Abstract
Shikonin derivatives exert powerful cytotoxic effects, induce apoptosis and escape multidrug resistance in cancer. However, the diverse mechanisms underlying their anticancer activities are not completely understood. Here, we demonstrated that shikonin-induced apoptosis is caused by reactive oxygen species (ROS)-mediated activation of Akt/ASK1/p38 mitogen-activated protein kinase (MAPK) and downregulation of p21Cip1. In the presence of shikonin, inactivation of Akt caused apoptosis signal-regulating kinase 1 (ASK1) dephosphorylation at Ser83, which is associated with ASK1 activation. Shikonin-induced apoptosis was enhanced by inhibition of Akt, whereas overexpression of constitutively active Akt prevented apoptosis through modulating ASK1 phosphorylation. Silencing ASK1 and MKK3/6 by siRNA reduced the activation of MAPK kinases (MKK) 3/6 and p38 MAPK, and apoptosis, respectively. Antioxidant N-acetyl cysteine attenuated ASK1 dephosphorylation and p38 MAPK activation, indicating that shikonin-induced ROS is involved in the activation of Akt/ASK1/p38 pathway. Expression of p21Cip1 was significantly induced in early response, but gradually decreased by prolonged exposure to shikonin. Overexpression of p21Cip1 have kept cells longer in G1 phase and attenuated shikonin-induced apoptosis. Depletion of p21Cip1 facilitated shikonin-induced apoptosis, implying that p21Cip1 delayed shikonin-induced apoptosis via G1 arrest. Immunohistochemistry and in vitro binding assays showed transiently altered localization of p21Cip1 to the cytoplasm by shikonin, which was blocked by Akt inhibition. The cytoplasmic p21Cip1 actually binds to and inhibits the activity of ASK1, regulating the cell cycle progression at G1. These findings suggest that shikonin-induced ROS activated ASK1 by decreasing Ser83 phosphorylation and by dissociation of the negative regulator p21Cip1, leading to p38 MAPK activation, and finally, promoting apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reactive oxygen species (ROS) are generated as by-products of cellular metabolism, primarily in the mitochondria, and are important regulators involved in a number of cellular processes. Depending on the cellular level, ROS could modulate various cellular responses, i.e., growth stimulation, growth arrest, apoptosis, and necrosis. With respect to apoptosis, excessive production of ROS is in general associated with induction of death. ROS can inactivate many protein tyrosine phosphatases and activate some kinases and transcription factors, which leads to cell cycle progression [1]. ROS can also regulate the Akt signaling pathway [2, 3]. Apoptosis signal-regulating kinase 1 (ASK1) has been reported to be phosphorylated by Akt at serine 83 (Ser83), which renders ASK1 inactive [4]. ASK1, a mammalian mitogen-activated protein kinase kinase (MKK) kinase, is activated in response to various cytotoxic stresses, including serum withdrawal, ROS, tumor necrosis factor, microtubule interfering agents, and cancer chemotherapeutic agents [5, 6]. Activated ASK1 activates downstream kinases such as c-Jun N-terminal protein kinase (JNK) and p38 pathways, resulting cell apoptosis [7, 8]. The association of p21Cip1 with ASK1 reportedly can block rapamycin- and VP16-induced apoptosis [9, 10]. It was also reported that ROS trigger proteasome-mediated degradation of p21Cip1 in human fibroblast cells and lung epithelial cells [11, 12].
Shikonin, a natural naphthoquinone derivative isolated from the traditional medical herb Lithospermum erythrorhizon, has been used as an ointment for wound healing. Shikonin and its derivatives reportedly possess numerous pharmacological properties such as antitumor, anti-inflammatory, antimicrobial, and antithrombotic effects [13–15]. Previous results have shown that the antitumor properties of shikonin derivatives were conferred by inhibiting cancer cell proliferation, inducing apoptosis, reducing angiogenesis, and circumvention of cancer drug resistance through induction of necroptosis [16–19]. In particular, shikonin derivatives may combat cancer through induction of ROS [16], upregulation of p53 and p27, decrease in anti-apoptotic proteins such as Bcl-2 and Bcl-xL [18, 20], regulation of pERK, JNK, and PKC-α activities [21, 22], activation of caspase [18, 23], and inhibition of topoisomerase-I, telomerase, pyruvate kinase M2 (PKM2), and polo-like kinase 1 (PLK1) [24–26]. Recently, shikonin derivatives have been of increasing interest as anticancer drugs with a broad spectrum because it is thought that they could inhibit cancer through activating multiple and diverse death mechanisms. Therefore, the diverse mechanisms underlying shikonin’s anticancer activities should be further evaluated in order to optimize the medical value of its derivatives.
In this study, we demonstrated that shikonin triggers ROS generation, and accumulated ROS inactivated Akt and led to the degradation of p21Cip1, which resulted in G1 arrest. Subsequently, ASK1 was activated by a decrease in Ser83 phosphorylation and dissociation of the negative regulator p21Cip1, leading to activation of p38 MAPK and finally promote apoptosis.
Materials and methods
Materials and cell culture
Shikonin, ROS inhibitor (NAC, N-acetyl cysteine), and sulforhodamine B (SRB) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002) and JNK inhibitor (SP600125) were purchased from Calbiochem (Darmstadt, Germany). The p38 inhibitor (SB203580) was purchased from LC laboratories (Woburn, MA, USA). siRNAs were purchased from Bioneer (Daejeon, South Korea). HA-tagged wild-type and S83A mutant ASK1 were kindly provided by Dr. H. Ha (Chungbuk National University, Cheongju, Korea). HA-tagged myr-Akt and DN-Akt were purchased from Addgene Co. (Cambridge, MA, USA). Antibodies against p53 and p-p53 were obtained from Calbiochem (Darmstadt, Germany). Antibodies against p-ATF2, ASK1, p-ASK1(S), p-p38, c-Jun, Akt, p-Akt1/2, MKK-3, MKK-6, p-MKK6, p-MKK3, and poly (ADP-ribose) polymerase (PARP) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against p21Cip1, Bax, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
A cervical cancer cell line HeLa, a colon cancer cell line Hct116, a hepatocellular carcinoma (HCC) cell line Hep3B and a lung cancer cell line A549 were maintained in RPMI 1640 supplemented with 1.5 g/L sodium bicarbonate, 5–10 % fetal bovine serum, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 mM l-glutamine in a humidified incubator containing 5 % CO2.
