Abstract
Physiological cell death is a key mechanism that ensures appropriate development and maintenance of tissues and organs in multicellular organisms. Most structures in the vertebrate embryo exhibit defined areas of cell death at precise stages of development. In this regard the areas of interdigital cell death during limb development provide a paradigmatic model of massive cell death with an evident morphogenetic role in digit morphogenesis. Physiological cell death has been proposed to occur by apoptosis, cellular phenomena genetically controlled to orchestrate cell suicide following two main pathways, cytochrome C liberation from the mitochondria or activation of death receptors. Such pathways converge in the activation of cysteine proteases known as caspases, which execute the cell death program, leading to typical morphologic changes within the cell, termed apoptosis. According to these findings it would be expected that caspases loss of function experiments could cause inhibition of interdigital cell death promoting syndactyly phenotypes. A syndactyly phenotype is characterized by absence of digit freeing during development that, when caused by absence of interdigital cell death, is accompanied by the persistence of an interdigital membrane. However this situation has not been reported in any of the KO mice or chicken loss of function experiments ever performed. Moreover histological analysis of dying cells within the interdigit reveals the synchronic occurrence of different types of cell death. All these findings are indicative of caspase alternative and/or complementary mechanisms responsible for physiological interdigital cell death. Characterization of alternative cell death pathways is required to explain vertebrate morphogenesis. Today there is great interest in cell death via autophagy, which could substitute or act synergistically to the apoptotic pathway. Here we discuss what is known about physiological cell death in the developing interdigital tissue of vertebrate embryos, paying special attention to the avian species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regulation and significance of interdigital cell death during the formation of the digits in vertebrate embryos
Programmed cell death is a basic mechanism employed in embryonic systems to sculpt the morphology and the structure of the developing organs (morphogenetic and histogenetic cell death) or to remove organs or tissues with transitory functions in the embryo (phylogenetic cell death; see [1]). The developing vertebrate limb is one of the best characterized paradigms where programmed cell death has a central role in morphogenesis [2]. The embryonic limb is a simple structure consisting of a core of mesodermal cells covered by an ectodermal jacket, which bulges as a bud in the lateral surface of the embryonic body (Fig. 1a). The mesodermal cells of the early limb bud have skeletogenic potential but are maintained undifferentiated and proliferating by the influence of a thickened region of the ectoderm occupying the distal margin of the bud, termed the Apical Ectodermal Ridge (AER; see Fig. 1b–e). The AER produces fibroblast growth factors (FGFs), which act on the subjacent mesodermal cells that constitute the Progress Zone (PZ). FGF8 is the main FGF of the AER, but FGF4, FGF9, FGF17 and FGF19 are also present, during development. Intense proliferation of the mesodermal cells of the distal region of the bud under the influence of the AER is accompanied by progressive differentiation at more proximal regions of the limb bud (Fig. 1f–g). This process forms the chondrogenic primordia of the limb in a characteristic proximal–distal sequence appearing first the primordium of the femur/humerus (stylopod), next those of the tibia-fibula/ulna-radius (zeugopod) and finally the carpal/tarsal pieces and the digits (autopod) (Fig. 1f).
Limb development and cell death areas. a–e Scanning electron micrographs that illustrate the early stages of limb development in a chicken embryo. Limbs form as small buds that emerge from the flanks of the developing vertebrate embryo (Arrows in a). Limb buds are simple structures consisting of a mass of mesodermal cells covered by an ectodermal jacket. As development takes place limb buds growth along the three axis (b–e). Early on, at the distal edge of the limb, a local thickening of the ectoderm forms the apical ectodermal ridge (AER), a key structure responsible of limb outgrowth (arrow in the frontal view of the limb bud in c). f illustrates on a chicken developing limb, the three regions that become specified along the proximal–distal axis within the limb, the Stylopod, Zeugopod and Autopod. g Schematic drawings representing limb bud development in order to illustrate the areas of cell death. As limbs growth, different areas of cell death appear concomitantly to skeletal elements differentiation. The initial undifferentiated mass of mesodermal cell contributes to limb bud growth as they proliferate within the Progress Zone (PZ) of the limb (1), which is influenced by the inductive signals from the apical ectodermal ridge (AER) (1). As differentiation of skeletal elements of the Stylopod (2) and the Zeugopod (3) starts in the core of the mesodermal tissue, the remaining mesoderm begin to be removed by cell death, appearing the Anterior Necrotic Zone (ANZ), the Posterior Necrotic Zone (PNZ) and the Opaque Patch (OP). In the same fashion within the auotopod (4), as digits differentiate, the interdigital mesoderm is removed by cell death in the Interdigital Necrotic Zones (INZs)
A remarkable feature is that, the mesodermal cells of the bud, which do not integrate into the developing cartilages, undergo apoptosis forming well defined regions of cell death around these chondrogenic aggregates (Fig. 1g). Among these areas of cell death, the most remarkable are those located between the developing digits, which have been initially termed the interdigital necrotic zones (INZs), as they were described when the term apoptosis was not yet established [3–5]. Other regions of cell death, such as the anterior and posterior necrotic zones (ANZ and PNZ) or the opaque patch (OP) are located in the peripheral margins of the limb bud, and between the zeugopodial cartilages [3–5]. Additionally cell death is also associated with the establishment of the muscle bellies, which develop from a distinct mesodermal cell population invading the limb from the adjacent somites [6]. Apoptosis is also present in the core of the prechondrogenic aggregates establishing the zone of joints formation [7].
All these areas of cell death are finely regulated and play a variety of roles including sculpturing the gross morphology of the limb [8–10] and the shape of the muscle bellies and tendons [6], and to establish the zones of joint formation [7]. The formerly termed interdigital necrotic zones (INZs) are regions of massive cell death, which remove the undifferentiated mesodermal cells located between the developing digits and cause the freeing of the digits from the hand or foot plate (see Fig. 2).
