Abstract
One of characteristics of diabetes mellitus (DM) is endothelial cell (EC) dysfunction and apoptosis which contributes to the development of vasculopathy. Advanced glycation end products (AGEs) continuously produced in the setting of DM play an important role in causing EC dysfunction and apoptosis. However, the underlying molecular mechanism remains largely elusive. Lactadherin, a secreted glycoprotein of milk-fat globule, is expressed by multiple cell types of arterial wall including ECs. Our previous proteomic studies showed that the expression of lactadherin was significantly increased in the aorta of diabetic rats as compared with control rats and treatment with grape seed procyanidin extracts significantly inhibited the lactadherin expression in diabetic rats. We hypothesized that lactadherin plays a critical role in AGEs-induced EC apoptosis; grape seed procyanidin B2 (GSPB2) and resveratrol protect against AGEs-induced EC apoptosis through lactadherin regulation. Our results showed that AGEs upregulated lactadherin expression and lactadherin RNA interference significantly attenuated AGEs-induced EC apoptosis. Overexpression of lactadherin increased EC apoptosis with up-regulation of Bax/Bcl-2 ratio, cytochrome c release, caspase-9 and caspase-3 activation suggesting the involvement of mitochondria apoptosis pathway. Mechanistically, overexpression of lactadherin reduced the phosphorylation of GSK3beta at baseline. Our study also revealed nine proteins interacting with lactadherin in HUVEC and study of these candidate proteins could unveil further underlying molecular mechanisms. In summary, our study identified lactadherin as a key player responsible for AGEs-induced EC apoptosis and antioxidants GSPB2 and resveratrol protect against AGEs-induced EC apoptosis by inhibiting lactadherin. Targeting lactadherin with antioxidant could be translated into clinical application in the fighting against DM complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Premature development of microvascular and macrovascular disease is the leading cause of morbidity and mortality associated with diabetes mellitus (DM) [1, 2]. Among the many metabolic abnormalities of DM, advanced glycation end products (AGEs) have been most consistently and significantly correlated with diabetic vascular complications by epidemiological studies. AGEs lead to endothelial dysfunction and apoptosis, which plays a critical role in the pathophysiology of vascular complications in DM [3–5].
In endothelial cells, AGEs induce mitochondrial dysfunction thus increased oxidative stress leading to cellular dysfunction and cell death [6, 7]. However, the molecular mechanism underlying AGEs-induced tissue damage and DM complications remains largely elusive, which has been the bottle neck in the development of effective therapeutic approaches to the prevention and treatment of DM and its complications. Protection of endothelial injury from AGEs in DM could be the first step in preventing the cardiovascular complications of DM.
In this study we provided strong evidence that lactadherin is an important player in the pathogenesis of endothelial dysfunction in AGE-induced endothelial dysfunction. Lactadherin is a secreted glycoprotein of milk-fat globule that shares structural domain homology with Del-1. Mouse lactadherin is also known as milk-fat globule-EGF factor 8 (MFG-E8). It consists of two C domains with homology to the C1 and C2 domains of coagulation factor V and factor VIII and one (in human) or two (in mouse) epidermal growth factor-like domains with an Arg-Gly-Asp (RGD) integrin-binding sequence [8, 9]. Lactadherin is produced in and around blood vessels. In arteries, lactadherin is mainly expressed by adventitial microvessels, medial smooth muscle cells and some luminal endothelial cells [9]. Our previous proteomic studies showed that the expression of lactadherin in the aorta of diabetic rats was significantly higher than those of control rats. We also found that treatment with grape seed procyanidin extracts (GSPE) significantly inhibited the expression of lactadherin in diabetic rats [10]. Among the signaling molecules in cell apoptosis, GSK3β is a pro-apoptotic kinase, as its overexpression sensitizes cells to apoptosis and pharmacological inhibition of GSK3β attenuates cytochrome c release from mitochondria thus inhibits apoptosis. Phosphorylation of GSK3β on residue serine-9 results in GSK3β inactivation [11].
Polyphenols are secondary metabolites of plants that are classified according to their structures. Some polyphenols are ubiquitous in plants, whereas others are restricted to particular families or species. Procyanidins and resveratrol are a complex family of polyphenol polymers widespread in nature and occur in processed products such as grape wines, a few fruits and vegetables [12, 13]. GSPE derived from grape seeds have been reported to possess anti-oxidant, anti-nonenzymatic glycosylation, anti-inflammation, and anti-tumor effects, among others [14–16]. Dimeric procyanidin B2 is one of the main components of GSPE, composed of two molecules of the flavan-3-ol (−)-epicatechin linked by a 4b → 8 bonds. Several studies have shown that procyanidin B2 and resveratrol have a variety of anti-inflammatory, anti-tumor effects and cardiovascular protective properties [17–19].
In our present study, we unveiled a novel molecular mechanism underlying AGE-induced endothelial cell apoptosis. Our data showed that lactadherin was a key mediator in endothelial cell apoptosis in response to AGE treatment and grape seed procyanidin B2 (GSPB2) and resveratrol were protective against AGE-induced endothelial apoptosis by regulating lacterherin. We also further investigated the role of lactaherin in the signaling cascade implicated in apoptotic process.