Construction and propagation of a recombinant adenovirus vector
To create a recombinant adenoviral vector expressing the p21 Cip1 gene, pENTR (Invitrogen) was used as transfer vector. The coding region of the p21 Cip1 gene was inserted into the transfer vector. LR clonase was used for site-directed recombination between the pENTR vector containing the p21 Cip1 gene and the recombinant adenoviral (rAd) vector pAd/CMV/V5-DEST (Invitrogen). After PacI digestion, the linearized rAd vector was transfected into HEK293A cells. Viruses were propagated, purified, and titrated by using a plaque assay.
Cytotoxicity assay
Growth inhibition of cells in the presence of shikonin was measured by using the SRB assay according to a previously described method [27] or WST-1 assay according to the manufacturer’s instructions. Cells were seeded into 96-well plates, incubated for 24 h, and treated with shikonin at different concentrations. After 48 h of incubation, cells were fixed with 10 % formalin solution and stained with 0.4 % SRB solution in 0.1 % acetic acid. The SRB dye bound to the cell matrix was dissolved in 10 mM Tris (pH 10.5), and absorbance was then measured at 530 nm using a Micro Plate Reader (Molecular Devices Emax Precision). For WST-1 assay, WST-1 reagent were added constituting 9 % of the well volume and the plates were incubated for another 0.5–1 h. The absorbance was measured on a scanning multiwell spectrophotometer at 440 nm with a 600-nm reference.
Flow cytometry analysis
Shikonin-treated cells or those infected with the adenovirus containing rAd and rAd-p21Cip1 were harvested and analyzed by flow cytometry as previously described [28]. Cells stained with propidium iodine (PI) were analyzed on the FACS Calibur (BD Biosciences, San Jose, CA, USA) and the sub-G0/G1 DNA content was analyzed using Modifit software (Verity Software House, Inc., Tosham, ME, USA).
Annexin V/PI double-staining assay was performed according to the previously described method [29]. Briefly, cells were plated in 6-well plates and infected with adenovirus containing rAd-p21Cip1 for 12 h, followed by shikonin treatment for 12 h. Then, cells were washed twice with pre-chilled phosphate buffered saline (PBS), resuspended in 100 μL of binding buffer, and annexin V-fluorescein isothiocyanate (FITC) and PI were added to the mixture. The mixture was maintained in the dark at room temperature for 20 min and then assessed using the FACS Calibur (BD Biosciences).
Immunoblot analysis and immunohistochemistry
Cells were washed with PBS and harvested in RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 1 mM Na3VO4, and protease inhibitor cocktail (Roche). Cells were then lysed by sonication on ice. Whole-cell extracts were collected by using previously described methods and analyzed by immunoblot analysis [28]. To determine the cellular location of p21Cip1, cells were cultured in 8-well microslides and infected with 1 × 1010 particle/mL of rAd-p21Cip1. After 14 h of incubation with the virus, cells were washed and cultured in fresh medium. At 36 h post infection, cells were treated with DMSO or shikonin. Cells were washed 3 times with PBS, fixed in 4 % paraformaldehyde solution for 30 min, permeabilized with 0.2 % Triton X-100, and incubated with a p21Cip1-specific antibody for 2 h followed by incubation with an FITC-conjugated secondary antibody (Santa Cruz) plus DAPI. The location of the p21Cip1 protein was observed by fluorescence microscopy (LSM5 Live DuoScan; Carl Zeiss).
Knockdown of gene expression using siRNAs
Knockdown of gene expression was performed using siRNAs according to a previously described method [28]. Validated siRNAs for human p21Cip1 (ID no. 1029367), ASK1 (ID no. 100233), MKK3 (ID no. 100205), MKK6 (ID no. 100215), p38 MAPK (5′-CAAATTCTCCGAGGTCTAA-3′), and a negative control siRNA (5′-CCTACGCCACCAATTTCGT-3′) were purchased from Bioneer (Daejeon, South Korea). For siRNA transfections, proliferating cells at about 30–50 % confluence were treated with the indicated siRNA using Hyperfect (Qiagen, CA, USA) according to the manufacturer’s instructions. Cells were treated with 20 nM of siRNA for 48 h, and the efficacy of siRNA treatment was verified by reverse transcription-polymerase chain reaction and immunoblot analysis.
Results
Shikonin induces apoptosis in cancer cell lines
Shikonin derivatives have been of increasing interest as anticancer drugs with a broad spectrum because it is thought that they could inhibit cancer through diverse mechanisms or by activating multiple death pathways. Therefore, the diverse mechanisms underlying the anticancer activities of shikonin should be further evaluated in order to optimize the medical value of its derivatives.
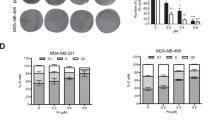
The effect of shikonin on growth inhibition in various human cancer cells was examined by conducting the SRB assay. It was shown that shikonin induced cancer cells apoptosis. Shikonin demonstrated cytotoxicity with a 50 % growth inhibition (GI50) range of 0.5–3.0 μM in most cancer cell lines tested (Fig. S1). The time-dependent effect of shikonin was evaluated in HeLa cells treated for different periods. The effects of shikonin on cell cycle and apoptosis were confirmed by FACS analysis, DNA fragmentation analysis, and immunoblot analysis (Fig. 1). The proportion cells in the G1 phase was increased by 71.9 % after 6 h of treatment with 2 μM shikonin, while only 56.4 % of control cells were in the G1 phase. The proportion of apoptotic cells increased by 9.8, 19.7, and 24.1 % at 12, 18, and 24 h, respectively, after treatment with 2 μM shikonin, whereas only 1.4 % of the control cells were apoptotic (Fig. 1a). As expected, both fragmentation of chromosomal DNA and cleavage of PARP, the hall markers of apoptosis, were increased by shikonin treatment concomitant with an increase in the proportion of sub-G1 cells (Fig. 1b–c). As previously reported in HepG2 cells, treatment of HeLa cells with shikonin also resulted in p53-dependent p21Cip1 induction followed by an increase in apoptotic protein Bax levels and PARP cleavage, indicating apoptosis. Therefore, we compared the apoptotic effects of shikonin and hydrogen peroxide, a representative ROS, because, H2O2 reportedly triggered apoptosis via the mitochondrial pathway involving upregulation of Bax in HeLa cells [30]. In agreement with previous reports, both agents caused a time-dependent increase in PARP cleavage concomitant with an increase in Bax, indicating the existence of a common biological mechanism (Fig. 1c). In addition, it seemed that shikonin-induced PARP cleavage followed the decrease in p21Cip1, but H2O2-induced PARP cleavage was observed even in HeLa cells that retained high p21Cip1 levels. It has been shown that high concentrations of H2O2 induce necrosis, whereas low concentrations induce apoptosis [31]. At apoptotic concentrations of H2O2, the increase in p21Cip1 protein did not appear to be an immediate response and the level remained higher in H2O2-treated cells compared with that in untreated cells for several days. Levels of p53 protein also remained elevated for up to 24 h [32]. Therefore, p21Cip1 probably plays a different role in the apoptotic pathway of both agents.