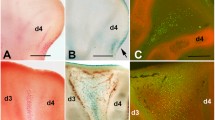
Interdigital Cell Death in the Developing Limb. a–c Images in a and b shows cell death occurring in the interdigital tissue of a mouse (a) and a chicken embryo manifested by TUNEL labeling (a) and Acridine Orange staining (b), respectively. Image in c shows the high levels of Cathepsin expression in the regressing interdigital tissue as manifested by Cathepsin D immunolabeling. d-f Electron microscopy images showing cell types that are found in the regressing avian interdigital tissue. d illustrates a typical apoptotic cell, e shows a characteristic necrotic cell while in f we can appreciate a macrophage
Morphogenetic role of INZ: interdigital tissue regression versus differential growth
INZ’s are present in most tetrapod embryos but their extent varies among species, exhibiting in most cases a close relationship with the final morphology of the digits. In the chick embryo, interdigital cell death is part of a complex process of tissue regression, which lasts more than 2 days. At the beginning of the process, interdigital mesenchymal cells die by apoptosis. However this is soon accompanied by regression of interdigital blood vessels [11] and disintegration of the extracellular matrix scaffold of the interdigit, which result in detachment of tissue fragments into the amniotic sac [12]. In birds with webbed digits, as the duck, INZ’s are rudimentary in comparison with that of the chick but the sequence of degenerative events is similar between both species [13]. Reptiles show a pattern of interdigital cell death similar to that of birds including differences in death intensity between species with free digits (i.e. lizards) and species with webbed digits (i.e. turtle) [14].
It has been proposed that the morphogenetic role of INZ′s in mouse embryos is less relevant than in birds [15, 16]. According to this interpretation there is no proper interdigital tissue degeneration in the mouse. Instead, cell death accompanies a differential growth process of the autopod characterized by intense growth of the digit tips and negative growth of the interdigital regions. However as in birds and reptilians, the morphology of the digits in mammals is closely associated with the pattern and the intensity of interdigital cell death. In this regard interdigital cell death is dramatically reduced or absent in the bat, which is a specie with a webbed digits phenotype [17]. In any case the presence of both free digits species (mice, chicken, lizard, etc.) and webbed digits species (bat, duck, turtle, etc.) within each class of vertebrate, provides researchers with potentially extraordinary tools to study the morphogenetic role of cell death during development [18].
Interdigital cell death is a characteristic feature of amniote embryos. In amphibians digits form largely by selective outgrowth of the digit tips in total absence of INZ [19, 20]. However, it has been reported that some amphibian species exhibit areas of interdigital cell death resembling that reptilian, avian or mammals [21].
Cytological features of INZ: loss of cell–matrix adhesion, apoptosis, cell fragmentation and phagocytosis
Cell death in the interdigital mesoderm follows a morphological pattern of apoptosis [7, 22]. Transmission electron microscopy studies have shown that most interdigital dying cells appear rounded and electron dense with the nucleus exhibiting a characteristic peripheral condensation of the chromatin (Fig. 2d). The apoptotic cells undergo fragmentation and the resulting debris is removed by phagocytosis. Removal is performed both by the neighboring healthy cells and by incoming macrophages of hematopoietic origin (Fig. 2f). Studies in PU.1 null mouse embryos indicate that local mesenchymal cells are able to substitute for professional phagocytes, and indeed interdigital tissue regression in these macrophageless mice follows almost the same schedule as in wild type animals [23].
Regardless of the predominant apoptotic morphology of the dying interdigital mesenchymal cells, it is remarkable that at peak stages of degeneration cells bearing morphological features of necrosis are relatively abundant in chick interdigits [24] (Fig. 2e). This fact may be interpreted as the coexistence of apoptotic and necrotic pathways or that apoptotic cells undergo secondary necrosis if phagocytic removal is delayed (see below for more details).
Interdigital mesenchyme isolated from the developing limb up to 12 h prior to the onset of INZ establishment exhibits considerable chondrogenic potential when cultured at high density [22]. This indicates that irreversible commitment to dye does not take place until a few hours prior to death. Immunocytological analysis of interdigital cells during the time period preceding overt apoptosis, combining a panel of nuclear and cytoplasmic markers with TUNEL labeling, allowed the identification of the earliest cell alterations associated with cell death [25]. At the nuclear level, degradation of acetylated histone 4 and alteration in the components of the nuclear membrane (lamin B and nucleoporin) accompanied by anomalies in the nucleo-cytoplasmic traffic are precocious degenerative events [25]. In the cytoplasm microtubules and actin microfilaments appeared conserved at initial stages of apoptosis, while vimentin intermediate filaments show clear signs of disorganization and degradation. The maintenance of N-CAM immunolabeling in TUNEL positive cells suggests that cell–cell adhesion is relatively conserved in dying cells. In this regard, interdigital mesenchyme bears specific domains of protocadherin gene expression [26]. However, alterations in components responsible for cell matrix adhesion, such as paxillin, were early events in the degenerative process [25]. Furthermore, the interdigital extracellular matrix scaffold is particularly complex in the stages preceding INZ [27] and undergoes intense remodeling by a variety of metalloproteinases specifically up-regulated in the regressing interdigits (Stromelysin-3 [28]; Adamts5 [29, 30]). On the basis of these findings it was proposed that the INZ might constitute an example of cell death associated with the loss of cell–matrix adhesion, often termed anoikis [25]. This interpretation is reinforced by the occurrence of syndactyly in mouse mutants with alterations in a variety of matrix components [30–35]. However, it cannot be forgotten that interdigital matrix may serve also a function in storing and targeting secreted growth factors implicated in the control of the INZ [36] (see also below).
