Abstract
Growth Arrest Specific 1 (GAS1) is a protein expressed when cells are arrested and during development. When ectopically expressed, GAS1 induces cell arrest and apoptosis of different cell lines, and we have previously demonstrated that the apoptotic process set off by GAS1 is caused by its capacity inhibiting the GDNF-mediated intracellular survival signaling. In the present work, we have dissected the molecular pathway leading to cell death. We employed the SH-SY5Y human neuroblastoma cell line that expresses GAS1 when deprived of serum. We observed, as we have previously described, that the presence of GAS1 reduces RET phosphorylation and inhibits the activation of AKT. We have now determined that the presence of GAS1 also triggers the dephosphorylation of BAD, which, in turn, provokes the release of Cytochrome-c from the mitochondria to the cytosol activating caspase-9, prompting the activity of caspase-3 and resulting in apoptosis of the cells. The apoptotic process is intrinsic, because there is no activation of caspase-8, thus this is consistent with apoptosis induced by the lack of trophic support. Interestingly, in cells where GAS1 has been silenced there is a significant delay in the onset of apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth Arrest Specific 1 (GAS1) was isolated from serum starved NIH-3T3 fibroblast, and thus associated with cell arrest [1]. GAS1 is an intronless gene, suggesting it originated from a retrotransposon, that codes for a protein linked to the outer cell membrane by a glycosyl phosphatydylinositol (GPI) anchor and possesses a high structural homology with the GDNF family of receptors [2–4]. It was also found that when ectopically expressed GAS1 could arrest different tumor cells and induced cell death [5–7]. Moreover, we demonstrated that the over-expression of GAS1 caused cell arrest and apoptosis of glioma cells [8–12], and that GAS1 was acting by inhibiting the GDNF-induced intracellular signaling pathway [4, 13, 14]. Because of its capacity to arrest cell growth and induce apoptosis, GAS1 becomes an attractive option as a potential adjuvant in the treatment of cancer.
Interestingly, GAS1 has also been related with SHH functioning. This interaction was originally interpreted as antagonistic [15], but further work has shown it to be a cooperative interaction, with the participation of the Cdo and Boc proteins [16–18]. GAS1 is expressed during development [19], and alterations of the GAS1–SHH functional interaction have been associated with developmental abnormalities, such as craniofacial malformations in rodents [20], and holoprosencephaly in humans [21–23]. The dual effects of GAS1 inhibiting growth and inducing apoptosis in tumor cells, and on the other hand, increasing the effect of SHH during development, appear to be contradictory, also considering that in tumor cells GAS1 induces cell arrest and apoptosis in a manner independent of the presence of SHH [11]. Taken all this information together we propose that GAS1 is a pleiotropic protein that exerts different effects depending on the cellular context where it is expressed; this suggests that GAS1 plays a critical role in the integration of different intercellular signaling systems [14].
Considering the potential importance of GAS1 in the treatment of cancer, and to better understand its function, we decided to carefully analyze the molecular pathway by which GAS1 leads to cell death. For the present experiments, we took advantage of the capacity of SH-SY5Y cells of expressing GAS1 when deprived of serum [13], and then proceeded to silence its expression to determine the consequences of the lack of GAS1 on cell survival. We observed, as we have previously described, that the presence of GAS1 reduces RET tyrosine 1062 phosphorylation and inhibits the activation of AKT [11, 13]. We have now determined that the presence of GAS1 also triggers the dephosphorylation of BAD, which, in turn, provokes the release of Cytochrome-c (Cyt-c) from the mitochondria to the cytosol activating caspase-9, resulting in the consequent activation of caspase-3 and in apoptosis of the cells. The apoptotic process is intrinsic, because there is no activation of caspase-8, thus this is consistent with apoptosis induced by the lack of trophic support.
Materials and methods
Cell culture and treatment
The SH-SY5Y neuroblastoma cell line (ATCC) (passages 5–15) was cultured in DMEM high glucose (Gibco) supplemented with 10% fetal bovine serum (Gibco), 1% antibiotic/antimycotic (Gibco) and maintained at 37°C, in a 95% air/5% CO2 atmosphere, with 100% relative humidity (proliferating conditions). To induce the expression of GAS1, complete medium was withdrawn and substituted with medium without serum for different periods, up to 96 h. Cultures of cells were observed using an Observer D1 (Carl Zeiss) microscope and images acquired with the Axion Vision software Version 4.8.1.0 (Carl Zeiss Imaging Solutions).