Shikonin induces apoptosis in cancer cell lines. a, b Time-dependent effects of shikonin on cell cycle arrest and apoptosis. HeLa cells were incubated for the indicated times in the presence of 2 μM shikonin and the occurrence of apoptosis was determined by using flow cytometry and DNA fragmentation assay. c Comparison of apoptotic effects of shikonin and H2O2, a representative ROS in HeLa cells. After the cells were treated with shikonin or 500 μM H2O2 (a representative ROS) for different times, the changes in the expression levels of proteins involved in apoptosis were examined. The occurrence of apoptosis was determined by PARP cleavage. d Effects of shikonin in various cancer cell lines. After the HeLa, Hct116, Hep3B and A549 cells were treated with shikonin with different doses, the changes in the expression levels of proteins involved in apoptosis were examined
To confirm if there is a general correlation between the p21Cip1 level and the start of apoptosis, the dose-dependent effect of shikonin was determined in HeLa, Hct116, Hep3B, and A549 cells (Fig. 1d). PARP cleavage was observed in HeLa, Hct116, and A549 cells that were treated with shikonin and that showed a decrease in the p21Cip1 level, whereas in Hep3B cells, PARP cleavage was observed even when high p21Cip1 levels were retained. These results suggest that the increase in the p21Cip1 level is probably responsible for the shikonin-induced cell cycle arrest and transiently delayed apoptotic response in many but not all cancer cells. This finding is in agreement with previous results, which showed that the up-regulation of p21Cip1 confers resistance to bortezomib-mediated apoptosis in HeLa cells [33].
ROS/p38 MAPK pathway, not JNK, is involved in shikonin-induced apoptosis
To identify signaling pathways activated by shikonin, the effects of specific protein kinase inhibitors (Akt inhibitor, LY294002; JNK inhibitor, SP600125 and p38 MAPK inhibitor, SB203580) or a ROS inhibitor (NAC) were analyzed by determining relative survival and microscopic observation (Figs. 2a, S2). HeLa and A549 cells were pretreated with or without inhibitors and subsequently incubated with shikonin. Treatment with 2 and 4 μM shikonin caused significant cell death in HeLa (58.93 ± 2.5 % and 84.79 ± 1.2 %, respectively) and A549 (52.73 ± 2.2 % and 77.5 ± 1.4 %, respectively). Pretreatment of the cells with 100 μM NAC markedly reduced the cytotoxic effect of 2 and 4 μM shikonin to 19.9 ± 3.3 % and 41.65 ± 2.78 % in HeLa and to 22.61 ± 2.5 % and 47.56 ± 1.5 % in A549, respectively. ROS generation by shikonin in HeLa cells was determined in the DCF assay (Fig. S3). In addition, pretreatment with SB203580 also significantly increased cell survival in HeLa cells treated with 2 μM shikonin (55.65 ± 2.42 % vs. 41.06 ± 2.50 % in DMSO) and 4 μM shikonin (32.33 % ± 1.45 vs. 15.21 % ± 1.21 in DMSO). Survival of A549 cells was also significantly enhanced by pretreatment with SB203580 in cells treated with 2 μM shikonin (63.66 ± 2.10 % vs. 47.26 ± 2.20 % in DMSO) and 4 μM shikonin (35.58 ± 2.11 % vs. 22.48 ± 1.21 % in DMSO). In contrast, pretreatment with a pharmacologic Akt inhibitor (LY294002, 10 μM) enhanced the cytotoxic effect of 2 and 4 μM shikonin to 81.25 ± 1.4 % and 93.98 ± 0.9 % in HeLa and to 71.52 ± 1.4 % and 87.77 ± 0.9 % in A549, respectively. Thus, the ROS inhibitor appeared to block shikonin-induced apoptosis, whereas the PI3 K inhibitor exerted a synergistic cytotoxic effect and enhanced shikonin-mediated cell death. Pretreatment with a JNK inhibitor (SP600125, 10 μM) did not affect cell death in comparison with the control vehicle.
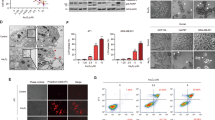
Akt is inactivated and involved in ROS-mediated ASK1 activation in shikonin-induced apoptosis. a Effects of different signaling pathway inhibitors on cell death and survival. After pretreatment for 2 h with 100 μM N-acetyl cysteine (NAC, a ROS inhibitor), 10 μM SB203580 (SB, a p38 inhibitor), 10 μM SP600125 (SP, a JNK inhibitor), or 10 μM LY294002 (LY, a Akt inhibitor), HeLa and A549 cells were treated with 2 and 4 μM shikonin for 24 h, and cell viability was determined with WST-1 assay. All means marked with asterisk (*p < 0.05, **p < 0.1) are significantly different from that of the control. b Effects of NAC and SB203580 on ASK1 phosphorylation at Ser83, p38 MAPK activation, and downstream signal transcription factors. HeLa cells were pretreated with or without NAC and SB203580 for 2 h and then coincubated with 2 μM shikonin for the indicated times, at which point protein lysates were prepared and subjected to immunoblot analysis. c Effects of p38 MAPK knockdown using siRNAs on ASK1 phosphorylation on Ser83 in the absence or presence of shikonin. The amount of phospho-ASK1 was determined by quantitation of the signals on films using Image J program (NIH) and expressed as a percentage relative to the amounts of GAPDH. d Effects of LY294002 on PARP cleavage and ASK1 phosphorylation at Ser83. e Effects of constitutively active Akt and dominant-negative Akt on PARP cleavage and ASK1 phosphorylation at Ser83. HeLa cells transfected with vehicle plasmid (pcDNA3) constitutively active Akt (myr-Akt1), or dominant-negative Akt (DN-Akt1) were incubated with shikonin for the indicated times. f Effect of ASK1 mutant (S83A) on shikonin-induced apoptosis. HeLa cells were transfected with plasmids containing wild-type ASK1 (HA-ASK1, WT) and mutant ASK1 (HA-ASK1, S83A) and 36 h later, cells were treated with 2 μM shikonin for the indicated times
To further investigate the involvement of ROS/p38 MAPK in shikonin-induced apoptosis, the expression levels of downstream factors of p38 MAPK were examined by immunoblot analysis after shikonin treatment in the presence of NAC and SB203580 in HeLa cells (Fig. 2b). As expected, PARP cleavage was significantly reduced in cells treated with NAC or SB203580. The activation of p38 MAPK was completely prevented in the presence of NAC and the activation of their target transcription factors, c-Jun and ATF-2, was significantly abrogated (Fig. 2b). Therefore, the ROS/p38 MAPK pathway, but not JNK, might be involved in the apoptotic pathway induced by shikonin. However, the inhibitory effect of SB203580 in shikonin-induced apoptosis was insufficient compared to that of NAC. Therefore, although p38 MAPK plays a role in regulating shikonin-induced apoptosis, the results suggest that other pathways may exist downstream of ROS.