Cell death machinery in the INZ: mitochondrial permeabilization, ROS, caspases and lysosomes
The apoptotic machinery responsible for cell death in the INZ has not yet been fully elucidated. The occurrence of soft tissue syndactyly in mice lacking both Bax and Bak function [37] and in mice lacking Bax and the BH3-only protein Bim [38] indicates that these pro-apoptotic members of the bcl-2 family play a critical role in the induction of INZ. Moreover, all these factors are functionally associated with the intrinsic apoptotic pathway associated with permeabilization of the outer mitochondrial membrane. Hence, it can be assumed that the mitochondria is the first target for the interdigital cell death triggering signals (see below). Consistent with a central role of mitochondrial dysfunction in the INZ, at peak stages of interdigital cell death cytochrome c is released into the cytosol and the mitochondrial apoptotic inducing factor AIF is translocated into the nucleus of cells in the regressing interdigital mesenchyme [25]. Furthermore, the interdigital antiapoptotic factor Bag-1 (Bcl-2 associated athanogene 1), which interacts and co-localizes in the mitochondria with BCL-2, is expressed in the interdigital mesoderm of the mouse limb at stages preceding apoptosis, becoming progressively down-regulated in correlation with the progress of the degenerative process [39].
The death effectors of the INZ acting downstream of mitochondrial permeabilization remain more elusive. The absence of an interdigital phenotype in mice with different genetic modifications to functionally abolish a large number of, if not all, potential candidate effectors is indicative of intense redundancy in these factors. In the canonical intrinsic apoptotic pathway, mitochondrial permeabilization is followed by cytosolic release of cytochrome c, which activates caspase 9 upon binding to the adaptor protein Apaf-1 and procaspase 9 to form the apoptosome. While there is evidence for the activation of caspase 9 in the INZ [40], syndactyly is not observed in knock out mice for either Apaf-1 [41] or Caspase 9 [42]. However, in Apaf1−/− mice interdigital cell death is delayed and acquires morphological features of necrosis [41], suggesting the activation of alternative cell death mediators to executor caspases in absence of apoptosome activation.
In the canonical mitochondrial apoptotic pathway, caspase 9 activates the executioner caspases 3, 6 and 7 (see [43]). As for caspase 9, there is evidence for the involvement of these executioner caspases in INZ [25, 44], but no interdigital phenotype is induced in mice deficient in either caspase 3 [45], caspase 6 or caspase 7 [46]. Together these findings indicate the existence of alternative cell death pathways able to substitute for the canonical apoptotic pathway mediated by caspases.
Caspase 2 is highly expressed by dying interdigital cells [25] and could be a good candidate to substitute for other apoptotic mediators following blockade of the intrinsic apoptotic pathway. In some systems caspase 2 is able to promote mitochondrial permeabilization [47], and can also activate caspase 3 [48]. However, silencing interdigital expression of caspase 2 with siRNA delays but do not inhibits cell death in the INZ [25]. Furthermore, syndactyly is not induced in mice deficient in this caspase [49].
In sum, all the above mentioned data indicates that apoptotic caspases acting downstream of mitochondrial permeabilization are active in INZ but they can not be the sole apoptotic factors. In this regard treatments with the pan-inhibitor of caspases Z-VAD-FMK only reduce but not inhibit cell death in the INZ [50].
There is evidence showing that formation of reactive oxygen species (ROS) and the release of lysosomal enzymes are also mechanisms accounting for cell death in the INZ. ROS are toxic compounds generated in the cells as consequence of aerobic metabolism. In physiological conditions ROS activity is modulated by antioxidant factors, and cell death and several degenerative diseases, including aging, can be caused by deficiencies in the capability of cells to neutralize these compounds [51]. At the stages of active cell death, the interdigital tissues exhibit high levels of reactive oxygen species (ROS) and, most important, treatments with antioxidants can reduce cell death in the INZ [52–54]. However, it is unlikely that ROS exert their influence in the INZ directly, as knock out mice deficient for enzymes responsible for neutralizing ROS are viable and lack an abnormal phenotype [54]. It is more likely that ROS appear as a consequence of other cell death pathways in regulating cell death in the INZ. Thus, the local interdigital increase in ROS might be due to the mitochondrial permeabilization secondary to Bax activation [55].
Lysosomes have been largely considered responsible for non-apoptotic cell death and phagocytic elimination of dead cells. However, there is increasing evidence for the involvement of lysosomes in death processes in which cell morphology corresponds with apoptosis. It has been proposed the term “apoptosis-like programmed cell death” to define this type of cell death [56]. Furthermore, analysis of cell death after treatment with lysosomotropic detergents revealed that the lysosomal induction of apoptotic versus necrotic features might be explained by differences in the intensity of lysosomal permeability [57, 58]. The role of lysosomes in cell death in the INZ is supported by the following facts: (1) lysosomal cathepsins genes (cathepsin B, D and L) are up-regulated in the non-macrophagic interdigital mesenchyme during the stages of interdigital tisssue regression; (2) the occurrence of lysosomal permeabilization in interdigital mesenchymal cells during interdigital tissue regression; (3) the induction of apoptosis in primary cultures of undifferentiated embryonic limb mesenchyme overexpressing cathepsin D; and, (4) by the increased reduction of cell death in the INZ in chick autopods subjected to combined treatments with caspase and cathepsin D inhbitors, in comparison with interdigits treated only with caspase inhibitors [50].
The mechanism responsible for lysosomal permeabilization in the INZ has not been yet analyzed. In some systems Bax activation has been shown to be associated with lysosomal permeabilization [59]. Moreover ROS are important factors able to induce lysosomal permeabilization [60, 61]. Hence, both mechanisms might reflect a crosstalk between different cell death pathways ensuring the elimination of the interdigital mesenchyme. In addition, lysosomal enzymes delivered into the cytosol might also induce mitochondrial permeabilization [62] and activation of the executioner caspases [63] forming an interactive loop able to reinforce the initial death signal. In this regard, it must be mentioned the absence of interdigital phenotype both in mice lacking different key members of the caspase-mediated death pathways (see above) and also in mice deficient in different cathepsin genes [64–66]. Fig. 3 illustrates a schematic representation of the interaction of different dying pathways accounting for cell death in the INZ.
