNEL staining was performed. As shown in Fig. 5a, apoptotic cells in the lung and liver were significantly increased after intestinal I/R, whereas LY333531 administration significantly reduced the number of apoptotic cells compared with the I/R group. To further confirm these results, we detected the expression of cleaved caspase-3 and the caspase-3 activity. As expected, intestinal I/R enhanced lung and liver cleaved-caspase-3 expression and the caspase-3 activity, compared with the sham group. However, these effects were significantly attenuated by LY333531 administration (Fig. 5b, c).
PKCβ suppression ameliorates lung and liver apoptosis after intestinal I/R. Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. a Representative TUNEL staining of lung and liver after intestinal I/R. Quantification of TUNEL staining. Results are presented as the mean ± SD of 10 frames per group from three animals per group. b Protein expression of cleaved-caspase-3 in the lung and liver. β-actin was used to normalize protein levels. Results are presented as the mean ± SD, n = 3. c Lung and liver caspase-3 activities. Results are presented as the mean ± SD, n = 10. **p < 0.01 versus sham; ## p < 0.01 versus I/R
LY333531 inhibits p66shc activation in the lung and liver after intestinal I/R
P66shc is involved in modulating oxidative stress and apoptosis [15, 27, 28]. P66shc silenced by small interfering RNA (siRNA) showed resistance to H2O2-induced caspase-3 activation and apoptosis in human alveolar epithelial cell line A549 and human normal liver cell line L02 (Suppl Fig. 1). To evaluate the activation of p66shc in the lung and liver after intestinal I/R, p66shc phosphorylation was measured. As shown in Fig. 6a, time-dependent phosphorylation of p66shc was observed after 45–180 min reperfusion. Next, we evaluated whether I/R-induced p66shc activation was PKCβ dependent. As a specific inhibitor of PKCβ, LY333531 showed a significant restraining effect on p66shc phosphorylation in the lung and liver after intestinal I/R, without impact on the sham or the sham + LY333531 groups (Fig. 6b). To further determine whether PKCβ, especially PKCβII, is specifically required for the activation of p66shc, we suppressed its expression using siRNA in A549 cells and L02 cells (Suppl Fig. 2a). The exposure of PMA (a classical PKC activator) markedly increased p66shc phosphorylation in A549 cells and L02 cells, which were inhibited significantly by PKCβII siRNA or LY333531 (Suppl Fig. 2b). As a whole, these findings suggest that intestinal I/R induced p66shc activation in the lung and liver may partially depend on PKCβ activation.
P66shc is phosphorylated after intestinal I/R, which is restrained by PKCβ suppression. a Relative expression of phosphorylated p66shc in the total protein in the lung and liver. b Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. Relative expression of phosphorylated p66shc in the total protein in the lung and liver. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham; # p < 0.05 versus I/R. Ser indicates serine
LY333531 inhibits p66shc mitochondrial translocation and binding to cytochrome-c
As verified by previous studies, the pro-oxidative and pro-apoptotic properties of p66shc depend on mitochondrial translocation and binding to cytochrome-c [27]. To assess whether intestinal I/R increases mitochondrial translocation of p66shc, Western blotting of p66shc in the mitochondrial lysates was performed. As shown in Fig. 7a, after 45–180 min reperfusion, mitochondrial p66shc expression significantly increased in a time-dependent manner. Additionally, LY333531 administration suppressed p66shc levels in the mitochondria after intestinal I/R (Fig. 7b). To further investigate the relationship between mitochondrial p66shc and cytochrome-c, co-immunoprecipitation analysis was performed. Cytochrome-c co-precipitated with p66shc significantly in the I/R group compared with sham animals. However, this effect was significantly attenuated by LY333531 administration (Fig. 8a). Taken together, these results suggest that LY333531 suppresses mitochondrial translocation and cytochrome-c binding of p66shc in the lung and liver after intestinal I/R.