Materials and methods
Materials
GSPB2 (>95% purity, Lot No: 20080915) and resveratrol (>98% purity, Lot No: 0810018-22) were provided by Jianfeng Inc (Tianjin, China). Bovine serum albumin (BSA), d-glucose, collagenase, trypsin/EDTA solution, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, USA). Fetal bovine serum (FBS) and RPMI 1640 were obtained from GIBCO (Grand Island, USA). Cell counting kit-8 (CCK-8) was obtained from Dojindo (Kumamoto, Japan). HUVEC were obtained from American Type Culture Collection (Rockville, USA). Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) in situ apoptosis detection kit (Roche Diagnostic, Indianapolis, IN). Cytochrome c enzyme-linked immunosorbent assay kit were purchased from eBioscience (San Diego, USA). Caspase-9 activity kit was purchased from R&D Systems (Minneapolis, USA). The antibody of lactadherin and prohibitin2 (PHB2) were purchased from Abcam (Cambridge, USA). The antibodies of GSK3β, phospho-GSK3β, cleaved caspase 3, caspase 3, Bax and Bcl-2 were purchased from Cell Signaling Technology (Beverly, MA). All other reagents were standard commercial high-purity materials.
AGE-BSA preparation
AGEs were prepared by incubating 50 mg/ml BSA with 0.5 mol/l glucose in 0.2 mol/l phosphate buffered saline (PBS, pH 7.4) in the dark at 37°C for 12 weeks [20, 21]. Before incubation, the solution was sterile filtered by passing it through a 0.2 mm filter. Unmodified BSA was incubated under the same conditions in the absence of glucose for control. After incubation, unincorporated sugars were removed by extensive dialysis. The AGEs concentration was determined by fluorescence spectrophotometry (Hitachi V-2001, Japan; 390 nm excitation wavelength, 450 nm emission wavelength). As a result, the AGEs concentration in AGE-modified BSA was 94.5 U/mg proteins, whereas that in control group was 0.5 U/mg proteins. Endotoxin concentrations were measured by the limulus amebocyte lysate assay (Endos), which revealed negligible values (<0.2 μg/l).
Cell cultures
HUVEC were cultured in complete medium RPMI 1640 containing 10% FBS at 37°C in a humidified atmosphere containing 50 ml/l CO2. GSPB2 and resveratrol were dissolved in DMSO and diluted so that the final concentration of DMSO was <0.1%.
Knockdown of lactadherin by siRNA, construction of lactadherin overexpression plasmids and transfection
Short interfering RNAs (siRNAs) for lactadherin and negative control siRNAs were designed and chemically synthesized from Shanghai GenePharma Co., Ltd (Shanghai, China). The siRNA sequence targeting lactadherin (GenBank accession no: NM_001114614) including: sense 5′-CGAGGAGAUUUCCCAAGAATT-3′, antisense 5′-UUCUUGGGAAAUCUCCUCGTT-3′. The sequence of negative control siRNA is: sense 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense 5′-ACGUGACACGUUCGGAGAATT-3′. For transient transfection, HUVEC were cultured in six-well plates overnight and transfected with siRNA against lactadherin using Lipofectamine2000 (Invitrogen Life Technologies, Carlsbad, CA) according to the instructions of the manufacturer. The expression of lactadherin was assayed 48 h after transfection by real-time PCR and western blotting.
Overexpression plasmids pGC-FU-lactadherin were constructed as follows from Shanghai GeneChem Co., Ltd (Shanghai, China). Briefly, full-length cDNA of lactadherin (GenBank accession no: NM_001114614) was amplified from a human cDNA library (open biosystem, USA) by PCR using forward primer (5′-CATTCTCCAGGCCCAGTGGCTCGACACAT-3′) and reverse primer (5′-GCCACTGGGCCTGGAGAATGGGAACATTGCC-3′). The sequences of lactadherin were confirmed by sequencing. The PCR products were then subcloned into pGC-FU expression vectors. The overexpression lentiviral vectors with lactadherin (LV-lactadherin) or green fluorescent protein (LV-GFP) were made. HUVEC (1 × 106) were transduced with lentiviral vectors at a multiplicity of infection (MOI = 10) according to the manufacturer’s instructions. Stably transfectant clones were characterized using real-time PCR and western blotting for the expression levels of lactadherin at 5 days.
Cell viability analysis
Cell viability was measured by MTT or CCK-8 colorimetric assay. HUVEC with overexpression or siRNA knockdown of lactaherin (1 × 106 cells/ml) were incubated for 48 h at 37°C in the presence or absence of GSPB2 and resveratrol (10 μmol/l). Moreover, HUVEC transfected with lactadherin siRNA were treated with 200 μg/ml of AGEs. MTT (0.5 mg/ml) or CCK-8 (20 μl) was added at 37°C for 4 h and cell viability was measured per instruction.
Detection of apoptosis by TUNEL assay
HUVEC with overexpression or siRNA knockdown of lactaherin were treated with or without GSPB2 and resveratrol (10 μmol/l) for 48 h. The apoptotic cells were determined by TUNEL assay using an in situ apoptosis detection kit according to the manufacturer’s instructions. The number of apoptotic cells was counted in ten randomly selected fields.
Cell morphology
Logarithmic phase cells were trypsinized and plated to the subconfluent density of 106/ml in 75 cm2 Petri dish and harvested after incubated with or without GSPB2 and resveratrol (10 μmol/l) for 48 h. The cells were fixed in 2.5% glutaraldehyde (v/v) and 2% osmium tetroxide (w/v), washed in PBS, post-fixed in osmium tetroxide, dehydrated in an ethanol series, embedded in epoxy resin, and examined with a HITACHI H-800 electron microscope as described [22].
Cytochrome c release and caspase-9 activity assay
After subcellular fractionation, the cytochrome c concentration in the cytosol was measured using the ELISA kit following the manufacturer’s instructions. The caspase-9 activity in cell lysates was measured using colorimetric assay kit, per the manufacturer’s instructions. The data are expressed as the mean optical density of the samples normalized to a percentage of the control value.