Interdigital Cell Death Pathway. The scheme illustrates the mitochondria and the lysosome as independent interconnected partners playing central roles in the cell death cascade of the interdigital cells. CytC: Cytochrome C; AIF: Apoptotic Inducing Factor; ICAD: Inhibitor of Caspase Activated DNase; CAD: Caspase Activated DNase; ROS: Reactive Oxigen Species; APAF1: Apoptosis Protease-Activated Factor 1
Molecular regulation of INZ: FGF, BMP and RA signaling
The hand/foot develop from the distal portion of the limb bud, which is termed the autopod. In this region the interdigits occupy the space delimited between two digit primordia. Each interdigit has a structure quite similar to the early limb bud, as they are formed by a core of undifferentiated mesodermal tissue, covered by ectoderm with a distinctive AER along their distal margin (see Fig. 4). As in the early limb bud, the tissue components of the interdigit establish complex interactions through the production of a variety of secreted signaling molecules that control cell fate and tissue differentiation. Among these secreted factors, Fibroblast Growth Factors (FGFs), Bone Morphogenetic Proteins (BMPs) and Retinoic acid metabolites (RA) have been identified as signals responsible for the regulation of cell fates in the INZ. However, their precise role and interactions remain uncertain.
Regulators of Interdigital Cell Death. This figure shows a schematic drawing of the interdigital tissue with the flanking forming digits where we represent the main factors involved in the stimulation of cell death. FGFs: Fibroblast Growth Factors; RA: Retinoic Acid; BMPs: Bone Morphogenetic Proteins;WNTs: Wnt factors; AER: Apical Ectodermal Ridge
FGF signaling
The AER produces FGF8 which maintains the subjacent mesoderm undifferentiated and proliferating accounting for limb outgrowth. The onset of cell death in the INZ coincides temporarily with the physiological cessation of AER function, which is accompanied by down-regulation of Fgf8 gene expression [18]. In concordance with a role for this secreted factor in the control of cell death, INZ formation is inhibited after local application of exogenous FGFs at cell death stages [67]. Furthermore, syndactyly is observed in the human syndromes caused by a constitutive activation of FGF receptors [68] and mice lacking Fgf4,8,9 and 17 genes in the AER have rudimentary limbs that exhibit ectopic regions of mesodermal cell death during development [69–71]. Together these findings are consistent with a role for FGFs as survival signals for undifferentiated limb mesoderm. However, there is also evidence showing that FGFs are required for cell death. Hence, local inhibition of FGF signaling causes syndactyly in chicks [72]. In addition, initial inhibition of interdigital cell death induced by local application of FGFs into the chick interdigit, is followed by a dramatic intensification of cell death 2 days after the treatment, when the source of exogenous FGFs loss its activity [72]. Consistent with this finding, local application of FGFs into the interdigits of the webbed digits of duck embryos [72] increases the sensitivity of the duck interdigital mesenchyme to undergo cell death by treatments with BMPs (see below).
The apparently contradictory effects of FGFs have been interpreted as evidence for a dual role of FGFs as a survival signal which, at the same time, sensitizes the limb mesoderm to the effect of the apoptotic triggering signals [72]. It is likely that apoptotic sensitivity of the undifferentiated mesenchymal cells is related to the regulation of N-Myc expression by FGFs [73].
BMP signaling
The family of Bone Morphogenetic Proteins (BMPs) includes cytokines that play crucial roles in limb development, thus their members are involved not only in early patterning [74–76] but also in cartilage formation [75, 77, 78], joint specification [79] or apoptotic cell death induction [77]. It has been suggested that different BMP receptors and crosstalk with different signaling molecules might be involved in the different responses to BMPs in the limb autopod [80, 81].
Numerous experiments performed in chick and mouse embryos point to BMPs as responsible molecules for the onset of cell death in the INZ. Genetic approaches in mice indicate that the apoptotic promoting effect of BMPs is secondary to the inhibition of Fgf gene expression in the AER [16, 70] but it cannot be discarded a direct cell-autonomous proapoptotic effect of BMPs on the mesodermal cells.
In the regressing interdigits there are two sources of BMPs, the AER and the mesoderm [82, 83]. The AER express Bmp2, Bmp4 and Bmp7 [74, 83, 84]. At initial stages of limb development these expression domains contribute to establish the dorso-ventral axis of the limb bud [74, 75], but during the stages of digit formation they appear to be implicated in the regression of the AER [18, 74, 83], which is followed by cell death in the subjacent mesoderm. Hence it has been proposed that ectodermal BMPs are central players in the control of INZ cell death by regulating the expression of Fgf8 in the AER [70, 74].
The interdigital mesoderm shows also prominent expression domains of Bmp2, Bmp 4, Bmp5 and, Bmp7 genes [84, 85] and gain-of-function experiments by local application of beads bearing any of those BMP proteins into the interdigital mesenchyme, prior to establishment of the INZ or in the early limb bud, induces massive cell death by apoptosis [77]. In a complementary fashion, loss-of-function experiments to block BMP signaling in the interdigits inhibit cell death and cause syndactyly [83, 86–89]. From these experiments it is difficult to establish whether the control of cell death in the INZ depends on the ectodermal or the mesodermal BMPs or even both, as local treatments with BMPs in the chick limb induces a precocious regression of the AER [18]. However, consistent with a role of mesodermal BMPs in the control of INZ formation, the functional activity of the mesodermal BMPs is finely tuned by two BMP antagonists, BAMBI [85, 90] and inhibitory-smads [91] expressed in the interdigits. Furthermore, the webbed interdigits of duck embryos express Bmp genes in a pattern similar to those of the chick, but their expression is accompanied by well defined expression domains of the secreted BMP antagonist Gremlin [86]. This feature is observed also in the webbed wing of bat embryos [17].