P66shc is translocated into mitochondria after intestinal I/R, which is restrained by PKCβ suppression. a Mitochondrial expression of p66shc in the lung and liver. b Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. Mitochondrial expression of p66shc in the lung and liver. VDAC was used to normalize mitochondrial protein levels. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham; ## p < 0.01 versus I/R
PKCβ suppression inhibits p66shc binding to cytochrome-c and attenuates the subsequent cytochrome-c release. Mice were subjected to 45 min of intestinal ischemia followed by 90 min reperfusion. a Mitochondrial lysate was immunoprecipitated with anti-cytochrome-c antibody and immunoblotted with anti-p66shc antibody. The same blot was re-hybridized with anti-cytochrome-c antibody. Cyt. C indicates cytochrome-c. b Protein expression of mitochondrial cytochrome-c and cytosolic cytochrome-c in the lung and liver. Results are presented as the mean ± SD, n = 3. **p < 0.01 versus sham; ## p < 0.01 versus I/R
LY333531 ameliorates cytochrome-c releasing and GSH system dysfunction
In the mitochondria, p66shc oxidizes cytochrome-c to generate ROS (H2O2). This process induces opening of mitochondrial permeability transition pore, with a subsequent release of cytochrome-c and H2O2 into the cytosol [16, 27]. Then, cytosolic cytochrome-c activates caspase-3 and results in apoptosis [29]. To confirm this process, we investigated mitochondrial and cytoplasmic cytochrome-c levels in each group. As shown in Fig. 8b, intestinal I/R caused a significant release of cytochrome-c from the mitochondria into the cytosol in the lung and liver, while control mice did not demonstrate this event. In contrast, PKCβ inhibition markedly restored mitochondrial localization of cytochrome-c.
P66shc induced H2O2 releasing also plays important role in promoting apoptosis [27]. In physiological conditions, cytosolic H2O2 released from the mitochondria will interact with GSH and ultimately convert into H2O by GSH-PX catalysis, thereby maintaining the homeostasis [30]. Once the GSH system is getting out-of-balance, apoptosis is more prone to occur. As shown in Table 2, in accordance with cytochrome-c release in the I/R group, lung and liver GSH content and GSH-PX activity dramatically decreased. In contrast, LY333531 administration showed an efficient recovery of GSH content and GSH-PX activity, compared with the I/R group.
Discussion
Intestinal I/R is a serious clinical dilemma with high morbidity and mortality [1, 2, 6, 31]. Multiple organ damage, including ALI and liver injury, are common complications of intestinal I/R and contribute to the high mortality rate [7, 8]. Only a limited number of pharmacologic treatment options provide benefit in intestinal I/R and remote organ injury. In this study, we provided the first evidence that (1) PKCβ (especially PKCβII) is selectively activated in the lung and liver after intestinal I/R; (2) PKCβ suppression by a specific inhibitor (LY333531) markedly reduced I/R-induced remote organ injury, as indicated by improved morphological damage, attenuated systemic inflammation, and decreased oxidative stress and apoptosis; (3) the protective effect of LY333531 is partially attributed to PKCβ dependent phospho-p66shc suppression and alleviation of p66shc-mediated oxidative stress and apoptosis.
Intestinal I/R is associated with severe damage to the mucosal structure and reduced epithelial function, eventually leading to the loss of barrier integrity and bacterial translocation [32]. Previous studies suggest that migratory bacteria result in the release of cytokines or chemokines, such as IL-6 and TNF-α, which exert a significantly harmful effect via direct inflammatory stimuli on remote organs. Therefore, patients who underwent intestinal I/R develop systemic inflammation in multiple organs [33]. We found that PKCβ suppression showed a less obvious inflammatory response compared with the I/R group. PKCβ suppression enhanced the mucosal integrity of the intestine (Fig. 3), thus reducing bacterial translocation and cytokines release. Our data indicates that PKCβ may play an important role in modulating inflammatory pathways. Consistent with our results, a recent study revealed that PKCβ suppression by LY333531 or hispidin (specific inhibitors of PKCβ) prevented nuclear factor κB activation in H2O2-treated cells, thus reducing the production of inflammatory cytokines and oxidative stress. In contrast, phorbol ester (an activator of PKC) treatment promoted PKCβ-associated inflammation [34].