Quantitative real-time PCR
Total RNA was extracted from cells and underwent quantitative analysis of mRNA expression of lactadherin by real-time PCR with an ABI Prism 7500 sequence detection system (Applied Biosystems, UK). The reaction reagent SYBR Green I Master Mix Kit (Stratagene) was used according to the manufacturer’s instructions. First strand cDNA was synthesized from total RNA per instruction (RT kit from MBI, Canada). The real-time PCR reaction components consisted of 10 μl of 2 × SYBR Green I Master Mix, 0.5 μl up-stream primers (10 μmol/l), 0.5 μl down-stream primers (10 μmol/l), 1 μl cDNA and 8 μl double distilled water. Sequences of the primer sets used were as follows: lactadherin (forward) 5′-CGTACACCTGCACGTGCCTTA-3′, (reverse) 5′-GGGACCCAATGCTGCAAAC-3′; β-actin (forward) 5′-TGGCACCCAGCA CAATGAA-3′, (reverse) 5′-CTAAGTCATAGTCCGCCTAGAAG CA-3′. The PCR conditions for all genes were as follows: 50°C for 2 min; preheating, 95°C for 10 min; followed by 40 cycles of denaturation (95°C for 15 s) and annealing/elongation (62°C for 50 s). Each sample was run in triplicate. Gene-specific mRNA was normalized to β-actin mRNA as an internal control. The amounts of lactadherin mRNA were expressed as fold change relative to those of untreated cells set as 1.
Western blotting
Equal amount of proteins were separated by SDS-PAGE (12%) and transferred onto a polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Proteins of interest were revealed by specific antibodies followed by secondary antibody. Signal detection was performed via exposing of the blots to enhanced DAB color reagent following the manufacturer’s recommendations. Densitometry was obtained for quantification of each identified protein band (Digital Protein DNA Imagineware, Huntington Station, NY, USA).
Co-immunoprecipitation
Sample preparation and LTQ-ESI-MS/MS analysis
HUVEC overexpressing lactadherin were lysed and the cell lysates (600 μl) were equally divided. The lysates were incubated with either anti-lactadherin antibody or IgG (2 μg/ml) overnight at 4°C. The samples were then treated with 30 μl of protein A/G-agarose beads for 2 h at 4°C. The beads were centrifuged and washed for 5 times. The beads were resuspended in loading buffer and boiled before subjected to 12% SDS-PAGE followed by LTQ-ESI-MS/MS analysis and database searching. The SDS-PAGE gels were scanned using GS-800 Calibrated Densitometer (Bio-Rad) and image analysis. Comparative studies was performed using the PDQuest Image Analysis software (Bio-Rad) per instruction. Different bands were identified when at least 100% of volume difference between matching spots consistently in all the triplicate experiments. These gels were fixed and stained with colloidal Coomassie brilliant blue (CBB). The specific bands of lactaherin immunoprecipitation (L-IP) group, as defined by the PDQuest Image analysis, were excised from the cCBB-stained gels for a modified in-gel trypsin digestion procedure. The peptides mixture was solubilized with 1% TFA for mass spectrometry analysis.
The peptide mixtures were measured using on a LTQ-Orbitrap Velos MS/MS (ThermoFinnigan, San Jose, CA, U.S.A.), using a surveyor high performance liquid chromatography (HPLC) system. The system was fitted with a C18 RP column (0.15 mm × 150 mm, Thermo Hypersil-Keystone). Mobile phase A (0.1% formic acid in water) and the mobile phase B (0.1% formic acid in ACN) were selected. The tryptic peptide mixtures were eluted using a gradient of 2–98% B over 60 min. The temperature of the heated capillary was set at 170°C. A voltage of 3.0 kV applied to the ESI needle resulted in a distinct signal. The normalized collision energy was 35.0. The number of ions stored in the ion trap was regulated by the automatic gain control. Voltages across the capillary and the quadrupole lenses were tuned by an automated procedure to maximize the signal for the ion of interest. The LTQ mass spectrometer was set so that one full MS scan was followed by ten MS/MS scans on the ten most intense ions from the MS spectrum. Dynamic Exclusion was set at repeat count 2; repeat duration 30 s, exclusion duration 90 s.
For protein identification and statistical validation, the acquired MS/MS spectra were automatically searched against the International Protein Index (IPI) HUMAN version 3.53 database using the Turbo SEQUEST program in the BioWorksTM 3.1 software suite. An accepted SEQUEST result had to have a DelCN score of at least 0.1 (regardless of charge state). Peptides with a +1 charge state were accepted if they were fully tryptic and had a cross correlation (Xcorr) of at least 1.9. Peptides with a +2 charge state were accepted if they had an Xcorr ≥ 2.2. Peptides with a +3 charge state were accepted if they had an Xcorr ≥ 3.75. Identifications were validated when considering at least two peptide sequences per protein or one peptide repeated 4 times. To sort out a single protein member from a protein group, we chose the protein with the highest sequence coverage.
Confirmation of lactadherin–PHB2 interaction
The proteins to be analyzed by immunoblotting were removed from the beads and separated by 1D gel electrophoresis as described above (Sample preparation). The gel was blotted and incubated with a polyclonal antibody to PHB2 overnight followed by incubation with the HRP-conjugated secondary antibody. Signal detection was performed via exposing of the blots to enhanced DAB color reagent.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis between groups was made using one-way analysis of variance (ANOVA) followed by Tukey’s HSD test for multiple comparisons. P value < 0.05 was considered statistically significant. All analyses were performed with SPSS for Windows software version 10.0 (SPSS, Chicago, USA).