RA signaling
Retinoic acid (RA) is the representative name of a set of active metabolites of vitamin A produced in the embryo by the enzymatic action of Raldh2. RA signals through a variety of nuclear receptors, which are ligand-inducible transcriptional regulators. RA is indispensable for embryonic development (see [92]) and plays important roles in the developing limb [93, 94]. During the formation of the digits, all the components of this signaling pathway including Raldh2; the retinoic acid receptors beta and gamma (RARbeta; RARgamma) and Cyp26b1, which encodes an enzyme responsible for the inactivation of retinoic acid, are all expressed in association with the developing digits. Of these genes, Raldh2 and RARbeta are specifically expressed in the interdigital mesoderm [95, 96]. In chick embryos local interdigital treatments with all-trans-RA induce premature cell death and regression of the AER, while local application of a RA antagonist inhibits interdigital cell death leading to syndactyly [97]. Mesenchymal cell death is also observed in mice treated with RA or genetically modified to silence Cyp26b1 [98, 99] and mice deficient in both RARbeta and RAR gamma exhibit soft tissue syndactyly [28].
Whether RA regulates cell death in the INZ directly or indirectly remains unclear. RA signaling up-regulates Bmp gene expression in the interdigital mesoderm [28, 97], and interdigital apoptosis induced by RA is attenuated or inhibited by combined treatment of RA with a BMP antagonist [97]. In addition, RA might also influence cell death through neutralizing the survival effect of FGF8 [16, 94] or inhibiting differentiation of interdigital mesoderm into cartilage [96]. Together these findings are suggestive of an indirect effect of RA on formation of the INZ. Consistent with this interpretation, interdigital expression of Bax which is a key regulator of INZ formation (see above) is not differentially regulated in RA loss-of-function experimental approaches [28] (but see also [16]).
Notch and Wnt signaling
While FGFs, BMPs and RA appear to be major regulators of INZ formation, other signaling pathways might also be implicated in this process as deduced by specific expression patterns in the regressing interdigits and/or by the occurrence of syndactyly when the pathway is genetically disturbed. One of the candidates is the Notch pathway signaling, which in fact is modulating apoptotic cell death in the developing limb at the level of the AER [100]. The formation and maintenance of the AER is one of the basic roles of the Notch pathway during limb development where it controls cell death modulating the level of Fgf8 expressing cells in the AER [100]. Consistent with this statement, mice deficient in the Notch ligands, Serrate or Jagged2, display syndactyly after showing expanded expression domains of Fgf8 and a considerable reduction on the expression of Bmps in the interdigital tissue [101–103].
Members of the Wnt signaling pathway, including the ligand Wnt5a and the receptor Fz4, are expressed in the regressing interdigital tissue [104] but a possible role in the control of cell death in the INZ still awaits clarification. Wnt factors might be considered survival signals during limb development. In this regard it has been suggested that Thalidomide, which is known to cause massive apoptotic cell death during limb development, may exert its teratogenic effects by the inhibition of WNT signaling after stimulation of ROS production [105]. In support for a role of WNT signaling in limb cell survival in physiological conditions, is the finding that one of the ways for BMPs to induce apoptotic cell death works through stimulation of Dickkopf1 (Dkk1), a WNT signaling inhibitor [106]. Thus, mice deficient in Dkk1, which is highly expressed in the regressing interdigital tissue, show syndactyly phenotype [107, 108]. Syndactyly is also found in mice mutants for other potential antagonist of WNT pathway as Megf7 [109] or Sfrp2 [110] supporting the antiapoptotic role for WNT signaling in the interdigital tissue.
Concluding remarks
The areas of interdigital cell death constitute one of the best illustrative models showing the implication of programmed cell death in embryonic morphogenesis. It is remarkable that at least in birds, this physiological degenerative process involves not only cell death but also tissue regression, including collapse of blood flow and tissue disintegration. This feature must cause important homeostatic modifications able to influence the functional properties of the enzymatic cascades implicated in the cell death mechanisms. In this regard, the changes in milieu pH conditions are particularly important, since they can drastically modulate not only the function of proteins but also their subcellular locations (see [111]). Hence it is not surprising that this degenerative process implicates multiple intracellular degenerative pathways, involving caspases, mitochondria, lysosomes and ROS. A mayor task for future research in the area is to unravel the molecular coordination and interactions between these different degenerative machineries.