GAS1 silencing
To silence GAS1 expression in a stable manner, we employed a complementary mRNA antisense approach. A 382 bp fragment comprising sequences in both the 5′UTR and coding regions (178–559 bp with respect to GAS1 mRNA) was amplified by PCR (forward primer 5′-CGGGGTACCAGAGCGGGACCAGAT-3′; reverse primer 5′-CGGGGTACCTGCCAGCAGATGAGG-3′) from the BAC clone RP11-208G24. The PCR fragment was cloned in both sense and antisense orientations in the pcDNA 3.0 plasmid, and sequenced. This fragment was then directionally subcloned into the pAdTrack-CMV expression plasmid which uses the CMV promoter and possesses a green fluorescent protein (GFP) reporter gene. The transfection of the different plasmids; backbone, GAS1 antisense and GAS1 sense, pAdTrack-CMV, pAdANTGas1, and pAdSENGas1, respectively, was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were selected with 600 μg/ml of G-418 (Gibco), maintained with 200 μg/ml of G-418, and finally selected by flow cytometry, using a Mo-Flo High-Performance Cell Sorter (Beckman Coulter).
RT-PCR assays
Total RNA was isolated using the Trizol reagent (Invitrogen). Briefly, 5 μg of total RNA were treated with DNAseI (Biolabs), reverse-transcribed with M-MLV reverse transcriptase (Invitrogen), and 2 μl of cDNA amplified by PCR. The amplification of GAS1 mRNA and β-ACTIN mRNA, as a control, were performed as previously described [9, 10]. We determined the expression of the (mRNAs) of members of the SHH pathway, SHH, PTCH1 and GLI1, as had been previously described [11, 24].
Cell viability assays
To determine cell viability, Trypan blue exclusion, and MTT assays were employed. For the Trypan blue assay, 1 × 105 cells were seeded in 24-well culture plates, and serum was withdrawn from the medium as previously described. The number of viable cells was determined at the indicated times. For the MTT assay, the same number of cells was employed, and the capability of cells to reduce MTT (Sigma) (5 mg/ml) to formazan was determined using a colorimetric assay with a Bio Rad 540 plate reader.
Release of cytochrome-c from mitochondria
The mitochondrial and cytosolic fractions were isolated using the Cyt-c Release Apoptosis Assay Kit (Calbiochem), and 10 μg of protein from each fraction were employed to determine the levels of Cyt-c by western blot analysis using a monoclonal antibody against Cyt-c (Calbiochem, catalog number JA5204, 1:200).
Western blot assays
Total protein was isolated in the presence of a protease inhibitor cocktail. Fifty micrograms of total protein were separated on 12 or 6% SDS-PAGE gels and transferred onto nitrocellulose. Membranes were blocked for 1 h at room temperature in 5% non-fat milk/TBST (0.1% Tween-20, TBS) and then incubated with primary polyclonal antibodies against: human GAS1 (ProSci, 1:500) [10, 13], GDNF (Santa Cruz Biotechnology, catalog number sc-328, 1:100), p-RET (Y1062, Santa Cruz Biotechnology, catalog number sc-20252-R, 1:700), t-RET (Santa Cruz Biotechnology, catalog number sc-13104, 1:700), t-AKT (Santa Cruz Biotechnology, catalog number sc-8312, 1:500), p-AKT (Santa Cruz Biotechnology, catalog number sc-7985-R 1:5,000), t-BAD (Cell Signaling, catalog number 9292, 1:500), p-BAD (Cell Signaling, catalog number 5286S, 1:250), Caspase-9 (Santa Cruz Biotechnology, catalog number sc-8355, 1:1,000), Caspase-3 (Cell Signaling, catalog number 9662, 1:500), Caspase-8 (Santa Cruz Biotechnology, catalog number sc-6134, 1:1,000), overnight at 4°C. Blots were washed and incubated with a goat anti-rabbit antibody (Jackson ImmunoResearch, catalog number 111-035-003, 1:5,000) or a rabbit anti-goat antibody (Jackson ImmunoResearch, catalog number 305-035-003, 1:5,000). As a positive control some blots were stripped and re-probed with a monoclonal anti-β-ACTIN antibody [25] and then with a goat anti-mouse (Invitrogen, catalog number 62-6520, 1:5,000). Secondary antibodies were coupled with peroxidase and proteins revealed with enhanced chemiluminescence (PerkinElmer). Images from films were digitally acquired with a BioDoc-It Imaging System (UVP), and densitometric analyses performed with the LabWorks software (UVP).