Results
Transduction efficiency with lactadherin siRNA and overexpression plasmids
We transduced the HUVEC with siRNA or lentivirus vector. Transduction conditions were optimized by using different MOI and the transduction efficiency was assessed by fluorescence microscopy, real-time PCR and western blotting. HUVEC carrying negative control siRNA (NC), HUVEC carrying siRNA against lactadherin (LsiRNA) and HUVEC carrying GFP (LV-C), HUVEC carrying both GFP and lactadherin genes (LV) were harvested. The transduction efficiency was about 95% or higher at day 5 or longer. Transgene expression was confirmed by fluorescent microscopic imaging for lactadherin protein expression (Fig. 1a, d). The protein expression of lactadherin in LsiRNA group decreased to more than 60% the level of the NC group at 48 h after transfection (Fig. 1b, c). Lactadherin mRNA and protein expression reached its highest level at day 5 after virus removal (Fig. 1e, f).
Lactadherin siRNA and overexpression plasmids transfection HUVEC. a Fluorescence micrograph displays lactadherin siRNA in HUVEC (×200). b RT-PCR analysis demonstrates lactadherin mRNA expression in HUVEC at 12, 24, and 48 h after transfection. c Western blot analysis demonstrates lactadherin protein expression in HUVEC at 24 and 48 h after transfection. d Fluorescence micrograph displays lactadherin-GFP overexpression in HUVEC (×200). e RT-PCR analysis demonstrates lactadherin mRNA expression in HUVEC at 24, 72, and 120 h after transfection. f Western blot analysis demonstrates lactadherin protein expression in HUVEC at 72 and 120 h after transfection. CC group normal control cells, NC group negative control siRNA cells, LsiRNA group siRNA against lactadherin cells, LV-C group HUVEC carrying GFP, LV group HUVEC carrying both GFP and lactadherin, HUVEC human umbilical vein endothelial cells
Effects of lactadherin on viability in HUVEC treated by AGEs
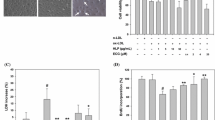
To investigate the role of lactadherin in HUVEC viability, we transfected HUVEC with lactadherin overexpression lentiviral vectors and siRNA, and estimated the cell viability using MTT and CCK-8 assay. The cell viability was decreased when HUVEC were exposed to AGEs (200 μg/ml) at 12, 24, 48, and 72 h. Meanwhile, siRNA against lactadherin significantly increased the cell viability compared with NC + AGEs group at 24, 48, and 72 (P < 0.05) (Fig. 2a). GFP or negative control siRNA did not affect cell viability. There were no differences in the cell viability between LsiRNA group and NC group. Lactadherin siRNA significantly attenuated AGEs treated the decrease of cell viability compared with NC + AGEs group (P < 0.05). The treatment of NC group with GSPB2 and resveratrol (10 μmol/l) significantly improved the AGEs treated cell viability for 48 h (P < 0.05) (Fig. 2b). Moreover, the overexpression of lactadherin significantly decreased the cell viability (P < 0.05). The cell viability was increased when LV group was exposed to GSPB2 and resveratrol (10 μmol/l) for 48 h (P < 0.05) (Fig. 2c).
Effects of lactadherin on cell viability treated by AGEs with MTT and CCK-8. a Effects of lactadherin siRNA on viability in HUVEC treated by AGEs at 12, 24, 48, and 72 h after transfection with MTT. b Effects of lactadherin siRNA, GSPB2 and resveratrol on viability in HUVEC treated by AGEs. c Effects of lactadherin overexpression, GSPB2 and resveratrol on viability in HUVEC treated by AGEs. Results are expressed as percent of untreated cells (100%) and are given as mean ± SD from five independent experiments. † P < 0.05, †† P < 0.01 compared with NC group; Δ P < 0.05, ΔΔ P < 0.01 compared with NC + AGEs group. *P < 0.05, **P < 0.01 compared with LV-C group; # P < 0.05, ## P < 0.01 compared with LV group. NC + AGEs group NC group with AGEs (200 μg/ml), LsiRNA + AGEs group LsiRNA group with AGEs (200 μg/ml), NC + AGEs +GSPB2 group NC + AGEs group treatment with GSPB2 (10 μmol/l), NC + AGEs + Res group NC + AGEs group treatment with resveratrol (10 μmol/l), LV + GSPB2 group LV group treatment with GSPB2 (10 μmol/l), LV + Res group LV group treatment with resveratrol (10 μmol/l), AGEs advanced glycation end products, GSPB2 grape seed procyanidin B2, CCK-8 cell counting kit-8
Role of lactadherin in apoptosis of HUVEC treated by AGEs and the anti-apoptotic effect of GSPB2 and resveratrol
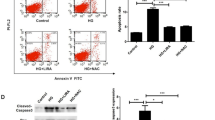
To examine whether lactadherin plays a role in AGEs-mediated apoptosis, LsiRNA group and NC group were treated with or without AGEs (200 μg/ml) and treated with GSPB2 and resveratrol (10 μmol/l) for 48 h. Induction of NC group with AGEs (200 μg/ml) resulted in a significant increase in the cell apoptosis, whereas siRNA against lactadherin significantly attenuated AGEs treated the cell apoptosis compared with NC + AGEs group (P < 0.05). The treatment of NC group with GSPB2 and resveratrol (10 μmol/l) also significantly decreased the AGEs treated cell apoptosis for 48 h (P < 0.05) (Fig. 3a). HUVEC overexpressing lactadherin was susceptible to cell apoptosis, while GSPB2 and resveratrol (10 μmol/l) significantly attenuated the cell apoptosis for 48 h (Fig. 3b). These data clearly show that lactadherin promote AGEs-mediated cell apoptosis.