ROS-mediated Akt inactivation is responsible for ASK1 activation in shikonin-induced apoptosis
Akt plays an essential role in regulating cell proliferation, promoting cell survival and inhibiting apoptosis [34, 35], which is consistent with the known apoptosis-regulating functions of the identified target proteins. Akt can phosphorylate ASK1 at serine 83 (Ser83) and inactivate the apoptotic function of the ASK1, leading to enhanced cell survival [4, 36]. The activity of Akt was transiently increased at the early time in cells treated with shikonin, but gradually decreased by prolonged exposure of shikonin and ASK1 was dephosphorylated at Ser83 (Fig. S4). In cells pretreated with NAC, the phosphorylation level of ASK1 remained significant, about 113 % at 9 h and 58 % at 12 h, after shikonin treatment compared to that of untreated cells, suggesting that ROS play a role upstream of ASK1 (Fig. 2b). On the other hand, in cells pretreated with SB203580, the phosphorylation of ASK1 decreased rapidly, to about 47 % at 9 h and 27 % at 12 h, after shikonin treatment, indicating that the decline of phospho-ASK1 was similar to that in shikonin-treated cells in the absence of an inhibitor (43 % at 9 h and 29 % at 12 h). However, it is still unclear whether p38 MAPK did not act upstream of ASK1 owing to the phosphorylation level of ASK1, which was increased by about three times in cells pretreated with SB203580 in the absence of shikonin compared to DMSO-treated cells (Fig. 2b). Because MAPKs are activated simultaneously by many stimuli and crosstalk can occur, it is possible that inhibition of p38 MAPK induces other kinase pathways as a compensatory mechanism in response to stress. It is known that the p38 MAPK inhibitor SB203580 has non-specific effects on MLK3-dependent JNK activation and Erk-dependent NF-kB activation, but knock-down of p38 MAPK by RNA interference did not affect other MAPK pathways, suggesting that non-specific effects of SB203580 are a specific property of this pharmaceutical inhibitor [37]. Therefore, we down-regulated the expression of p38 MAPK by using siRNA instead of SB203580 and evaluated its effect on ASK1 phosphorylation. As shown in Fig. 2c, knock-down of p38 MAPK did not show non-specific induction of ASK1 phosphorylation on Ser83. The phosphorylation level of ASK1 in the p38 knocked down cells in the absence of shikonin was similar to that of cells transfected with control siRNA (94 vs. 100 %). After shikonin treatment, the level of ASK1 dephosphorylation on Ser83 in the p38 knocked down cells (46 % at 9 h and 14 % at 12 h) was similar to that of cells transfected with control siRNA (52 % at 9 h and 13 % at 12 h), indicating that p38 MAPK worked as a downstream kinase of ASK1 in shikonin-induced apoptosis.
Since inhibition of PI3 K with LY294002 enhanced the cytotoxic effect of shikonin, we aimed to determine the role of Akt on ASK1 phosphorylation. Cells were pretreated with Akt inhibitor, LY294002, and the amount phospho-ASK1 (Ser83) was investigated. In cells pretreated with LY294002, phosphorylation of ASK1 at Ser83 was significantly reduced in comparison with untreated cells and increased PARP cleavage was noted following shikonin treatment (Fig. 2d). To confirm the role of Akt, cells were transfected with the constitutively active form of Akt [myristoylated (myr)-Akt1] or dominant-negative form of Akt (DN-Akt1). Overexpression of myr-Akt1 triggered the phosphorylation of ASK1 at Ser83 and reduced shikonin-induced PARP cleavage, whereas DN-Akt1 enhanced ASK1 dephosphorylation and PARP cleavage (Fig. 2e). These results imply that the activation of Akt attenuated the apoptotic effect of shikonin via ASK1 phosphorylation on Ser83. To investigate whether the dephosphorylation of ASK1 on Ser83 was involved in shikonin-induced apoptosis, cells were transfected with vectors expressing wild-type or S83A mutant ASK1. After 36 h of transfection, cells were incubated in media with or without 2 μM shikonin. Expression of S83A mutant ASK1, which is defective in ASK1 inactivation by Akt, resulted in significantly enhanced apoptosis compared with the expression of wild-type ASK1 (Fig. 2f). Recently, many pathways and signals related to shikonin-induced apoptosis are being validated in various cancer cells. Our experimental evidence suggests that shikonin led to dephosphorylation of ASK1 at Ser83 via Akt inactivation, which eventually facilitated apoptosis, implying that the ROS/Akt/ASK1 signal cascade is one of the mechanisms of shikonin-induced cell death.
ASK1/MKK3(6)/p38 MAPK signal cascade is activated in shikonin-induced apoptosis
To investigate the intracellular signal mechanisms and downstream effector molecules of ASK1 on shikonin-induced apoptosis, ASK1 was knocked down using siRNA that specifically silences ASK1 and the phenotypes were analyzed. Apoptotic cell death by shikonin was decreased by pretreatment with ASK1-specific siRNA compared to control siRNA (Fig. 3a). Since ASK1 is reportedly located upstream of the MKK4(7)/JNK and MKK3(6)/p38 MAPK pathways [8], and because our inhibitor study revealed that the JNK pathway is not involved in shikonin-induced apoptosis, we next investigated if the knockdown of ASK1 influenced the activation of p38 MAPK signaling cascade by using phospho-specific antibodies. Figure 3b shows that shikonin treatment resulted in the activation of p38 MAPK and its upstream activators MKK3 and MKK6. These bands were markedly reduced by the knockdown of ASK1 using siRNA, suggesting that ASK1 is responsible for the activation of the p38 MAPK cascade followed by apoptotic cell death.