Apoptosis assays
Apoptosis was determined using two different assays. Annexin-V (Pacific Blue conjugate)/Propidium iodide (Invitrogen, catalog number A35122) assays were performed with flow cytometry, according to the manufacturer’s instructions, using a CyAN ADP instrument (DAKO Cytomation) and data were analyzed with the Summit 4.3 software. DNA damage was estimated using a TUNEL immunocytochemical assay according to the manufacturer′s instructions (Roche), and cells counted using an Olympus BX-51 microscope.
Statistical analysis
Linear regression analysis was performed and slopes compared with a t test using the SPSS 17 software; a P < 0.05 was considered significant.
Results
Effects of GAS1 on cell viability
To determine the capacity of our experimental approach of a complementary antisense mRNA to effectively silence the expression of GAS1 we employed SH-SY5Y cells, derived from a human neuroblastoma. We observed that there is no expression of GAS1 mRNA in either SH-SY5Y cells or cells transfected with the antisense GAS1 in normal serum (proliferating conditions), however, after serum withdrawal there is a clear expression of GAS1 in SH-SY5Y cells that persists for up to 96 h after serum withdrawal, whereas no GAS1 mRNA is detected in cells expressing antisense GAS1 (Fig. 1a). We also determined the levels of GAS1 protein, as assayed by western blot analysis, and observed that the expression of GAS1 mRNA antisense eliminates the expression of GAS1 induced by serum starvation to background noise levels (Fig. 1b, d). Moreover, neither the transfection of the pAd-SENGas1vector nor of the backbone plasmid had any effect on the expression of GAS1 after serum withdrawal (Supplementary Fig. 1). We also studied the effect of the GAS1 silencing procedure on cells in proliferating conditions, normal serum, and observed what appears to be normal morphology of the cells expressing the antisense mRNA (Fig. 1c). Photographs of SH-SY5Y cells transfected with pAd-Track-CMV, pAd-ANTGas1 and pAd-SENGas1 are shown in Supplementary Fig. 2, and we can observe similar morphologies in normal serum, and that cells are GFP positive. From these experiments, we conclude that the method employed to silence GAS1 expression is very effective, and it causes no evident damage to transfected cells.
Silencing of GAS1 in SH-SY5Y cells. a Shows the expression of GAS1 as determined by RT-PCR in control, non-transfected SH-SY5Y cells, and in cells transfected with pAd-ANTGas1 (SH-ANTGas1 cells), in conditions of proliferation (0 h), and at different times after serum withdrawal. b Shows a representative experiment of the presence of GAS1 protein as ascertained by western blot analysis in control and transfected SH-SY5Y cells, in conditions of proliferation and at different times after serum withdrawal. c Shows photographs of cultures of control and transfected cells in conditions of proliferation. d Shows the densitometric analysis of the expression of GAS1 in control SH-SY5Y and cells transfected with pAd-ANTGas1 in conditions of proliferation and at different times after serum withdrawal from three independent experiments. Results are expressed as arbitrary units, normalized with the respective β-ACTIN control (mean + SEM). Bar is 50 μm
Serum withdrawal causes cell death, and we wanted to determine the effect of GAS1 silencing on cell viability. For this series of experiments, we assayed cell viability employing two different methods, using the MTT assay, which reflects the functional capacity of mitochondria, and Trypan blue assays which report the integrity of the membranes. We compared the viability of SH-SY5Y cells, cells in which GAS1 had been silenced, cells transfected with the pAdTrack-CMV plasmid and cells expressing the sense sequence of GAS1, at different times after serum withdrawal. Figure 2a shows a high level of viable GAS1-silenced cells, as assayed by MTT, whereas SH-SY5Y cells and the different controls show an important loss of cell viability which is significantly different from the antisense cells. A similar trend is observed when using the Trypan blue assay, because we can observe a significantly higher number of viable antisense-expressing cells, when compared with all other controls, which behave in the same manner (Fig. 2b). Cells of all groups, in conditions of proliferation and at different times after serum withdrawal are shown in Supplementary Fig. 3; where we can observe the preserved survival of SH-ANTGas1 cells compared to all others after serum withdrawal, approximately 90 and 20%, respectively. These data show that the silencing of GAS1 preserves the number of viable cells, and that this result is specifically caused by the lack of GAS1, because the decrease in the number of viable cells is the same in control SH-SY5Y cells and in cells transfected with the backbone plasmid, or with the GAS1 sense sequence.