Effects of lactadherin on cell apoptosis treated by AGEs with TUNEL assay (×400). a Effects of lactadherin siRNA, GSPB2 and resveratrol on apoptosis in HUVEC treated by AGEs. b Effects of lactadherin overexpression, GSPB2 and resveratrol on apoptosis in HUVEC treated by AGEs. Black arrows showed TUNEL positive cells. The bar graph at the bottom shows the percentage of apoptotic cells. Results represent mean ± SD of five independent experiments. † P < 0.05, †† P < 0.01 compared with NC group; Δ P < 0.05, ΔΔ P < 0.01 compared with NC + AGEs group. *P < 0.05, **P < 0.01 compared with LV-C group; # P < 0.05, ## P < 0.01 compared with LV group
The morphological changes of HUVEC overexpressing lactadherin
To support these results, we performed electronic microscope analysis of HUVEC overexpressing lactadherin treated and not treated with GSPB2 and resveratrol (10 μmol/l). Under electronic microscope, typical apoptosis phenomena were found in the HUVEC overexpressing lactadherin: apoptotic body, assemblage of heterochromatin and edema of mitochondria were found in many apoptotic cells. While GSPB2 and resveratrol (10 μmol/l) significantly attenuated the apoptotic phenomena and organelle necrosis for 48 h (Fig. 4).
Effects of lactadherin on cytosol cytochrome c concentration, caspases and Bax/Bcl-2
To further examine the molecule mechanism of the apoptosis pathway, we examined the cytosol cytochrome c concentration, caspase-9 activity, caspase-3 cleavage and Bax/Bcl-2 induced by lactadherin in HUVEC by western blotting. The uncleaved caspase-3 levels were unchanged in LV-C group, LV group, LsiRNA group and NC group. Stimulation of NC group with AGEs (200 μg/ml) resulted in a significant increase in the cytosol cytochrome c concentration, caspase-9 activity, cleaved caspase-3 and Bax/Bcl-2 ratio, whereas siRNA against lactadherin significantly attenuated AGEs treated the cytosol cytochrome c concentration, caspase-9 activity, cleaved caspase-3 and Bax/Bcl-2 ratio compared with NC + AGEs group (P < 0.05). The treatment of NC group with GSPB2 and resveratrol (10 μmol/l) significantly improved the AGEs treated the cytosol cytochrome c concentration, caspase-9 activity, caspase-3 cleavage and Bax/Bcl-2 ratio for 48 h (P < 0.05) (Fig. 5a–f). The cytosol cytochrome c concentration, caspase-9 activity, caspase-3 cleavage and Bax/Bcl-2 ratio significantly increased in LV group compared with those in LV-C group, while GSPB2 and resveratrol (10 μmol/l) significantly inhibited the cytosol cytochrome c concentration, caspase-9 activity, caspase-3 cleavage and Bax/Bcl-2 ratio in HUVEC overexpressing lactadherin for 48 h (Fig. 5g–l). These results suggest that mitochondria pathway is involved in lactadherin-mediated endothelial cell apoptosis in response to AGEs treatment.
Effects of lactadherin on cytosol cytochrome c concentration, caspase-9 activity, cleaved caspase-3 and Bax/Bcl-2 ratio in HUVEC treated by AGEs. a–c Effects of lactadherin siRNA, GSPB2 and resveratrol on cytosol cytochrome c concentration, caspase-9 activity, cleaved caspase-3 and Bax/Bcl-2 ratio in HUVEC treated by AGEs. g–i Effects of lactadherin overexpression, GSPB2 and resveratrol on cytosol cytochrome c concentration, caspase-9 activity, cleaved caspase-3 and Bax/Bcl-2 ratio in HUVEC treated by AGEs. d–f, j–l Data were expressed as the expression ratio of cleaved caspase 3/caspase 3, Bax/β-actin, Bax/Bcl-2. Each data point represents mean ± SD of five independent experiments. † P < 0.05, †† P < 0.01 compared with NC group; Δ P < 0.05, ΔΔ P < 0.01 compared with NC + AGEs group. *P < 0.05, **P < 0.01 compared with LV-C group; # P < 0.05, ## P < 0.01 compared with LV group
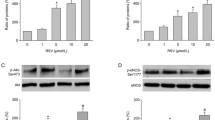
Effect of lactadherin on the levels of phospho-GSK3β
Given the well-established role of GSK3β in cell apoptosis, we determined the effect of lactadherin on phosphorylation of GSK-3β (phospho-GSK3β) in HUVEC. Treatment of NC group with AGEs (200 μg/ml) resulted in a significant decrease in the levels of phospho-GSK3β, whereas siRNA against lactadherin significantly reversed the decreased levels of phospho-GSK3β in response to AGEs. Treatment with GSPB2 and resveratrol (10 μmol/l) significantly attenuated the AGEs-induced decrease of phospho-GSK3β level for 48 h (P < 0.05) (Fig. 6a, b). The level of phospho-GSK3β significantly decreased in LV group compared with LV-C group, while GSPB2 and resveratrol (10 μmol/l) significantly reversed the decreased phospho-GSK3β in HUVEC overexpressing lactadherin (Fig. 6c, d). Since GSK3β phosphorylation inhibits GSK3β activity, these results suggested that AGEs increases GSK3β activity in a lactadherin-dependent manner.