Previous studies demonstrated that the oxidative stress response induced by intestinal I/R plays a pivotal role in the pathogenesis of remote organ damage [9, 19]. After ROS exposure, cells undergo organelle disintegration, membrane lipid breakdown, and DNA damage [35, 36]. P66shc is an essential regulator of mitochondrial and cytoplasmic oxidative stress, since p66shc knockout mice had decreased ROS generation and had anti-oxidative features [14, 15]. Previous study identified that oxidative stress induces the phosphorylation of p66shc, which allowing it to be recognized by prolyl isomerase Pin1 and isomerized. After dephosphorylated by protein serine/threonine phosphatase, p66shc translocates into mitochondria and promotes the ROS generation [14]. In this study, we found that significant oxidative stress was induced in the lung and liver after intestinal I/R, which accompanied p66shc phosphorylation. However, LY333531 administration attenuated p66shc phosphorylation, while also alleviating oxidative stress in these organs. Moreover, MnSOD is a mitochondrial antioxidative enzyme regulated by foxo3a [28]. It has been known that phosphorylation of p66shc promotes phosphorylation of foxo3a, with foxo3a translocation into the cytosol in an inactive form. Indeed, p66shc suppression blocks foxo3a phosphorylation and leads to a resistance to oxidative stress, in accordance with MnSOD upregulation [28]. In the current work, MnSOD expression was actually attenuated in the I/R group. In contrast, phospho-p66shc suppression by LY333531 enhanced MnSOD expression, thereby strengthening the anti-oxidative capacity. Furthermore, we showed that p66shc phosphorylation facilitated mitochondrial translocation and binding to cytochrome-c, recognized as the trigger event of H2O2 release [27]. Although cytoplasmic GSH could turn the H2O2 into H2O with the catalysis of GSH-PX and maintain the balance of intracellular redox state, we observed H2O2 over production, GSH exhaustion and GSH-PX inactivation in this study. However, the PKCβ suppressed mice displayed restraining effects on p66shc translocation as well as binding to cytochrome-c. As a result, GSH and GSH-PX were recovered and oxidative stress was relieved. Taken together, PKCβ suppression by LY333531 may restrain p66shc-associated oxidative stress in remote organs after intestinal I/R.
Apoptosis and oxidative stress are inseparable parts during various pathophysiological processes. Given exposure to ROS, mitochondrial proteins, lipids, and DNA are believed to be primary targets of oxidative stress damage, leading to alteration or loss of cellular functions, and causing inhibition of proliferation and induction of apoptosis [35, 36]. It has been shown that apoptosis plays a critical role in multiple organ dysfunction in critically ill patients [37]. P66shc acts as a pro-apoptotic protein which plays a pivotal role in modulating the intracellular redox state, increasing susceptibility to oxidative stress, and resulting in apoptosis elicited by oxidative damage eventually. Several researchers have delineated a functional link between p66shc and caspase-3 [38], which plays a crucial role in cell apoptosis by resulting in DNA fragmentation, cytoskeleton degradation, and the formation of apoptotic bodies. P66shc is an indispensable protein that regulates mitochondrial-mediated apoptosis induction in mammals. The realization of its pro-apoptotic function mainly depends on binding of cytochrome-c and its oxidation in the mitochondria. During oxidization, cytochrome-c is released into the cytosol and triggers caspase-9 dependent caspase-3 activation, thus inducing apoptosis [27, 29, 39]. Conversely, mutations in p66shc impair its ability to mediate apoptosis by restraining the interaction between p66shc and mitochondrial cytochrome-c [29]. Consistent with these observations, our data showed that expression of cleaved-caspase-3 was upregulated after intestinal I/R, and accompanied by cytochrome-c release. Meanwhile, TUNEL data showed significant apoptosis in the lung and liver. In contrast, phospho-p66shc suppression by PKCβ inhibition was associated with dysfunction of the cytochrome-c-caspase-3 network and improved apoptosis. Taken together, intestinal I/R activated the cascade from PKCβ dependent p66shc phosphorylation, cytochrome-c release, and caspase-3 activation. However, LY333531 administration inhibited PKCβ activation, while also suppressing p66shc phosphorylation, translocation, and further interaction with cytochrome-c. Thus, cytochrome-c release was reduced and apoptosis was alleviated.