Effect of GAS1 on cell viability. a Shows the viability of cells as determined by the MTT assay, in conditions of proliferation and at different times after serum withdrawal. b Shows the percentage of viable cells according to the Trypan blue assay, in conditions of proliferation and at different times after serum withdrawal. Cells evaluated are: SH-SY5Y cells, control non-transfected, and: cells transfected with GAS1 antisense (SH-ANTGas1); transfected with GAS1 in the sense orientation (SH-SENGas1) and transfected with the pAdTrack-CMV plasmid (SH-Track). Data represent mean + SEM, N = 3; ***P < 0.001, SH-ANTGas1cells different from all other groups
Effects of GAS1 silencing the GDNF signaling pathway
Our previous work showed that GAS1 induced apoptosis by blocking the GDNF-mediated signaling pathway. Here, we wanted to describe in greater detail the molecular pathway leading to cell death. We took advantage of the property of SH-SY5H cells to express GAS1 after serum withdrawal, and to analyze the results comparing them with cells in which GAS1 had been silenced.
We first demonstrated that both, control and GAS1-silenced cells, expressed GDNF (Fig. 3). We then proceeded to determine the activity of the intracellular components of the GDNF pathway on both cell types, at different times after serum withdrawal. We first observed decreased levels of phosphorylation of RET in SH-SY5Y cells after serum withdrawal, whereas RET phosphorylation is preserved in GAS1-silenced cells (Fig. 3). An even more intense effect was observed regarding AKT activity; in this case the levels of phosphorylated AKT were undetectable in control cells, after serum withdrawal, but in cells that do not express GAS1 a robust signal is seen (Fig. 3). Another intermediate in the GDNF-induced signaling is BAD, here we observe that in response to serum withdrawal, there is a striking decrease of protein phosphorylation, but the level remains high in GAS1-silenced cells (Fig. 3). With these experiments, we demonstrate that in the presence of GAS1, there is a blockade of the GDNF-induced signaling pathway, an effect that is overcome when GAS1 is silenced.
Signaling pathway leading to apoptosis. The presence and activity of different components of the GDNF signaling pathway as assayed by western blot analysis, in control SH-SY5Y and SH-ANTGas1 transfected cells, in conditions of proliferation (0 h) and at different times after serum withdrawal. Left panel shows a representative experiment; from top to bottom: expression of GDNF, and β-ACTIN as its control; RET phosphorylated on Y-1062 (p-RET) and total RET as its control (t-RET); phosphorylated AKT (p-AKT) and total AKT (t-AKT) as its control; phosphorylated BAD (p-BAD) and total BAD (t-BAD) as its control. The right panels show the densitometric analysis of the components of the pathway from three independent experiments. Results are expressed as arbitrary units normalized with their respective controls (mean + SEM)
Since there is evidence indicating the effect of GAS1 on the SHH pathway we determined, by RT-PCR, the expression of the mRNAs of SHH, its receptor PTCH1, and GLI1 the most important mediator of this pathway, and observed they are present in SH-SY5Y cells and in silenced cells both in the presence and absence of serum (Supplementary Fig. 4).