Effects of lactadherin on phospho-GSK3β in HUVEC treated by AGEs. a Effects of lactadherin siRNA, GSPB2 and resveratrol on phospho-GSK3β in HUVEC treated by AGEs. c Effects of lactadherin overexpression, GSPB2 and resveratrol on phospho-GSK3β in HUVEC treated by AGEs. b, d Data were expressed as the expression ratio of phospho-GSK3β/total-GSK3β and given as mean ± SD from five independent experiments. † P < 0.05, †† P < 0.01 compared with NC group; Δ P < 0.05, ΔΔ P < 0.01 compared with NC + AGEs group. *P < 0.05, **P < 0.01 compared with LV-C group; # P < 0.05, ## P < 0.01 compared with LV group
Identification of proteins interacting with lactadherin
SDS-PAGE was performed and the bands of proteins with differential expression between L-IP groups and control groups were revealed (Fig. 7a). In total, five bands were detected on the three gels as determined by the PDQuest Image Analysis Software. Nine protein spots were successfully identified by LTQ-ESI-MS/MS, including Swiss-Prot accession number, protein description, theoretical Mr/PI, unique peptides and sequence coverage, etc (Table 1). The interaction proteins were prohibitin-2, prohibitin, annexin A2, glyceraldehyde-3-phosphate dehydrogenase, serpin B3, elongation factor 1-alpha 2, ADP/ATP translocase 2, isoform M1 of pyruvate kinase isozymes M1/M2, and myoferlin. We also used co-immunoprecipitation to specifically test for the presence of these proteins. Figure 7b showed that the PHB2 was detected in the lactadherin immunoprecipitate, which was undetectable in the control samples.
Interaction proteins of lactadherin. a SDS-PAGE map of the lactadherin protein complex from the overexpressing lactadherin cell extracts. b Confirmation of novel, high confidence lactadherin-PHB2 interactions by co-immunoprecipitation. L-IP immunoprecipitation of lactadherin groups were incubated overnight at 4°C with primary antibody (anti-lactadherin, 2 μg/ml). C control groups were incubated overnight at 4°C without the antibody. Bands 1, 6, 7, 9, 11 are the different expression of bands between L-IP group and C group
Discussion
Although classical paradigms defining the pathophysiology of vascular disease have focused on the abnormal regulation of cell growth in response to growth factors, it has been recently postulated that the regulation of cell death by apoptosis may be another determinant of vascular structure and lesion formation [23]. It has become increasingly clear that the process of cell death by apoptosis is a relatively ubiquitous phenomenon observed in a variety of cell types, including endothelial cells. Disruption or dysfunction of endothelial cells induced by AGEs has been hypothesized to play a pivotal role in the progression and/or development of vascular disease in diabetes [5, 24, 25]. In this study, we demonstrated that AGEs induced endothelial cell death through the induction of apoptosis. However, little is known about the molecular mechanisms on AGEs induced endothelial cell death.
The present study demonstrated that elevated AGEs induces apoptosis of HUVEC and up-regulates lactadherin in HUVEC. Several pathways have been demonstrated in mediating cell apoptosis, such as death receptors, mitochondria and endoplasmic reticulum pathway. Our study shows that EC apoptosis induced by AGEs is at least in part through the mitochondrial pathway. The lactadherin siRNA inhibited HUVEC apoptosis from AGEs exposure by inhibition of Bax/Bcl-2 ratio and cleaved caspase-3. These results suggest that upregulation of lactadherin plays a critical role in apoptosis of endothelial cells induced by AGEs and inhibition of lactadherin might prevent endothelial dysfunction in the early stage of diabetes.
Previous studies have shown that lactadherin is abundantly expressed in atherosclerotic plaques. Our results showed for the first time to the best of our knowledge that lactadherin plays a critical role in apoptosis of endothelial cells induced by AGEs. Some studies had reported that MFG-E8 helped phagocytosis of apoptotic cells by thioglycollate-elicited peritoneal macrophages and by tangible body macrophages [26, 27]. It has recently been reported that glucose of high concentration upregulated lactadherin in the adiposomes. ROS production was increased when cultured in the high-glucose medium, and ROS scavenger N-acetyl cysteine decreased lactadherin in the adiposomes. Expression of lactadherin was up-regulated in epididymal adipose tissues of diet-induced obese C57BL/6 mice as well as genetically obese ob/ob and db/db mice [28]. Endothelial cell apoptosis is critically involved in vascular remodeling in normal development and in pathological neovascularization [9, 29].
It is well known that the mitochondria play a pivotal role in cell death and cell survival, and that mitochondrial dysfunction constitutes a critical event in the apoptotic process [30]. Here, we demonstrated that the overexpression lactadherin may be attributable to an inappropriate increase in the cytosol cytochrome c concentration, caspase-9 activity, ratio of bax to bcl-2 and cleaved caspase-3. Cytochrome c release is a key step in activation of caspase cascade for initiation of apoptosis. This leads to caspase-9 oligomerization and activation and to caspase-3 cleavage. Caspase-3 is one of the key executioners of apoptosis, and its activation is a good marker for apoptosis. Moreover, apoptotic death signals converge on the mitochondria through the activation of pro-apoptotic members of the Bcl-2 family, such as Bax, while Bcl-2 serves as an anti-apoptotic protein. The apoptosis-related proteins Bax and Bcl-2 and caspases appear to be important for the progression of apoptotic cell death in mitochondrial pathway. The homo and heterodimerization of Bcl-2 family proteins is important for transduction and integration of apoptotic signals and control of the permeability of mitochondria and endoplasmic reticulum membranes. Bcl-2 interacts with activated Bax during apoptosis in an effective manner to neutralize the proapoptotic activity of Bax, auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. The formation of a Bax/Bcl-2 heterodimer can inhibit Bcl-2 homodimerization, once Bax/Bcl-2 ratio elevated can trigger apoptotic cell death. The elevated Bax/Bcl-2 ratio could activate cleavage of caspase-3 and then trigger the apoptosis of endothelial cells [31, 32]. Glycogen synthase kinase-3β (GSK3β) is a constitutively active enzyme. GSK3β is an important downstream target of the Akt signaling pathway. Phosphorylation of GSK3β on the inactivating residue serine-9 by Akt results in GSK3β inactivation [33]. Pro-apoptotic functions of GSK3β have been shown in apoptosis by withdrawal of growth factors, DNA damage, mitochondrial toxins, oxidant stress and other conditions that trigger apoptosis via the mitochondrial pathway [34–37]. In our study, we showed that AGEs increases GSK3β activity in HUVEC through a lactadherin dependent manner.