ROS-mediated activation of the ASK1/MKK3(6)/p38 MAPK pathway in shikonin-induced apoptosis. a, b Effects of ASK1 knockdown using siRNA on shikonin-induced apoptosis and activation of downstream kinases. Cells were transfected with 50 nM ASK1 siRNA and control siRNA, and 36 h later, cells were treated with 2 μM shikonin for 24 h or for the indicated times. Cells in which ASK was knocked down with siRNA were incubated with shikonin for the indicated times, at which point protein lysates were prepared and subjected to immunoblot analysis. c–e Effects of siRNAs against MKK3 and MKK6 on shikonin-induced apoptosis and p38 MAPK activation. Cells pretreated with siRNAs against MKK3 and/or MKK6 were incubated with shikonin for 24 h for cell survival analysis or for 12 h for immunoblot analysis. Each bar represents the mean ± SE of the three independent experiments. All means marked with asterisk (*p < 0.05) are significantly different from that of the control
To confirm the roles of MKK3 and MKK6, we also investigated whether the knockdown of MKK3 and MKK6 rescues cells from apoptosis by determining the relative survival and by measuring PARP cleavage and p38 phosphorylation of cells treated with the respective siRNA (Fig. 3c–e). siRNA-mediated MKK3 and MKK6 knockdowns resulted in increased survival to 2.0 and 1.8-fold, respectively, and attenuated phosphorylation of p38 MAPK and PARP cleavage, even in the presence of shikonin. When both MKK3 and MKK6 were knocked down simultaneously, there was a 2.8-fold increase in cell survival after shikonin treatment (Fig. 3c, d). Thus, ROS-induced activation of p38 MAPK may be mediated via the sequential activation of ASK1 and MKK3/6.
Decreased p21Cip1 levels are required for efficient induction of apoptosis
p21Cip1 was originally identified as a mediator of p53-induced growth arrest and became later known as a modulator of apoptosis. Since shikonin-induced PARP cleavage occurred after degradation of p21Cip1, we aimed to evaluate whether p21Cip1 overexpression could enhance or inhibit shikonin-induced apoptosis in HeLa or A549 cells. HeLa cells were infected with an adenovirus containing the rAd-p21cip1 vector and apoptotic cells were quantified by annexin V/PI double-staining analysis (Fig. 4a). Apoptotic cells were quantified by counting annexin V-positive cells, as described under “Materials and Methods.” At 12 h post infection with rAd-p21Cip1 or rAd-empty vector, cells were treated with 2 μM shikonin for 12 h. The number of both early (29.74 %) and late (8.43 %) apoptotic cells induced by shikonin treatment was decreased to 10.33 and 4.46 %, respectively, in rAd-p21Cip1 infected cells. Analysis of relative survival also showed clear inhibition of shikonin-induced apoptosis (Fig. 4b–c). The correlation between the p21Cip1 level and apoptosis was further verified by immunoblot and cell cycle analysis. As shown in Fig. 4d, the p21Cip1 level was higher at 6 h but was lower at 12 h after shikonin treatment, at which time cleaved PARP was detected in both HeLa and A549 cells. However, in cells expressing ectopic p21Cip1, high levels of p21Cip1 were retained until 12 h after shikonin treatment, and no PARP cleavage was noted. In addition, FACS analysis revealed that cells infected with rAd-p21Cip1 were still arrested in the G1 phase (until 12 h after shikonin treatment), whereas cells infected with the rAd-empty vector rapidly progressed to apoptosis after a transient G1 arrest (Fig. 4e). To further confirm whether G1 arrest caused by p21Cip1 plays a role in the delay of shikonin-induced cell death, we investigated the effect of p21Cip1 depletion on apoptosis upon shikonin treatment. HeLa cells treated with p21Cip1-specific or control siRNA were exposed to shikonin (2 μM) for 9 h. Apoptosis was then measured by immunoblot analysis of the cleavage of PARP. Knock-down of p21Cip1 did not induce apoptosis per se but, following 9 h of incubation with shikonin, p21Cip1 knock-down resulted in significant PARP cleavage (Fig. 4f). Taken together, these results indicate that an increase in the level of p21Cip1 is responsible for cancer cell resistance to shikonin and down-regulation of p21Cip1 is necessary for inducing apoptosis.
Increase in p21cip1 levels is responsible for cell cycle arrest and transiently delayed apoptosis in the presence of shikonin. a Cells infected with an adenovirus containing the rAd-p21cip1 vector were treated with 2 μM shikonin for 12 h and the effects of p21Cip1 overexpression on the degree of apoptosis were determined. Annexin V/PI double staining analysis in HeLa cells. b Cells morphology. c Quantitative analysis. Data are represented as mean ± SEM of the percentage of apoptotic cells from three separate experiments. All means marked with asterisk (p < 0.05) are significantly different from that of the control. d Immunoblot analysis of PARP cleavage and p21cip levels. e Flow cytometry analysis for cells in the G1 phase and sub-G1 phase. f Effects of p21Cip1 knockdown using siRNAs on shikonin-induced apoptosis. HeLa cells were transfected with p21Cip1-directed siRNA, or with a control siRNA. Forty-eight hours later, cells were treated with shikonin for 9 h and the protein extracts analyzed by immunoblot analysis
p21Cip1 localization might be important to determine the cell’s fate after exposure to shikonin
It was reported that Akt is transiently activated to promote survival when cells are treated with H2O2, which is followed by Akt dephosphorylation with increasing treatment time, resulting in apoptotic cell death. In addition, p21Cip1 protein has to be re-localized to the cytoplasm via Akt kinase activity for cell survival and protection against cytotoxic damages [34, 38]. Koster et al. [39] reported that the localization of p21Cip1 in the cytoplasm was critical for cisplatin resistance, since re-localization of p21Cip1 to the nucleus by inhibition of Akt sensitized endothelial cell lines to cisplatin. Cytoplasmic p21Cip1 acts by direct binding and inhibition of apoptotic molecules such as ASK1, JNK, or caspase-3 [40–42]. Therefore, we examined the increase of Akt phosphorylation at early time point in the presence of shikonin, and the changes of p21Cip1 localization in the cell and the change of the p21Cip1 level, because the down regulation of p21Cip1 and dephosphorylation of ASK1 at Ser83 following inhibition of Akt seems to be responsible for shikonin-induced apoptosis. Cells were infected with an adenovirus carrying rAd-p21Cip1 for 12 h, and incubated in the presence or absence of LY294002 for 2 h prior to shikonin treatment for indicated times. Consistent with previous results with H2O2, phosphorylation of Akt and ASK1 was also transiently increased at 1–3 h, but gradually decreased by prolonged exposure to shikonin thereafter (Fig. 5a). The localization of p21Cip1 was monitored by immunostaining with an antibody against p21Cip protein. Ectopically expressed p21Cip1 in HeLa cells was primarily present in the nucleus, but was distributed in both the cytoplasm and nucleus at 3 h after shikonin treatment and finally re-localized into the nucleus following prolonged exposure to shikonin. In cells pretreated with LY294002, p21Cip1 protein was still present in the nucleus at 3 h after shikonin treatment and (Fig. 5b). Immunoprecipitation using a p21Cip1 antibody revealed that the level of ASK1 protein, which interacted with p21cip, was increased in cells exposed to shikonin and decreased in cells pretreated with LY294002 prior to shikonin treatment (Fig. 5c). It was previously reported that cytoplasmic p21Cip1 is able to interact to phosphorylated ASK1 on Ser83 for inhibiting apoptosis. Therefore, the increase of ASK1 binding to p21Cip1 represented the phosphorylation of ASK1 on Ser83 is increased and apoptotic activity of ASK1 was decreased. Moreover, pretreatment with LY294002 markedly prevented the export of p21Cip from the nucleus, which facilitated apoptosis. These results suggest that re-localization of p21Cip1 to the nucleus by inhibition of Akt sensitized HeLa cells to shikonin. Akt is involved in the cytoplasmic localization of p21Cip1 at early time points in the presence of shikonin and cytoplasmic p21Cip1 binds to ASK1, inhibiting apoptosis.