GAS1 and apoptosis
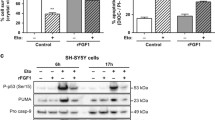
A critical component of the apoptotic process is the release of Cyt-c from the mitochondria to the cytosol, an event demonstrating mitochondrial membrane permeabilization. In this work, we show that in conditions of serum withdrawal, control cells release Cyt-c from the mitochondria to the cytosol, an event that does not occur in GAS1-silenced cells, where Cyt-c remains in the mitochondria, even after serum has been removed from the culture medium (Fig. 4).
Release of Cyt-c and activation of caspases by GAS1. Levels of Cyt-c in mitochondria and cytosol and assessment of the activation of caspases by western blot assays in control SH-SY5Y cells and GAS1-antisense expressing cells in conditions of proliferation (0 h) and at different times after serum withdrawal. From top to bottom: Cyt-c in mitochondria (Cyt–Mit) and in cytosol (Cyt–Cyt); assay for caspase-9; assay for caspase-3, and lower panel assay for caspase-8
The release of Cyt-c is necessary for the activation of caspases leading to apoptotic cell death. Figure 4 shows, that in the presence of GAS1, there is activation of both caspase-9 and caspase-3 in serum-starved SH-SY5Y cells, but that this does not occur in the cells that do not express GAS1, where there is no activation of these caspases. On the other hand, caspase-8 is not activated in response to serum withdrawal in neither cell type, control or GAS1 silenced cells. Finally, to demonstrate that serum withdrawal is inducing apoptotic death, we ascertained this type of cell death by two different methods. The first one was to determine the translocation of phosphatidylserine to the external side of the cell membrane, an early event of apoptosis, and the second was to establish the oligonucleosomal fragmentation of DNA, a late event in apoptosis, as revealed by a TUNEL assay. The number of viable SH-SY5Y cells is significantly decreased from 48 to 96 h after serum deprivation, when compared with cells not expressing GAS1, as determined by the Annexin V/propidium iodide assays; so that 96 h after serum withdrawal only 16% of SH-SY5Y cells remain viable, whereas 54% of SH-ANTGas1 cells are viable (Fig. 5a). The same tendency is observed when determining the amount of TUNEL-positive cells. There is a significantly higher number of TUNEL-positive control cells after removing the serum from the culture medium, compared with GAS1-silenced cells. This difference is over tenfold 96 h after serum withdrawal (Fig. 5b). Based on the results of these experiments, we surmise that GAS1 induces cell death by an intrinsic apoptotic process that can be significantly delayed or reduced by silencing GAS1, thus showing that GAS1 is a key factor in the death induced by serum withdrawal of SH-SY5Y cells.
Determination of apoptosis. a Apoptosis determined by Annexin V/propidium iodide by flow cytometry in SH-SY5Y control and SH-ANTGas1 transfected cells, in conditions of proliferation (0 h) and at different times after serum withdrawal. b Number of TUNEL positive cells in SH-SY5Y control and SH-ANTGas1 transfected cells, in conditions of proliferation and at different times after serum withdrawal. Data represent means + SEM, N = 3; ***P < 0.001, SH-ANTGas1cells different from SH-SY5Y control cells
Discussion
GAS1 has been associated with cell arrest, and when ectopically expressed it induces arrest and apoptosis of different tumor cell lines and primary cultures of human gliomas [5–12]. Furthermore, the expression and levels of GAS1 are related with the malignancy of tumors and with their metastatic potential, suggesting that GAS1 is a relevant tumor biomarker and thus of clinical relevance [26–31].
The effects of GAS1 inducing growth arrest and apoptosis are caused by its capacity inhibiting the GDNF-mediated survival pathway due to its structural similarity with the GDNF-α-receptors [4, 14]. Based on this concept, we showed that GAS1 decreases the phosphorylation of the GDNF co-receptor RET, and the activation of AKT [11, 13]. The fact that SH-SY5Y cells where GAS1 had been silenced showed a significant larger number of viable cells, compared with all different controls that do not affect GAS1 expression, proves that it is the absence of GAS1 what is protecting the cells from damage and death. Furthermore, cells transfected with either the backbone plasmid or the GAS1 sense vector are not different regarding their viability compared with the control non-transfected SH-SY5Y cells. This indicates that the effects on cell survival and mitochondrial failure are caused by the lack of GAS1, and that neither the transfection procedure nor the expression of unrelated RNAs influence cell functioning.