Moreover, GSPB2 and resveratrol significantly attenuated the AGEs-mediated cell apoptosis by inhibition of lactadherin. Antioxidant therapy is therefore considered to be a promising strategy to prevent the glycosylated damage to the vascular endothelium in the early stage of diabetic vascular complications [38–40]. Our previous studies showed that GSPE possesses potent anti-nonenzymatic glycation and anti-inflammation effects, inhibiting expression of high level vascular cell adhesion molecule 1 induced by AGEs in endothelial cells [20, 41, 42].
Our study also demonstrates that there are nine interaction proteins of lactadherin, such as prohibitin-2, prohibitin, etc. Prohibitin2, also designated as repressor of estrogen receptor activity is a 37 kDa protein exhibiting high homology to the putative tumor suppressor protein prohibitin [43]. PHB1 and 2 are evolutionarily conserved membrane proteins that are localized in the mitochondrial membrane. PHB2 are structurally related and assemble into large, ring-like complexes. Recent studies suggested that PHBs have been showed to be involved not only in the regulation of transcription but also cellular senescence, apoptosis, and mitochondrial respiratory activity and maintenance of mitochondrial morphology [44–46]. Prohibitins bind to several mitochondrial proteins such as voltage-dependent anion channel 2, adenine nucleotide translocator 2, and anti-apoptotic factor HS-associated protein X-1, and regulate their functions [47]. The results reconfirmed that the mitochondria apoptosis pathway played a pivotal role in the cell apoptosis by lactadherin.
Taken together, our study suggests that up-regulation of lactadherin plays a critical role in endothelial cells apoptosis induced by AGEs. The down-regulation of lactadherin inhibits cell apoptosis from AGEs exposure. Moreover, GSPB2 or resveratrol might have benefits in the early stage of diabetic endothelial dysfunction by inhibition of lactadherin. The present study provides the first evidence that lactadherin can modulate cell apoptosis and is a molecular target of GSPB2 and resveratrol.
References
Lin EH, Rutter CM, Katon W, Heckbert SR, Ciechanowski P, Oliver MM et al (2010) Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care 33:264–269
Cull CA, Jensen CC, Retnakaran R, Holman RR (2007) Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation 116:2119–2126
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Zhou YJ, Yang HW, Wang XG, Zhang H (2009) Hepatocyte growth factor prevents advanced glycation end products-induced injury and oxidative stress through a PI3K/Akt-dependent pathway in human endothelial cells. Life Sci 85:670–677
Xiang M, Yang M, Zhou C, Liu J, Li W, Qian Z (2006) Crocetin prevents AGEs-induced vascular endothelial cell apoptosis. Pharmacol Res 54:268–274
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC et al (2009) RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol 20:742–752
Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT (2005) Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem 280:12087–12095
Andersen MH, Berglund L, Petersen TE, Rasmussen JT (2002) Annexin-V binds to the intracellular part of the beta(5) integrin receptor subunit. Biochem Biophys Res Commun 292:550–557
Silvestre JS, Théry C, Hamard G, Boddaert J, Aguilar B, Delcayre A et al (2005) Lactadherin promotes VEGF-dependent neovascularization. Nat Med 11:499–506
Li XL, Li BY, Gao HQ, Cheng M, Xu L, Li XH et al (2010) Proteomics approach to study the mechanism of action of grape seed proanthocyanidin extracts on arterial remodeling in diabetic rats. Int J Mol Med 25:237–248
Choi SE, Kang Y, Jang HJ, Shin HC, Kim HE, Kim HS et al (2007) Involvement of glycogen synthase kinase-3beta in palmitate-induced human umbilical vein endothelial cell apoptosis. J Vasc Res 44:365–374
Miura T, Chiba M, Kasai K, Nozaka H, Nakamura T, Shoji T et al (2008) Apple procyanidins induce tumor cell apoptosis through mitochondrial pathway activation of caspase-3. Carcinogenesis 29:585–593
Zunino S (2009) Type 2 diabetes and glycemic response to grapes or grape products. J Nutr 139:1794S–1800S
Houde V, Grenier D, Chandad F (2006) Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J Periodontol 77:1371–1379
Khanna S, Venojarvi M, Roy S, Sharma N, Trikha P, Bagchi D et al (2002) Dermal wound healing properties of redox-active grape seed proanthocyanidins. Free Radic Biol Med 33:1089–1096
Vayalil PK, Mittal A, Katiyar SK (2004) Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NF kappa B. Carcinogenesis 25:987–995
Chen DM, Cai X, Kwik-Uribe CL, Zeng R, Zhu XZ (2006) Inhibitory effects of procyanidin B(2) dimer on lipid-laden macrophage formation. J Cardiovasc Pharmacol 48:54–70
Mackenzie GG, Adamo AM, Decker NP, Oteiza PI (2008) Dimeric procyanidin B2 inhibits constitutively active NF-kappaB in Hodgkin’s lymphoma cells independently of the presence of IkappaB mutations. Biochem Pharmacol 75:1461–1471
Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z (2006) Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol 291:H1694–H1699
Zhang FL, Gao HQ, Wu JM, Ma YB, You BA, Li BY et al (2006) Selective inhibition by grape seed proanthocyanidin extracts of cell adhesion molecule expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol 48:47–53