The Akt/ASK1 signaling pathway and p21cip1 localization are involved in shikonin-induced apoptosis in HeLa cells. a The change of phosphorylation status of Akt and ASK1 by shikonin. b Effects of shikonin treatment on p21cip1 localization. HeLa cells were transfected with p21cip1 using adenovirus containing rAd-p21cip1 vector. Cells were pretreated with LY294002 for 2 h or left untreated prior to shikonin exposure for 3 h or 9 h. Cellular localization of p21cip1 was detected using an anti-p21cip1 antibody. After extensive washing, samples were further incubated with FITC-conjugated anti-mouse IgG plus DAPI and examined by fluorescence microscopy. c In vitro immunoprecipitation assay. Total cell lysates were immunoprecipitated with an anti-p21cip1 antibody and immunoblotted with anti-ASK1 and anti-p21cip1 antibodies. Experimental conditions are the same as in panel A described above (Color figure online)
Discussion
The purpose of this work was to elucidate the mechanism underlying the anticancer activities of shikonin to optimize the medical value of shikonin and its derivatives. Many studies suggest that the derivatives of shikonin have the potential to be used as anticancer drugs since they might meet the criterion of possessing apoptosis-inducing activity as well as causing acceptable toxic side effects. Medicinal mixtures that contain shikonin are reported to be safe and effective in the treatment of late-stage cancer patients. The efficacy of shikonin in cancer treatment has been tested in vivo in animal models. The survival time of sarcoma 180 tumor-bearing mice treated with 6 mg/kg 2-hyim-DMNQ-S33, a shikonin derivative, was prolonged by 239 % compared to control animals [22]. Many efforts have been made in elucidating the precise molecular mechanisms and therapeutic targets of shikonin derivatives with regard to their anti-tumor activity. Some of the molecular and biochemical pathways involved in shikonin-induced apoptosis were investigated in several cancer cells. Previous studies have shown that ROS represent the most important mediator of shikonin-induced apoptosis in Bcr/Abl-positive chronic leukemia cell through JNK activation and in hepatocellular carcinoma cells through inhibition of the Akt and receptor-interacting protein (RIP)/NF-kB pathway [13, 43]. Although shikonin could induce apoptosis in various cancer cell types, its various modes of action and molecular mechanisms remain to be elucidated. Here, we demonstrated that shikonin induced apoptosis in HeLa cell via the ROS/Akt/ASK1 pathway and downregulation of p21Cip1.
Reactive oxygen species is the most important mediator of many anti-cancer agents [44]. We also found that accumulation of ROS is a critical component in shikonin-induced HeLa cell death. As shown in Fig. 3, inhibitor studies revealed that shikonin-induced apoptosis in HeLa cells is associated with inhibition of Akt and activation of p38 through excessive ROS accumulation. The JNK pathway is probably not involved in shikonin-induced apoptosis in HeLa cells.
This study addressed the involvement of the Akt/ASK1/p38 signal cascade in shikonin-induced apoptosis. Akt kinases are activated in response to many growth factors and mitogens and control cellular signaling molecules that are responsible for preventing cell death. Previous studies showed that shikonin derivatives significantly decreased phosphorylation of Akt signaling proteins in rat brain microglia [45] and induced apoptosis in HCC cells through inactivation of Akt [13]. The activity of Akt is mainly regulated by its phosphorylation via the PI3 K/Akt pathway. Oxidative stress causes activation of ASK1 and cell apoptosis. The activity of ASK1 is regulated in various ways, including phosphorylation, protein interaction, and oligomerization. Phosphorylation of ASK1 at Ser83 by Akt decreases its activity [4]. The Ser83 residue of ASK1 is fully phosphorylated in unstressed conditions, which renders ASK1 inactive, whereas upon ROS including H2O2, ASK1 becomes dephosphorylated at Ser83, which results in its activation [46, 47]. Our data showed that phosphorylation of ASK1 at Ser83, which attenuates its activity and promotes cell survival, was decreased in shikonin-treated cells and knockdown of ASK1 with specific siRNA attenuated shikonin-induced apoptosis (Fig. 3). Both apoptosis and dephosphorylation of ASK1 at Ser83 by shikonin were facilitated in cells pretreated with Akt inhibitor, which was alleviated by the constitutive overexpression of the active form of Akt and enhanced by dominant negative Akt1 (Fig. 2). Therefore, we conclude that shikonin-induced apoptosis of HeLa cells is related to the activation of ASK1 due to decreased Akt activity. ASK1 is one of the MKK kinase that activates p38 and JNK via activation of MKKs, MKK4/MKK7 and MKK3/MKK6 [8]. As shown in Fig. 4, the increase in phosphorylation of MKK3/MKK6, which is the downstream substrate of ASK1, was in concordance with shikonin-induced apoptosis. Activation of ASK1, which can selectively activate the p38 pathway, leading to apoptosis, as well as knockdown of ASK1 and MKK3/6 with specific siRNA significantly inhibited p38 phosphorylation induced by shikonin. The p38 inhibitor, but not JNK, attenuated the cytotoxic effect of shikonin. Thus, ROS may act as upstream-mediating molecules of the Akt/ASK1/p38 signaling pathway in shikonin-induced apoptosis in HeLa cells.