PKC has become an attractive target in the pathogenesis of various diseases [10]. In the case of ischemic or I/R injury, previous studies have reported activation of PKCε, PKCδ, and PKCβII in cardic ischemia or I/R [40–42], activation of PKCβII associated with the response to single-lung I/R [11], and activation of PKCα related to hepatic I/R [43]. Thus, the activation of individual PKC isoforms in particular disease is tissue-specific. Despite this feature of PKC activation, we found firstly that the activated PKC isoform in the remote organ, specifically PKCβII and not the other isoforms, is the same as that in the intestine after intestinal I/R. This finding is interesting and makes PKCβII/p66shc pathway as a specific therapeutic target not only in attenuating the primary damage of intestinal I/R, but also in improving the secondary injury in the remote organs.
In recent years, specific regulators of PKC (agonist or inhibitor) have been used in many animal models [10]. As a highly selective inhibitor of PKCβ, LY333531 protected against several ROS and apoptosis associated pathological processes [11, 44]. Moreover, LY333531 is now the focus of testing in human clinical trials [45–47]. We propose that the activation of p66shc-mediated pro-oxidative and pro-apoptosis signaling pathway is particularly dependent on PKCβ; hence, administration of LY333531 to antagonize PKCβ may be a novel strategy to prevent intestinal I/R-induced remote organ injury via attenuation of the PKCβ-p66shc pathway.
In summary, these findings highlight a novel signaling pathway regulating the remote organ injury induced by intestinal I/R. As shown in Fig. 9, PKCβII is strongly activated in remote organs after intestinal I/R. PKCβ suppression by LY333531 protects against lung and liver injury induced by intestinal I/R, partially through the p66shc-cytochrome-c axis. Our results have identified a novel therapeutic target in the management of remote organ injury induced by intestinal I/R.
Proposed mechanism of LY333531 for preventing remote organ injury induced by intestinal I/R. I/R stress activates PKCβII to induce phosphorylation of p66shc, allowing transfer of the protein from the cytosol to the mitochondria. In the mitochondria, p66shc binds to cytochrome-c and oxidizes it, and catalyzes the reduction of H2O2. This latter induces the release of cytochrome-c and H2O2 into the cytosol. Besides, phosphorylated p66shc inhibits expression of MnSOD. LY333531 inhibits activation of PKCβII, thereby suppressing the above mentioned process. Cyt. C indicates cytochrome-c
References
Reino DC, Palanyge D, Feketeova E, Bonitz RP, Xu DZ, Lu Q, Sheth SU, Pena G, Ulloa L, De Maio A (2012) Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock 38:107–114
Tadros T, Traber DL, Heggers JP, Herndon DN (2003) Effects of interleukin-1alpha administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Ann Surg 237:101–109
Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP (2007) Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol 293:R1538–R1544
Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE (2012) Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26:1727–1735
Hsieh YH, McCartney K, Moore TA, Thundyil J, Gelderblom M, Manzanero S, Arumugam TV (2011) Intestinal ischemia-reperfusion injury leads to inflammatory changes in the brain. Shock 36:424–430
Tendler DA (2003) Acute intestinal ischemia and infarction. Semin Gastrointest Dis 14:66–76
Matsuo S, Yang WL, Aziz M, Jacob A, Wang P (2013) Cyclic arginine-glycine-aspartate attenuates acute lung injury in mice after intestinal ischemia/reperfusion. Crit Care 17:R19
Horie Y, Wolf R, Miyasaka M, Anderson DC, Granger DN (1996) Leukocyte adhesion and hepatic microvascular responses to intestinal ischemia/reperfusion in rats. Gastroenterology 111:666–673