Bhatwadekar AD, Ghole VS (2005) Rapid method for the preparation of an AGE-BSA standard calibrator using thermal glycation. J Clin Lab Anal 19:11–15
Zhang Y, He L, Zhou Y (2008) Taspine isolated from Radix et Rhizoma Leonticis inhibits growth of human umbilical vein endothelial cell (HUVEC) by inducing its apoptosis. Phytomedicine 15:112–119
Nakagami H, Morishita R, Yamamoto K, Yoshimura SI, Taniyama Y, Aoki M et al (2001) Phosphorylation of p38 mitogen-activated protein kinase downstream of bax-caspase-3 pathway leads to cell death induced by high d-glucose in human endothelial cells. Diabetes 50:1472–1481
Yamagishi S, Nakamura K, Matsui T (2009) Regulation of advanced glycation end product (AGE)-receptor (RAGE) system by PPAR-gamma agonists and its implication in cardiovascular disease. Pharmacol Res 60:174–178
Hodgkinson CP, Laxton RC, Patel K, Ye S (2008) Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol 28:2275–2281
Hanayama R, Tanaka M, Miwa K, Nagata S (2004) Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol 172:3876–3882
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417:182–187
Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N et al (2007) Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology 148:3850–3862
Neutzner M, Lopez T, Feng X, Bergmann-Leitner ES, Leitner WW, Udey MC (2007) MFG-E8/lactadherin promotes tumor growth in an angiogenesis-dependent transgenic mouse model of multistage carcinogenesis. Cancer Res 67:6777–6785
Yang Z, Mo X, Gong Q, Pan Q, Yang X, Cai W et al (2008) Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis 13:1331–1343
Zhang Z, Lapolla SM, Annis MG, Truscott M, Roberts GJ, Miao Y et al (2004) Bcl-2 homodimerization involves two distinct binding surfaces, a topographic arrangement that provides an effective mechanism for Bcl-2 to capture activated Bax. J Biol Chem 279:43920–43928
Tan C, Dlugosz PJ, Peng J, Zhang Z, Lapolla SM, Plafker SM et al (2006) Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem 281:14764–14775
Chen L, Zhang Y, Sun X, Li H, Le Sage G, Javer A et al (2009) Synthetic resveratrol aliphatic acid inhibits TLR2-mediated apoptosis and an involvement of Akt/GSK3beta pathway. Bioorg Med Chem 17:4378–4382
Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA et al (2004) Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24:9993–10002
King TD, Bijur GN, Jope RS (2001) Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res 919:106–114
King TD, Jope RS (2005) Inhibition of glycogen synthase kinase-3 protects cells from intrinsic but not extrinsic oxidative stress. Neuroreport 16:597–601
Miura T, Miki T (2009) GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J 73:1184–1192
Chew GT, Watts GF (2004) Coenzyme Q10 and diabetic endotheliopathy: oxidative stress and the ‘recoupling hypothesis’. QJM 97:537–548
Choi YJ, Lim SS, Jung JY, Choi JS, Kim JK, Han SJ et al (2008) Blockade of nitroxidative stress by roasted licorice extracts in high glucose-exposed endothelial cells. J Cardiovasc Pharmacol 52:344–354
Chao CL, Hou YC, Chao PD, Weng CS, Ho FM (2009) The antioxidant effects of quercetin metabolites on the prevention of high glucose-induced apoptosis of human umbilical vein endothelial cells. Br J Nutr 101:1165–1170
Ma L, Gao HQ, Li BY, Ma YB, You BA, Zhang FL (2007) Grape seed proanthocyanidin extracts inhibit vascular cell adhesion molecule expression induced by advanced glycation end products through activation of peroxisome proliferators-activated receptor gamma. J Cardiovasc Pharmacol 49:293–298
Zhang FL, Gao HQ, Shen L (2007) Inhibitory effect of GSPE on RAGE expression induced by advanced glycation end products in endothelial cells. J Cardiovasc Pharmacol 50:434–440
Pabona JM, Velarde MC, Zeng Z, Simmen FA, Simmen RC (2009) Nuclear receptor co-regulator Krüppel-like factor 9 and prohibitin 2 expression in estrogen-induced epithelial cell proliferation in the mouse uterus. J Endocrinol 200:63–73
Steglich G, Neupert W, Langer T (1999) Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol Cell Biol 19:3435–3442
Osman C, Merkwirth C, Langer T (2009) Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122:3823–3830
Ross JA, Nagy ZS, Kirken RA (2008) The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem 283:4699–4713
Kasashima K, Ohta E, Kagawa Y, Endo H (2006) Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. J Biol Chem 281:36401–36410
Acknowledgments
This work was supported by National Natural Science Foundation of China (30873145, 81000340), Outstanding Young Scientist Research Award Fund of Shandong Province (BS2009YY046), China Postdoctoral Science Foundation (20100471520) and Natural Science Foundation of Shandong Province (Y2008C100, ZR2010HQ067). We wish to thank the personnel in Medical Science Academy of Shandong and the personnel in Research Center for Proteome Analysis, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences. We also thank Professor Ji-yuan Ding for his assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bao-ying Li, Xiao-li Li and Qian Cai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, By., Li, Xl., Cai, Q. et al. Induction of lactadherin mediates the apoptosis of endothelial cells in response to advanced glycation end products and protective effects of grape seed procyanidin B2 and resveratrol. Apoptosis 16, 732–745 (2011). https://doi.org/10.1007/s10495-011-0602-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-011-0602-4