In addition, we observed that the p21Cip level was increased in cells treated with sub-lethal concentrations of shikonin, leading to cell cycle arrest at the G1 phase and rapid decrease in PARP cleavage. It was known that p21Cip1 could prevent apoptosis by direct binding and inhibition of molecules known to be involved in the apoptotic process, e.g., caspase-3, JNK, or ASK1 [40–42]. Thus, we hypothesized that p21Cip1 functions as a suppressor of apoptosis and initiates cell cycle arrest in order to allow DNA repair. p21Cip1 could directly bind to ASK1 to inhibit shikonin-induced apoptosis in HeLa cells at sub-lethal doses of shikonin. In fact, we found that overexpression of p21Cip1 alleviated the effect of shikonin on apoptosis and induced prolonged G1 arrest, and depletion of p21Cip1 significantly enhanced shikonin-induced cell death. Shikonin treatment also transiently led to rapid nuclear export of p21Cip1 and bound to ASK1 in the cytoplasm. Both cytoplasmic localization and interaction of ASK1 with p21Cip was abrogated by inhibition of Akt. These results suggest that Akt activity is important for cytoplasmic localization of p21Cip1 and apoptosis resistance in early responses to shikonin. Nuclear localization of p21Cip1 was necessary to initiate apoptosis induced by prolonged exposure to shikonin. On the basis of recent evidence, p21Cip1 levels increased in response to sub-lethal doses (<500 μM) of H2O2, which induced multi-phase cell cycle arrest in human lung carcinoma H1299 cells and mouse fibroblasts [48, 49] and resulted in dephosphorylation of ASK1 at Ser83. However, H2O2-induced PARP cleavage was observed even in HeLa cells that retained high p21Cip1 levels, while shikonin-induced PARP cleavage followed the decrease in p21Cip1. Levels of p21Cip1 following H2O2 treatment remained increased for several days and p53 levels also remained elevated for up to 24 h. Thus, p21Cip1 seems to be responsible for cell cycle arrest. However, down-regulation of p21Cip1 may not be important and other apoptotic pathways such as the mitochondrial pathway have been shown to be mainly involved in H2O2-induced cell death [30].
In conclusion, we suggest that increased p21Cip1 levels under moderate oxidative stress conditions protect cells from shikonin-induced apoptosis via interaction of p21Cip1 with ASK1 in the cytoplasm. Elevated ROS levels due to prolonged exposure to shikonin resulted in inactivation of Akt, dephosphorylation of ASK1 on Ser83, and re-localization of p21Cip to the nucleus. Subsequently, ASK1 dissociated from p21Cip1 is activated, and the MKK3/6/p38 MAPK pathway is activated in shikonin-induced apoptosis.
References
Meng TC, Fukada T, Tonks NK (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9:387–399
Huang C, Li J, Ding M, Leonard SS, Wang L, Castranova V et al (2001) UV induces phosphorylation of protein kinase B (Akt) at Ser-473 and Thr-308 in mouse epidermal Cl 41 cells through hydrogen peroxide. J Biol Chem 276:40234–40240
Wang X, McCullough KD, Franke TF, Holbrook NJ (2000) Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem 275:14624–14631
Kim AH, Khursigara G, Sun X, Franke TF, Chao MV (2001) Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 21:893–901
Gotoh Y, Cooper JA (1998) Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J Biol Chem 273:17477–17482
Wang TH, Wang HS, Ichijo H, Giannakakou P, Foster JS, Fojo T et al (1998) Microtubule-interfering agents activate c-Jun N-terminal kinase/stress-activated protein kinase through both Ras and apoptosis signal-regulating kinase pathways. J Biol Chem 273:4928–4936
Ichijo H (1999) From receptors to stress-activated MAP kinases. Oncogene 18:6087–6093
Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T et al (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90–94
Huang S, Shu L, Dilling MB, Easton J, Harwood FC, Ichijo H et al (2003) Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1). Mol Cell 11:1491–1501
Schepers H, Geugien M, Eggen BJ, Vellenga E (2003) Constitutive cytoplasmic localization of p21(Waf1/Cip1) affects the apoptotic process in monocytic leukaemia. Leukemia 17:2113–2121
Boncoeur E, Tabary O, Bonvin E, Muselet C, Fritah A, Lefait E et al (2006) Oxidative stress response results in increased p21WAF1/CIP1 degradation in cystic fibrosis lung epithelial cells. Free Radic Biol Med 40:75–86
Xie S, Wang Q, Luo L, Ruan Q, Liu T, Jhanwar-Uniyal M et al (2002) Proteasome-dependent downregulation of p21(Waf1/Cip1) induced by reactive oxygen species. J Interferon Cytokine Res 22:957–963
Gong K, Li W (2011) Shikonin, a Chinese plant-derived naphthoquinone, induces apoptosis in hepatocellular carcinoma cells through reactive oxygen species: A potential new treatment for hepatocellular carcinoma. Free Radic Biol Med 51:2259–2271
Tanaka S, Tajima M, Tsukada M, Tabata M (1986) A comparative study on anti-inflammatory activities of the enantiomers, shikonin and alkannin. J Nat Prod 49:466–469
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui QC et al (2009) Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer 124:2450–2459
Chen CH, Chern CL, Lin CC, Lu FJ, Shih MK, Hsieh PY et al (2003) Involvement of reactive oxygen species, but not mitochondrial permeability transition in the apoptotic induction of human SK-Hep-1 hepatoma cells by shikonin. Planta Med 69:1119–1124
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y et al (2007) Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 6:1641–1649
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK, Pan MH (2004) Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem 52:6330–6337
Xuan Y, Hu X (2009) Naturally-occurring shikonin analogues–a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett 274:233–242
Wu Z, Wu L, Li L, Tashiro S, Onodera S, Ikejima T (2004) p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375–S2 cells. J Pharmacol Sci 94:166–176
Chang IC, Huang YJ, Chiang TI, Yeh CW, Hsu LS (2010) Shikonin induces apoptosis through reactive oxygen species/extracellular signal-regulated kinase pathway in osteosarcoma cells. Biol Pharm Bull 33:816–824
Kim SH, Kang IC, Yoon TJ, Park YM, Kang KS, Song GY et al (2001) Antitumor activities of a newly synthesized shikonin derivative, 2-hyim-DMNQ-S-33. Cancer Lett 172:171–175
Kretschmer N, Rinner B, Deutsch AJ, Lohberger B, Knausz H, Kunert O et al (2012) Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma Cells. J Nat Prod 75:865–869
Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X (2011) Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 30:4297–4306
Lu Q, Liu W, Ding J, Cai J, Duan W (2002) Shikonin derivatives: synthesis and inhibition of human telomerase. Bioorg Med Chem Lett 12:1375–1378
Masuda Y, Nishida A, Hori K, Hirabayashi T, Kajimoto S, Nakajo S et al (2003) Beta-hydroxyisovalerylshikonin induces apoptosis in human leukemia cells by inhibiting the activity of a polo-like kinase 1 (PLK1). Oncogene 22:1012–1023