References
Zakeri Z, Lockshin RA (2008) Cell death: history and future. Adv Exp Med Biol 615:1–11
Saunders JW Jr (1966) Death in embryonic systems. Science 154:604–612
Zuzarte-Luis V, Hurle JM (2005) Programmed cell death in the embryonic vertebrate limb. Semin Cell Dev Biol 16:261–269
Fernandez-Teran MA, Hinchliffe JR, Ros MA (2006) Birth and death of cells in limb development: a mapping study. Dev Dyn 235:2521–2537
Zuzarte-Luis V, Montero JA, Torre-Perez N, Garcia-Porrero JA, Hurle JM (2007) Cathepsin D gene expression outlines the areas of physiological cell death during embryonic development. Dev Dyn 236:880–885
Rodriguez-Guzman M, Montero JA, Santesteban E, Ganan Y, Macias D, Hurle JM (2007) Tendon-muscle crosstalk controls muscle bellies morphogenesis, which is mediated by cell death and retinoic acid signaling. Dev Biol 302:267–280
Mori C, Nakamura N, Kimura S, Irie H, Takigawa T, Shiota K (1995) Programmed cell death in the interdigital tissue of the fetal mouse limb is apoptosis with DNA fragmentation. Anat Rec 242:103–110
Hinchliffe JR, Ede DA (1973) Cell death and the development of limb form and skeletal pattern in normal and wingless (Ws) chick embryos. J Embryol Exp Morphol 30:753–772
Chen Y, Zhao X (1998) Shaping limbs by apoptosis. J Exp Zool 282:691–702
Bouldin CM, Harfe BD (2009) Aberrant FGF signaling, independent of ectopic hedgehog signaling, initiates preaxial polydactyly in dorking chickens. Dev Biol 334:133–141
Hurle JM, Colvee E, Fernandez-Teran MA (1985) Vascular regression during the formation of the free digits in the avian limb bud: a comparative study in chick and duck embryos. J Embryol Exp Morphol 85:239–250
Hurle JM, Fernandez-Teran MA (1983) Fine structure of the regressing interdigital membranes during the formation of the digits of the chick embryo leg bud. J Embryol Exp Morphol 78:195–209
Hurle JM, Fernandez-Teran MA (1984) Fine structure of the interdigital membranes during the morphogenesis of the digits of the webbed foot of the duck embryo. J Embryol Exp Morphol 79:201–210
Fallon JF, Cameron J (1977) Interdigital cell death during limb development of the turtle and lizard with an interpretation of evolutionary significance. J Embryol Exp Morphol 40:285–289
Salas-Vidal E, Valencia C, Covarrubias L (2001) Differential tissue growth and patterns of cell death in mouse limb autopod morphogenesis. Dev Dyn 220:295–306
Hernandez-Martinez R, Castro-Obregon S, Covarrubias L (2009) Progressive interdigital cell death: regulation by the antagonistic interaction between fibroblast growth factor 8 and retinoic acid. Development 136:3669–3678
Weatherbee SD, Behringer RR, Rasweiler JJ, Niswander LA (2006) Interdigital webbing retention in bat wings illustrates genetic changes underlying amniote limb diversification. Proc Natl Acad Sci USA 103:15103–15107
Ganan Y, Macias D, Basco RD, Merino R, Hurle JM (1998) Morphological diversity of the avian foot is related with the pattern of Msx gene expression in the developing autopod. Dev Biol 196:33–41
Cameron JA, Fallon JF (1977) The absence of cell death during development of free digits in amphibians. Dev Biol 55:331–338
Vlaskalin T, Wong CJ, Tsilfidis C (2004) Growth and apoptosis during larval forelimb development and adult forelimb regeneration in the newt (Notophthalmus Viridescens). Dev Genes Evol 214:423–431
Franssen RA, Marks S, Wake D, Shubin N (2005) Limb Chondrogenesis of the Seepage Salamander, Desmognathus Aeneus (Amphibia: Plethodontidae). J Morphol 265:87–101
Garcia-Martinez V, Macias D, Ganan Y, Garcia-Lobo JM, Francia MV, Fernandez-Teran MA, Hurle JM (1993) Internucleosomal DNA fragmentation and programmed cell death (Apoptosis) in the interdigital tissue of the embryonic chick leg bud. J Cell Sci 106(Pt 1):201–208
Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P (2000) Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 127:5245–5252
García-Martínez V, Climent V (1985) Apoptosis and necrosis in the areas of interdigital cell death of the developing chick embryo limb bud. An Desarr 29:119–129
Zuzarte-Luis V, Berciano MT, Lafarga M, Hurle JM (2006) Caspase redundancy and release of mitochondrial apoptotic factors characterize interdigital apoptosis. Apoptosis 11:701–715
Makarenkova H, Sugiura H, Yamagata K, Owens G (2005) Alternatively spliced variants of protocadherin 8 exhibit distinct patterns of expression during mouse development. Biochim Biophys Acta 1681:150–156
Hurle JM, Corson G, Daniels K, Reiter RS, Sakai LY, Solursh M (1994) Elastin exhibits a distinctive temporal and spatial pattern of distribution in the developing chick limb in association with the establishment of the cartilaginous skeleton. J Cell Sci 107(Pt 9):2623–2634
Dupe V, Ghyselinck NB, Thomazy V, Nagy L, Davies PJ, Chambon P, Mark M (1999) Essential roles of retinoic acid signaling in interdigital apoptosis and control of BMP-7 expression in mouse autopods. Dev Biol 208:30–43
McCulloch DR, Le Goff C, Bhatt S, Dixon LJ, Sandy JD, Apte SS (2009) Adamts5, the gene encoding a proteoglycan-degrading metalloprotease, is expressed by specific cell lineages during mouse embryonic development and in adult tissues. Gene Expr Patterns 9:314–323
McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS (2009) ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell 17:687–698
Miner JH, Cunningham J, Sanes JR (1998) Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol 143:1713–1723
Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F (2001) Regulation of limb patterning by extracellular microfibrils. J Cell Biol 154:275–281
Debeer P, Schoenmakers EF, Twal WO, Argraves WS, De Smet L, Fryns JP, Van De Ven WJ (2002) The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J Med Genet 39:98–104
Smyth I, Du X, Taylor MS, Justice MJ, Beutler B, Jackson IJ (2004) The extracellular matrix gene frem1 is essential for the normal adhesion of the embryonic epidermis. Proc Natl Acad Sci USA 101:13560–13565
Bose K, Nischt R, Page A, Bader BL, Paulsson M, Smyth N (2006) Loss of nidogen-1 and -2 results in syndactyly and changes in limb development. J Biol Chem 281:39620–39629
Ramirez F, Sakai LY (2010) Biogenesis and Function of Fibrillin Assemblies. Cell Tissue Res 339:71–82
Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K et al (2000) The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 6:1389–1399
Hutcheson J, Scatizzi JC, Bickel E, Brown NJ, Bouillet P, Strasser A, Perlman H (2005) Combined loss of proapoptotic genes bak or bax with bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med 201:1949–1960
Crocoll A, Herzer U, Ghyselinck NB, Chambon P, Cato AC (2002) Interdigital apoptosis and downregulation of BAG-1 expression in mouse autopods. Mech Dev 111:149–152
Nakanishi K, Maruyama M, Shibata T, Morishima N (2001) Identification of a caspase-9 substrate and detection of its cleavage in programmed cell death during mouse development. J Biol Chem 276:41237–41244
Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P (1999) Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol 9:967–970
Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA (1998) Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94:325–337
Pop C, Salvesen GS (2009) Human caspases: activation, specificity, and regulation. J Biol Chem 284:21777–21781
Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM (1995) Peptide inhibitors of the ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron 15:385–393
Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA (1996) Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 384:368–372
Zheng TS, Hunot S, Kuida K, Flavell RA (1999) Caspase knockouts: matters of life and death. Cell Death Differ 6:1043–1053
Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (2002) Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem 277:13430–13437
Grossmann J, Walther K, Artinger M, Kiessling S, Scholmerich J (2001) Apoptotic signaling during initiation of detachment-induced apoptosis (“Anoikis”) of primary human intestinal epithelial cells. Cell Growth Differ 12:147–155
Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, Varmuza S, Latham KE, Flaws JA, Salter JC et al (1998) Defects in regulation of apoptosis in caspase-2-deficient mice. Genes Dev 12:1304–1314
Zuzarte-Luis V, Montero JA, Kawakami Y, Izpisua-Belmonte JC, Hurle JM (2007) Lysosomal cathepsins in embryonic programmed cell death. Dev Biol 301:205–217
Covarrubias L, Hernandez-Garcia D, Schnabel D, Salas-Vidal E, Castro-Obregon S (2008) Function of reactive oxygen species during animal development: passive or active? Dev Biol 320:1–11
Salas-Vidal E, Lomeli H, Castro-Obregon S, Cuervo R, Escalante-Alcalde D, Covarrubias L (1998) Reactive oxygen species participate in the control of mouse embryonic cell death. Exp Cell Res 238:136–147
Shan SW, Tang MK, Cai DQ, Chui YL, Chow PH, Grotewold L, Lee KK (2005) Comparative proteomic analysis identifies protein disulfide isomerase and peroxiredoxin 1 as new players involved in embryonic interdigital cell death. Dev Dyn 233:266–281
Schnabel D, Salas-Vidal E, Narvaez V, Sanchez-Carbente Mdel R, Hernandez-Garcia D, Cuervo R, Covarrubias L (2006) Expression and regulation of antioxidant enzymes in the developing limb support a function of ros in interdigital cell death. Dev Biol 291:291–299
Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL (2002) A bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J Neurosci 22:6480–6490
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:589–598
Li W, Yuan X, Nordgren G, Dalen H, Dubowchik GM, Firestone RA, Brunk UT (2000) Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett 470:35–39
Kirkegaard T, Jaattela M (2009) Lysosomal involvement in cell death and cancer. Biochim Biophys Acta 1793:746–754
Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ (2006) Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol 290:G1339–G1346
Castino R, Bellio N, Nicotra G, Follo C, Trincheri NF, Isidoro C (2007) Cathepsin D-bax death pathway in oxidative stressed neuroblastoma cells. Free Radic Biol Med 42:1305–1316
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27:6434–6451
Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G (2003) Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 197:1323–1334
Ishizaki Y, Jacobson MD, Raff MC (1998) A role for caspases in lens fiber differentiation. J Cell Biol 140:153–158
Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossmann H, Koster A, Hess B, Evers M, von Figura K (1995) Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J 14:3599–3608
Deussing J, Roth W, Saftig P, Peters C, Ploegh HL, Villadangos JA (1998) Cathepsins B and D are dispensable for major histocompatibility complex class II-mediated antigen presentation. Proc Natl Acad Sci USA 95:4516–4521
Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, Schmidt P, Schmahl W, Scherer J, Anton-Lamprecht I et al (2000) Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J 14:2075–2086
Macias D, Ganan Y, Ros MA, Hurle JM (1996) In vivo inhibition of programmed cell death by local administration of FGF-2 and FGF-4 in the interdigital areas of the embryonic chick leg bud. Anat Embryol (Berl) 193:533–541
Wilkie AO, Patey SJ, Kan SH, van den Ouweland AM, Hamel BC (2002) FGFs, their receptors, and human limb malformations: clinical and molecular correlations. Am J Med Genet 112:266–278
Sun X, Mariani FV, Martin GR (2002) Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418:501–508
Pajni-Underwood S, Wilson CP, Elder C, Mishina Y, Lewandoski M (2007) BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development 134:2359–2368
Mariani FV, Ahn CP, Martin GR (2008) Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature 453:401–405
Montero JA, Ganan Y, Macias D, Rodriguez-Leon J, Sanz-Ezquerro JJ, Merino R, Chimal-Monroy J, Nieto MA, Hurle JM (2001) Role of FGFs in the control of programmed cell death during limb development. Development 128:2075–2084
Ota S, Zhou ZQ, Keene DR, Knoepfler P, Hurlin PJ (2007) Activities of N-Myc in the developing limb link control of skeletal size with digit separation. Development 134:1583–1592
Maatouk DM, Choi KS, Bouldin CM, Harfe BD (2009) In the limb AER Bmp2 and Bmp4 are required for dorsal-ventral patterning and interdigital cell death but not limb outgrowth. Dev Biol 327:516–523
Pizette S, Abate-Shen C, Niswander L (2001) BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development 128:4463–4474