Cui T, Miksa M, Wu R, Komura H, Zhou M, Dong W, Wang Z, Higuchi S, Chaung W, Blau SA (2010) Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med 81:238–246
Mochly-Rosen D, Das K, Grimes KV (2012) Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11:937–957
Fujita T, Asai T, Andrassy M, Stern DM, Pinsky DJ, Zou YS, Okada M, Naka Y, Schmidt AM, Yan SF (2004) PKCbeta regulates ischemia/reperfusion injury in the lung. J Clin Invest 113:1615–1623
Xu J, Li T, Yang GM, Liu LM (2010) Protein kinase C isoforms responsible for the regulation of vascular calcium sensitivity and their relationship to integrin-linked kinase pathway after hemorrhagic shock. J Trauma 69:1274–1281
Chen Z, Wang G, Zhai X, Hu Y, Gao D, Ma L, Yao J, Tian X (2014) Selective inhibition of protein kinase C β2 attenuates the adaptor P66 Shc-mediated intestinal ischemia-reperfusion injury. Cell Death Dis 5:e1164
Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR (2007) Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315:659–663
Migliaccio E, Giorgio M, Pelicci PG (2006) Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal 8:600–608
Cosentino F, Francia P, Camici GG, Pelicci PG, Luscher TF, Volpe M (2008) Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol 28:622–628
Fadini GP, Albiero M, Menegazzo L, Boscaro E, Pagnin E, Iori E, Cosma C, Lapolla A, Pengo V, Stendardo M (2010) The redox enzyme p66Shc contributes to diabetes and ischemia-induced delay in cutaneous wound healing. Diabetes 59:2306–2314
Haga S, Terui K, Fukai M, Oikawa Y, Irani K, Furukawa H, Todo S, Ozaki M (2008) Preventing hypoxia/reoxygenation damage to hepatocytes by p66(shc) ablation: up-regulation of anti-oxidant and anti-apoptotic proteins. J Hepatol 48:422–432
Wang GZ, Yao JH, Jing HR, Zhang F, Lin MS, Shi L, Wu H, Gao DY, Liu KX, Tian XF (2012) Suppression of the p66shc adapter protein by protocatechuic acid prevents the development of lung injury induced by intestinal ischemia reperfusion in mice. J Trauma Acute Care Surg 73:1130–1137
Lane JS, Todd KE, Lewis MP, Gloor B, Ashley SW, Reber HA, McFadden DW, Chandler CF (1997) Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery 122:288–294
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN (1970) Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101:478–483
Mikawa K, Nishina K, Takao Y, Obara H (2003) ONO-1714, a nitric oxide synthase inhibitor, attenuates endotoxin-induced acute lung injury in rabbits. Anesth Analg 97:1751–1755
Eckhoff DE, Bilbao G, Frenette L, Thompson JA, Contreras JL (2002) 17-Beta-estradiol protects the liver against warm ischemia/reperfusion injury and is associated with increased serum nitric oxide and decreased tumor necrosis factor-alpha. Surgery 132:302–309
Lei S, Li H, Xu J, Liu Y, Gao X, Wang J, Ng KF, Lau WB, Ma XL, Rodrigues B (2013) Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes 62:2318–2328
Jing H, Shen G, Wang G, Zhang F, Li Y, Luo F, Yao J, Tian XF (2012) MG132 alleviates liver injury induced by intestinal ischemia/reperfusion in rats: involvement of the AhR and NFkappaB pathways. J Surg Res 176:63–73
Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P, Zhang JH, Sun X, Yuan H (2009) Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res 43:478–484
Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122:221–233
Nemoto S, Finkel T (2002) Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450–2452
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Ustundag B, Kazez A, Demirbag M, Canatan H, Halifeoglu I, Ozercan IH (2000) Protective effect of melatonin on antioxidative system in experimental ischemia-reperfusion of rat small intestine. Cell Physiol Biochem 10:229–236