In the present work we sought to carefully examine the molecular pathway by which GAS1 induces apoptosis, and precisely define the type of apoptosis by which tumor cells die. To accomplish these objectives we used SH-SY5Y cells that express GAS1 when deprived of serum, and studied the specific effects of GAS1 by silencing it in serum deprived cells. Here, we confirmed previous results showing that GAS1 reduces the phosphorylation of RET and inhibits the activity of AKT. We followed the intracellular signaling cascade downstream and observed that in the presence of GAS1, BAD is dephosphorylated allowing it to translocate to the mitochondria where it binds to BcL-XL and facilitates the release of Cyt-c to the cytosol, a critical event in the apoptotic pathway. In the presence of GAS1 there is activation of both caspase-9 and caspase-3 which lead to apoptosis, demonstrated in this case both by the translocation of phosphatidyl serine to the outer cell membrane leaflet, and by the oligonucleosomal fragmentation of DNA. We observed that caspase-8 was not involved in this process, thus indicating this is an intrinsic apoptotic pathway. The present data are consistent with the fact that RET acts as a dependence receptor that induces caspase-3-mediated apoptosis in the absence of GDNF [32]. We showed that cells continue to produce GDNF even in the absence of serum (Fig. 3), indicating that in GAS1-silenced cells the endogenous production of GDNF maintains the survival pathway active. In Fig. 6, we submit a schematic representation of the model we propose to explain the molecular mechanism by which GAS1 induces cell death of tumor cells.
Proposed model of the action of GAS1 inducing apoptosis. The panel represents the GDNF family of ligands (GFLs) signaling pathway; in this representation (left side) the binding of GDNF to GFRα1 recruits RET to form a molecular complex allowing the autophosphorylation of RET which in turn interacts with PI3K to activate AKT, this permits AKT to phosphorylate BAD so it remains in the cytosol. On the right side we represent the effects of GAS1 on this signaling pathway. The presence of GAS1 impedes the autophosphorylation of RET on tyrosine 1062, decreasing the activity of AKT; this facilitates the dephosphorylation of BAD which translocates to the mitochondria and participates in the release of Cyt-c to the cytosol where it forms part of the apoptosome that activates caspase-9 and then caspase-3, leading to apoptosis of the cell. Phosphorylation is represented by circles on the illustration
GAS1 is a protein involved with two major signaling pathways, namely GDNF and SHH, and in this paper, we have analyzed a potential interaction between the two intracellular signaling cascades. Abnormal SHH signaling has been associated with the origin and maintenance of neuroblastomas, and several of the components of this pathway have been detected in primary human neuroblastomas and cell lines, including SH-SY5Y [33–36]. In the present work, we detected the expression of the mRNAs for SHH, PTCH1 and GLI1, in both SH-SY5Y cells and cells expressing GAS1 mRNA antisense, in conditions of proliferation and after serum withdrawal (Supplementary Fig. 4). These data show that the SHH pathway is active, because we can observe the expression of GLI1, its major functional mediator, and that neither the expression of GAS1, nor its silencing affect SHH signaling. From this information, we conclude that GAS1 is capable of inducing apoptosis in tumor cells in a manner independent of the SHH pathway, as we had previously observed for human primary gliomas [11].
In tumor cells, GAS1 acts as an antitumoral agent, and is also an important biomarker of the malignity of tumors. Moreover, when ectopically expressed, GAS1 has strong antitumoral effects, making it a potentially interesting therapeutic agent. In this paper, we present evidence showing that GAS1 induces an intrinsic apoptotic process, due to its capacity inhibiting the GDNF-induced survival pathway. Succinctly, GAS1 causes cell death by withdrawing the GDNF trophic support, an effect consistent with the previously described results of GDNF stimulating C6 cell proliferation [37]. Thus, we provide evidence to better understand the mechanism of action of GAS1 causing tumor cell death, and to assist providing tools to design new therapeutic strategies for treating cancer, as well as identifying potential target molecules involved in cell death.