Bastida MF, Sheth R, Ros MA (2009) A BMP-Shh negative-feedback loop restricts Shh expression during limb development. Development 136:3779–3789
Macias D, Ganan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM (1997) Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development 124:1109–1117
Zou H, Wieser R, Massague J, Niswander L (1997) Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev 11:2191–2203
Merino R, Macias D, Ganan Y, Economides AN, Wang X, Wu Q, Stahl N, Sampath KT, Varona P, Hurle JM (1999) Expression and function of Gdf-5 during digit skeletogenesis in the embryonic chick leg bud. Dev Biol 206:33–45
Montero JA, Hurle JM (2007) Deconstructing digit chondrogenesis. Bioessays 29:725–737
Montero JA, Lorda-Diez CI, Ganan Y, Macias D, Hurle JM (2008) Activin/TGFbeta and BMP crosstalk determines digit chondrogenesis. Dev Biol 321:343–356
Francis-West PH, Robertson KE, Ede DA, Rodriguez C, Izpisua-Belmonte JC, Houston B, Burt DW, Gribbin C, Brickell PM, Tickle C (1995) Expression of genes encoding bone morphogenetic proteins and sonic hedgehog in talpid (ta3) limb buds: their relationships in the signalling cascade involved in limb patterning. Dev Dyn 203:187–197
Wang CK, Omi M, Ferrari D, Cheng HC, Lizarraga G, Chin HJ, Upholt WB, Dealy CN, Kosher RA (2004) Function of BMPs in the apical ectoderm of the developing mouse limb. Dev Biol 269:109–122
Geetha-Loganathan P, Nimmagadda S, Huang R, Scaal M, Christ B (2006) Expression pattern of BMPs during chick limb development. Anat Embryol (Berl) 211(Suppl 1):87–93
Zuzarte-Luis V, Montero JA, Rodriguez-Leon J, Merino R, Rodriguez-Rey JC, Hurle JM (2004) A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Dev Biol 272:39–52
Merino R, Rodriguez-Leon J, Macias D, Ganan Y, Economides AN, Hurle JM (1999) The BMP antagonist gremlin regulates outgrowth, chondrogenesis and programmed cell death in the developing limb. Development 126:5515–5522
Yokouchi Y, Sakiyama J, Kameda T, Iba H, Suzuki A, Ueno N, Kuroiwa A (1996) BMP-2/-4 mediate programmed cell death in chicken limb buds. Development 122:3725–3734
Zou H, Niswander L (1996) Requirement for BMP signaling in interdigital apoptosis and scale formation. Science 272:738–741
Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA (2002) In vivo evidence that BMP signaling is necessary for apoptosis in the mouse limb. Dev Biol 249:108–120
Grotewold L, Plum M, Dildrop R, Peters T, Ruther U (2001) Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev 100:327–330
Vargesson N, Laufer E (2009) Negative Smad expression and regulation in the developing chick limb. PLoS One 4:e5173
Ross SA, McCaffery PJ, Drager UC, De Luca LM (2000) Retinoids in embryonal development. Physiol Rev 80:1021–1054
Mercader N, Leonardo E, Piedra ME, Martinez-A C, Ros MA, Torres M (2000) Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 127:3961–3970
Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G (2009) Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol 19:1050–1057
Dolle P, Ruberte E, Kastner P, Petkovich M, Stoner CM, Gudas LJ, Chambon P (1989) Differential expression of genes encoding alpha, beta and gamma retinoic acid receptors and CRABP in the developing limbs of the mouse. Nature 342:702–705
Kuss P, Villavicencio-Lorini P, Witte F, Klose J, Albrecht AN, Seemann P, Hecht J, Mundlos S (2009) Mutant Hoxd13 induces extra digits in a mouse model of synpolydactyly directly and by decreasing retinoic acid synthesis. J Clin Invest 119:146–156
Rodriguez-Leon J, Merino R, Macias D, Ganan Y, Santesteban E, Hurle JM (1999) Retinoic acid regulates programmed cell death through BMP signalling. Nat Cell Biol 1:125–126
Ahuja HS, James W, Zakeri Z (1997) Rescue of the limb deformity in hammertoe mutant mice by retinoic acid-induced cell death. Dev Dyn 208:466–481
Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H (2004) Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell 6:411–422
Francis JC, Radtke F, Logan MP (2005) Notch1 signals through Jagged2 to regulate apoptosis in the apical ectodermal ridge of the developing limb bud. Dev Dyn 234:1006–1015
Sidow A, Bulotsky MS, Kerrebrock AW, Bronson RT, Daly MJ, Reeve MP, Hawkins TL, Birren BW, Jaenisch R, Lander ES (1997) Serrate2 is disrupted in the mouse limb-development mutant syndactylism. Nature 389:722–725
Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T (1998) Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev 12:1046–1057
Pan Y, Liu Z, Shen J, Kopan R (2005) Notch1 and 2 cooperate in limb ectoderm to receive an early Jagged2 signal regulating interdigital apoptosis. Dev Biol 286:472–482
Chimal-Monroy J, Montero JA, Ganan Y, Macias D, Garcia-Porrero JA, Hurle JM (2002) Comparative analysis of the expression and regulation of Wnt5a, Fz4, and Frzb1 during digit formation and in micromass cultures. Dev Dyn 224:314–320
Knobloch J, Ruther U (2008) Shedding light on an old mystery: thalidomide suppresses survival pathways to induce limb defects. Cell Cycle 7:1121–1127
Grotewold L, Ruther U (2002) The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J 21:966–975
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP et al (2001) Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1:423–434
Grotewold L, Ruther U (2002) Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int J Dev Biol 46:943–947
Johnson EB, Hammer RE, Herz J (2005) Abnormal development of the apical ectodermal ridge and polysyndactyly in Megf7-deficient mice. Hum Mol Genet 14:3523–3538
Morello R, Bertin TK, Schlaubitz S, Shaw CA, Kakuru S, Munivez E, Hermanns P, Chen Y, Zabel B, Lee B (2008) Brachy-syndactyly caused by loss of Sfrp2 function. J Cell Physiol 217:127–137
Garcia-Moreno B (2009) Adaptations of proteins to cellular and subcellular pH. J Biol 8:98
Acknowledgments
Thanks are due to Montse Fernandez-Calderón and Sonia Pérez-Mantecón for excellent technical support. JAM and JMH work is supported respectively by grants BFU2005-04393 and BFU2008-03930 from the Spanish Sciences and Innovation Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montero, J.A., Hurlé, J.M. Sculpturing digit shape by cell death. Apoptosis 15, 365–375 (2010). https://doi.org/10.1007/s10495-009-0444-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-009-0444-5