Allford M, Bew S (2010) Ventricular fibrillation in an ex-premature neonate following reperfusion of ischemic gut incarcerated within an inguinal hernia. Paediatr Anaesth 20:763–766
Zhi-Yong S, Dong YL, Wang XH (1992) Bacterial translocation and multiple system organ failure in bowel ischemia and reperfusion. J Trauma 32:148–153
Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G (1999) Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg 229:478–486
Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC (2010) Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol 24:2030–2037
Suzuki YJ, Forman HJ, Sevanian A (1997) Oxidants as stimulators of signal transduction. Free Radic Biol Med 22:269–285
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Papathanassoglou ED, Moynihan JA, Ackerman MH (2000) Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? a review and a theoretical framework. Crit Care Med 28:537–549
Arany I, Faisal A, Clark JS, Vera T, Baliga R, Nagamine Y (2010) p66SHC-mediated mitochondrial dysfunction in renal proximal tubule cells during oxidative injury. Am J Physiol Renal Physiol 298:F1214–F1221
Seo BN, Ryu JM, Yun SP, Jeon JH, Park SS, Oh KB, Park JK, Han HJ (2013) Delphinidin prevents hypoxia-induced mouse embryonic stem cell apoptosis through reduction of intracellular reactive oxygen species-mediated activation of JNK and NF-κB, and Akt inhibition. Apoptosis 18:811–824
Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D (2003) Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 108:2304–2307
Tanaka M, Terry RD, Mokhtari GK, Inagaki K, Koyanagi T, Kofidis T, Mochly-Rosen D, Robbins RC (2004) Suppression of graft coronary artery disease by a brief treatment with a selective epsilonPKC activator and a deltaPKC inhibitor in murine cardiac allografts. Circulation 110:194–199
Kong L, Andrassy M, Chang JS, Huang C, Asai T, Szabolcs MJ, Homma S, Liu R, Zou YS, Leitges M, Yan SD, Ramasamy R, Schmidt AM, Yan SF (2008) PKCbeta modulates ischemia-reperfusion injury in the heart. Am J Physiol Heart Circ Physiol 294:H1862–H1870
Baiocchi L, Tisone G, Russo MA, Longhi C, Palmieri G, Volpe A, Almerighi C, Telesca C, Carbone M, Toti L, De Leonardis F, Angelico M (2008) TUDCA prevents cholestasis and canalicular damage induced by ischemia-reperfusion injury in the rat, modulating PKCalpha-ezrin pathway. Transpl Int 21:792–800
Wei L, Sun D, Yin Z, Yuan Y, Hwang A, Zhang Y, Si R, Zhang R, Guo W, Cao F (2010) A PKC-beta inhibitor protects against cardiac microvascular ischemia reperfusion injury in diabetic rats. Apoptosis 15:488–498
Sheetz MJ, Aiello LP, Davis MD, Danis R, Bek T, Cunha-Vaz J, Shahri N, Berg PH (2013) The effect of the oral PKC beta inhibitor ruboxistaurin on vision loss in two phase 3 studies. Invest Ophthalmol Vis Sci 54:1750–1757
Tesfaye S, Tandan R, Bastyr ER, Kles KA, Skljarevski V, Price KL (2007) Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care 30:2626–2632
Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW (2007) Kidney outcomes in long-term studies of ruboxistaurin for diabetic eye disease. Clin J Am Soc Nephrol 2:631–636
Acknowledgments
This work was supported by grants from the Chinese National Natural Science Foundation (No. 81171850, 81372037).
Conflict of interests
The authors have no competing interests to declare.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, G., Chen, Z., Zhang, F. et al. Blockade of PKCβ protects against remote organ injury induced by intestinal ischemia and reperfusion via a p66shc-mediated mitochondrial apoptotic pathway. Apoptosis 19, 1342–1353 (2014). https://doi.org/10.1007/s10495-014-1008-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-1008-x