References
Schneider C, King RM, Philipson L (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell 54:787–793
Hatinen T, Holm L, Airaksinen MS (2007) Loss of neurturin in frog–comparative genomics study of GDNF family ligand-receptor pairs. Mol Cell Neurosci 34:155–167
Stebel M, Vatta P, Ruaro ME, Del Sal G, Parton RG, Schneider C (2000) The growth suppressing gas1 product is a GPI-linked protein. FEBS Lett 481:152–158
Schueler-Furman O, Glick E, Segovia J, Linial M (2006) Is GAS1 a co-receptor for the GDNF family of ligands? Trends Pharmacol Sci 27:72–77
Evdokiou A, Cowled PA (1998) Growth-regulatory activity of the growth arrest-specific gene, GAS1, in NIH3T3 fibroblasts. Exp Cell Res 240:359–367
Evdokiou A, Cowled PA (1998) Tumor-suppressive activity of the growth arrest-specific gene GAS1 in human tumor cell lines. Int J Cancer 75:568–577
Mellstrom B, Cena V, Lamas M, Perales C, Gonzalez C, Naranjo JR (2002) Gas1 is induced during and participates in excitotoxic neuronal death. Mol Cell Neurosci 19:417–429
Zamorano A, Lamas M, Vergara P, Naranjo JR, Segovia J (2003) Transcriptionally mediated gene targeting of gas1 to glioma cells elicits growth arrest and apoptosis. J Neurosci Res 71:256–263
Zamorano A, Mellstrom B, Vergara P, Naranjo JR, Segovia J (2004) Glial-specific retrovirally mediated gas1 gene expression induces glioma cell apoptosis and inhibits tumor growth in vivo. Neurobiol Dis 15:483–491
Benitez JA, Arregui L, Vergara P, Segovia J (2007) Targeted-simultaneous expression of Gas1 and p53 using a bicistronic adenoviral vector in gliomas. Cancer Gene Ther 14:836–846
Dominguez-Monzon G, Benitez JA, Vergara P, Lorenzana R, Segovia J (2009) Gas1 inhibits cell proliferation and induces apoptosis of human primary gliomas in the absence of Shh. Int J Dev Neurosci 27:305–313
Lopez-Ornelas A, Mejia-Castillo T, Vergara P, Segovia J (2011) Lentiviral transfer of an inducible transgene expressing a soluble form of Gas1 causes glioma cell arrest, apoptosis and inhibits tumor growth. Cancer Gene Ther 18:87–99
Lopez-Ramirez MA, Dominguez-Monzon G, Vergara P, Segovia J (2008) Gas1 reduces Ret tyrosine 1062 phosphorylation and alters GDNF-mediated intracellular signaling. Int J Dev Neurosci 26:497–503
Dominguez-Monzon G, Gonzalez-Ramirez R, Segovia J (2011) Molecular mechanisms of action of Gas1 and its possible therapeutic applications. Curr Signal Transduct Ther 6:106–112
Lee CS, Buttitta L, Fan CM (2001) Evidence that the WNT-inducible growth arrest-specific gene 1 encodes an antagonist of sonic hedgehog signaling in the somite. Proc Natl Acad Sci USA 98:11347–11352
Allen BL, Tenzen T, McMahon AP (2007) The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev 21:1244–1257
Martinelli DC, Fan CM (2007) Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev 21:1231–1243
Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP (2011) Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell 20:775–787
Lee KK, Leung AK, Tang MK, Cai DQ, Schneider C, Brancolini C, Chow PH (2001) Functions of the growth arrest specific 1 gene in the development of the mouse embryo. Dev Biol 234:188–203
Seppala M, Depew MJ, Martinelli DC, Fan CM, Sharpe PT, Cobourne MT (2007) Gas1 is a modifier for holoprosencephaly and genetically interacts with sonic hedgehog. J Clin Invest 117:1575–1584
Martinelli DC, Fan CM (2009) A sonic hedgehog missense mutation associated with holoprosencephaly causes defective binding to GAS1. J Biol Chem 284:19169–19172
Ribeiro LA, Quiezi RG, Nascimento A, Bertolacini CP, Richieri-Costa A (2010) Holoprosencephaly and holoprosencephaly-like phenotype and GAS1 DNA sequence changes: report of four Brazilian patients. Am J Med Genet A 152A:1688–1694