Kim DM, Won M, Chung CS, Kim S, Yim HJ, Jung SH et al (2010) JNK-mediated transcriptional upregulation of RhoB is critical for apoptosis of HCT-116 colon cancer cells by a novel diarylsulfonylurea derivative. Apoptosis 15:1540–1548
Ahn J, Choi JH, Won M, Kang CM, Gyun MR, Park HM et al (2011) The activation of p38 MAPK primarily contributes to UV-induced RhoB expression by recruiting the c-Jun and p300 to the distal CCAAT box of the RhoB promoter. Biochem Biophys Res Commun 409:211–216
Kim BK, Kim HM, Chung KS, Kim DM, Park SK, Song A et al (2011) Upregulation of RhoB via c-Jun N-terminal kinase signaling induces apoptosis of the human gastric carcinoma NUGC-3 cells treated with NSC12618. Carcinogenesis 32:254–261
Singh M, Sharma H, Singh N (2007) Hydrogen peroxide induces apoptosis in HeLa cells through mitochondrial pathway. Mitochondrion 7:367–373
Troyano A, Sancho P, Fernandez C, de Blas E, Bernardi P, Aller P (2003) The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ 10:889–898
Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN (1998) Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J 332(Pt 1):43–50
Gareau C, Fournier MJ, Filion C, Coudert L, Martel D, Labelle Y et al (2011) p21(WAF1/CIP1) upregulation through the stress granule-associated protein CUGBP1 confers resistance to bortezomib-mediated apoptosis. PLoS ONE 6:e20254
Lawlor MA, Alessi DR (2001) PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114:2903–2910
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC (2001) Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 3:245–252
Osaki M, Oshimura M, Ito H (2004) PI3 K-Akt pathway: its functions and alterations in human cancer. Apoptosis 9:667–676
Birkenkamp KU, Tuyt LM, Lummen C, Wierenga AT, Kruijer W, Vellenga E (2000) The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway. Br J Pharmacol 131:99–107
Levkau B, Koyama H, Raines EW, Clurman BE, Herren B, Orth K et al (1998) Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell 1:553–563
Koster R, di Pietro A, Timmer-Bosscha H, Gibcus JH, van den Berg A, Suurmeijer AJ et al (2010) Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest 120:3594–3605
Fan G, Ma X, Wong PY, Rodrigues CM, Steer CJ (2004) p53 dephosphorylation and p21(Cip1/Waf1) translocation correlate with caspase-3 activation in TGF-beta1-induced apoptosis of HuH-7 cells. Apoptosis 9:211–221
Shim J, Lee H, Park J, Kim H, Choi EJ (1996) A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature 381:804–806
Zhan J, Easton JB, Huang S, Mishra A, Xiao L, Lacy ER et al (2007) Negative regulation of ASK1 by p21Cip1 involves a small domain that includes Serine 98 that is phosphorylated by ASK1 in vivo. Mol Cell Biol 27:3530–3541
Mao X, Yu CR, Li WH, Li WX (2008) Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res 18:879–888
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Nam KN, Son MS, Park JH, Lee EH (2008) Shikonins attenuate microglial inflammatory responses by inhibition of ERK, Akt, and NF-kappaB: neuroprotective implications. Neuropharmacology 55:819–825
Hsieh CC, Papaconstantinou J (2006) Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J 20:259–268
Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y et al (2006) Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene 25:3699–3707
Barnouin K, Dubuisson ML, Child ES, Fernandez de Mattos S, Glassford J, Medema RH et al (2002) H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem 277:13761–13770
Chung YW, Jeong DW, Won JY, Choi EJ, Choi YH, Kim IY (2002) H(2)O(2)-induced AP-1 activation and its effect on p21(WAF1/CIP1)-mediated G2/M arrest in a p53-deficient human lung cancer cell. Biochem Biophys Res Commun 293:1248–1253
Acknowledgments
This work was supported, in part, by Basic Science Research (RBM3301213) and by the Bio & Medical Technology Development Program (No. 2012053532) of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, and a Grant from the Korea Research Council of Fundamental Science and Technology (KGM2011211).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiwon Ahn and Misun Won contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10495_2013_835_MOESM1_ESM.ppt
Fig. S1 IC50 of shikonin in different human cancer cell lines. Various human cell lines, i.e., WI38 (normal lung), PC3 (prostate cancer), MIA-paca2 (pancreatic cancer), MCF7 (breast cancer), HT29 (colon cancer), Hep3B (liver cancer), HeLa (cervical cancer), HCT116 (colon cancer), A549 (small lung cancer), and MDA-MB-231 (breast cancer), were maintained in RPMI 1640 supplemented with 1.5 g/L sodium bicarbonate, 5 % fetal bovine serum, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 mM l-glutamine in a humidified incubator containing 5 % CO2. Cells were treated with varying concentrations of shikonin (0.01–100 μM) for 48 h and cell viability was analyzed by the SRB assay. Results are presented as means ± SE of triplicate experiments (P < 0.05). Fig. S2 Cell morphology. Effects of different signaling pathway inhibitors on cell death and survival. After pretreatment for 2 h with 100 μM N-acetyl cysteine (NAC, a ROS inhibitor), 10 μM SB203580 (SB, a p38 inhibitor), 10 μM SP600125 (SP, a JNK inhibitor), or 10 μM LY294002 (LY, an Akt inhibitor), HeLa cells were treated with 2 or 4 μM shikonin for 24 h. Pretreatment of cells with NAC and SB203580 reduced the cytotoxic effect of shikonin. In contrast, pretreatment with LY294002 enhanced the cytotoxic effect of shikonin. Fig. S3 Shikonin generates ROS in HeLa cells. HeLa cells were treated with 2 μM shikonin for 6 h and the amount of generated ROS was determined by ROS assay kit (Cell Biolab, CA, USA). Briefly, cells were incubated with 100 μL of DCFH-DA in complete medium for 30 min at 37 °C to allow cellular incorporation. After, cells were incubated with a new medium containing shikonin with or without 100 μM NAC. ROSdependent DCF fluorescence was monitored at 37 °C with an emission wavelength set at 530 nm and an excitation wavelength set at 480 nm in a fluorescence microplate reader (SpectraMax, Molecular Devices, USA). Hydrogen peroxide was used as positive control for ROS. Each bar represents the mean ± SE of the three independent experiments. Fig. S4 Shikonin induced Akt dephosphorylation at Ser473 and ASK1 phosphorylation at Ser83. HeLa cells were treated with 2 μM shikonin for different times, followed by immunoblot analysis of Akt, phospho-Akt (Ser473), and phospho-ASK1 (Ser83). Supplementary material 1 (PPT 1822 kb)
Rights and permissions
About this article
Cite this article
Ahn, J., Won, M., Choi, JH. et al. Reactive oxygen species-mediated activation of the Akt/ASK1/p38 signaling cascade and p21Cip1 downregulation are required for shikonin-induced apoptosis. Apoptosis 18, 870–881 (2013). https://doi.org/10.1007/s10495-013-0835-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0835-5