Pineda-Alvarez DE, Roessler E, Hu P, Srivastava K, Solomon BD, Siple CE, Fan CM, Muenke M (2011) Missense substitutions in the GAS1 protein present in holoprosencephaly patients reduce the affinity for its ligand, SHH. Hum Genet. doi:10.1007/s00439-011-1078-6
Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA (2007) Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA 104:5895–5900
Garcia-Tovar CG, Perez A, Luna J, Mena R, Osorio B, Aleman V, Mondragon R, Mornet D, Rendon A, Hernandez JM (2001) Biochemical and histochemical analysis of 71 kDa dystrophin isoform (Dp71f) in rat brain. Acta Histochem 103:209–224
Gobeil S, Zhu X, Doillon CJ, Green MR (2008) A genome-wide shRNA screen identifies GAS1 as a novel melanoma metastasis suppressor gene. Genes Dev 22:2932–2940
Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, Pellegata NS, de la Chapelle A (2001) Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA 98:15044–15049
Bettuzzi S, Davalli P, Astancolle S, Carani C, Madeo B, Tampieri A, Corti A (2000) Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulfated glycoprotein 2) in human prostate cancer specimens. Cancer Res 60:28–34
Rizzi F, Belloni L, Crafa P, Lazzaretti M, Remondini D, Ferretti S, Cortellini P, Corti A, Bettuzzi S (2008) A novel gene signature for molecular diagnosis of human prostate cancer by RT-qPCR. PLoS One 3:e3617
Zhao L, Pan Y, Gang Y, Wang H, Jin H, Tie J, Xia L, Zhang Y, He L, Yao L, Qiao T, Li T, Liu Z, Fan D (2009) Identification of GAS1 as an epirubicin resistance-related gene in human gastric cancer cells with a partially randomized small interfering RNA library. J Biol Chem 284:26273–26285
Jiang Z, Xu Y, Cai S (2011) Down-regulated GAS1 expression correlates with recurrence in stage II and III colorectal cancer. Hum Pathol 42:361–368
Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P (2000) The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 19:4056–4063
Hamann J, Wernicke C, Lehmann J, Reichmann H, Rommelspacher H, Gille G (2008) 9-Methyl-beta-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. Neurochem Int 52:688–700
Mao L, Xia YP, Zhou YN, Dai RL, Yang X, Duan SJ, Qiao X, Mei YW, Hu B, Cui H (2009) A critical role of sonic hedgehog signaling in maintaining the tumorigenicity of neuroblastoma cells. Cancer Sci 100:1848–1855
Shahi MH, Schiapparelli P, Afzal M, Sinha S, Rey JA, Castresana JS (2011) Expression and epigenetic modulation of sonic hedgehog-GLI1 pathway genes in neuroblastoma cell lines and tumors. Tumour Biol 32:113–127
Schiapparelli P, Shahi MH, Enguita-German M, Johnsen JI, Kogner P, Lazcoz P, Castresana JS (2011) Inhibition of the sonic hedgehog pathway by cyplopamine reduces the CD133+/CD15+ cell compartment and the in vitro tumorigenic capability of neuroblastoma cells. Cancer Lett 310:222–231
Wiesenhofer B, Weis C, Humpel C (2000) Glial cell line-derived neurotrophic factor (GDNF) is a proliferation factor for rat C6 glioma cells: evidence from antisense experiments. Antisense Nucleic Acid Drug Dev 10:311–321
Acknowledgments
We want to thank Victor H. Rosales for his assistance with the flow cytometry assays, and Dr. J.M. Hernández (Cinvestav) for the kind gift of the antibody against β-ACTIN, and Dr. B. Vogelstein (Johns Hopkins University) for providing us with the pAdTrack-CMV plasmid. This work was partially supported by Conacyt (Mexico) Grant 127357 (J.S).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zarco, N., González-Ramírez, R., González, R.O. et al. GAS1 induces cell death through an intrinsic apoptotic pathway. Apoptosis 17, 627–635 (2012). https://doi.org/10.1007/s10495-011-0696-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0696-8