Abstract
Yeast-based functional screening of a human glioblastoma cDNA library identified ras-related nuclear protein (Ran) as a novel suppressor of Bcl-2-associated X protein (Bax), a pro-apoptotic member of the Bcl-2 family of proteins. Yeast cells that expressed human Ran were resistant to Bax-induced cell death. In U373MG glioblastoma cells, stable overexpression of Ran significantly attenuated apoptotic cell death induced by the chemotherapeutic agent paclitaxel. FACS analysis demonstrated that Ran is involved in paclitaxel-induced cell cycle arrest. Stable overexpression of Ran also markedly inhibited the phosphorylation of Bcl-2 by paclitaxel, and inhibited the translocation of Bax, the release of cytochrome c and activation of caspase-3. Paclitaxel-induced phosphorylation of c-JUN N-terminal kinase (JNK), but not p38, extracellular signal-regulated kinase and Akt, was markedly suppressed in U373MG cells that stably expressed Ran. These results suggest that Ran suppresses paclitaxel-induced cell death through the downregulation of JNK-mediated signal pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Programmed cell death, or apoptosis, is a physiological process for eliminating infected, mutated, or damaged cells that has a critical role in normal development and the maintenance of tissue homeostasis in nearly all multi-cellular organisms [1]. Apoptosis is a tightly regulated process, and the deregulation has been implicated in a number of diseases, including cancer, neurodegenerative disorders, and autoimmunity [2]. Apoptosis is initiated by both intrinsic and extrinsic mechanisms [3]. The extrinsic pathway is initiated by the binding of ligands to transmembrane receptors, the so-called death receptors of the tumor necrosis factor family, and results in the activation of caspase-10. The intrinsic pathway is triggered by a variety of stresses, and results in the activation of caspase-9. The activation of caspase-9 triggers the execution phase of apoptosis. The intrinsic apoptotic pathway is regulated by the Bcl-2 family of proteins, which comprises both anti-apoptotic (Bcl-2, Bcl-xL, Bcl-w) and pro-apoptotic (Bax, Bak, Bid, Bad) proteins. Specifically, the translocation of Bax, one of the pro-apoptotic members of the Bcl-2 family, to the mitochondrial membrane triggers the activation of the intrinsic pathway of apoptosis [4].
Although yeast do not contain homologues of the mammalian Bcl-2 proteins, the expression of mammalian Bax induces cell death in yeast, similar to mammalian cells. Yeast that express Bax under the control of an inducible promoter exhibit a conditional lethal Bax-dependent phenotype [5–7]. This system has been used successfully to identify novel anti-apoptotic genes by yeast-based functional screening, including several inhibitors of Bax-induced apoptosis, such as Bax inhibitor-1 (BI-1), Ku70, sphingomyelin synthase 1, ascorbate peroxidase, and glutathione S-transferase [5–9].
Paclitaxel was isolated from the bark of Taxus brevifolia and exhibits potent antiproliferative activity in cancer cells [10, 11]. Cells treated with paclitaxel undergo cell-cycle arrest in G2/M phase due to the stabilization of the microtubule network and tubulin dimers, and the inhibition of microtubule disassembly [12]. Following G2/M arrest, cells either undergo apoptosis or exit from mitosis. Paclitaxel can also trigger apoptosis through caspase-dependent and -independent pathways via the activation of mitogen-activated protein (MAP) kinases, including c-Jun NH2-terminal kinase (JNK) [13–15]. Recently, it was reported that paclitaxel regulates the expression of apoptosis-related proteins, including Bax, Bcl-2, and Bcl-xL. In particular, paclitaxel increased the ratio of Bax to Bcl-2 through the phosphorylation-mediated degradation of Bcl-2 [15].
Ran is a small GTP binding protein of the Ras superfamily that is essential for the translocation of RNA and proteins through the nuclear pore complex [16, 17]. Small GTP-binding proteins function as molecular “switches” in the regulation of numerous cellular processes by catalyzing the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) [18]. Ran plays important roles in nucleo-cytoplasmic transport and other cellular processes, including mitotic spindle assembly and post-mitotic nuclear envelope assembly [19, 20]. A role for Ran in the regulation of cell cycle transitions has recently emerged, based on the observation that mutations of Ran network members cause premature progression of the cell cycle [21]. Ran is overexpressed in most cancer cell lines and cancer tissues at both the mRNA and protein levels, which suggests that it may be associated with malignant transformation and/or the enhanced proliferation of cancer cells [22].
In the current study, yeast-based functional screening of a U373MG cDNA library was carried out to identify novel suppressors of Bax-mediated apoptosis. We identified Ran as a suppressor of Bax-induced yeast cell death, and examined whether Ran is involved in paclitaxel-induced apoptosis in mammalian cells.
Materials and methods
Materials
Paclitaxel, hygromycin, and propidium iodide (PI) were purchased from Sigma–Aldrich Co. (St. Louis, MO). 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) was purchased from Calbiochem (La Jolla, CA). Monoclonal anti-Bax antibodies, and polyclonal antibodies for Bcl-2, caspase-3, cytochrome c, β-actin, and Heat shock protein 60 (Hsp60) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies specific for JNK, phosphorylated-JNK (phospho-JNK), p38, phospho-p38, extracellular signal regulated kinase (ERK), phospho-ERK, and Flag, as well as HRP-conjugated goat anti-rabbit and anti-mouse IgG were purchased from Cell Signaling (Beverly, MA). Other reagents were of the highest grade available.
Yeast-based functional screening
A U373MG glioblastoma cDNA library was constructed using pADGAL4-2.1 (Eugentech, Daejeon, Korea). The yeast strain used in these studies, Saccharomyces cerevisiae strain W303-1a/Bax (Mata ade2-1 can1-100 leu2-3.112 trp1-1 ura3-1), contained the inducible plasmid pGilda-Bax, as previously described [6]. Cells (strain W303-1a/Bax) were grown to mid-log phase in glucose-based synthetic dropout (SD) medium [0.67% Yeast Nitrogen Base-w/o amino acid, 2% glucose] supplemented with amino acids [l-arginine 20 mg/l, leucine 20 mg/l, lysine 30 mg/l, methionine 20 mg/l, tryptophan 20 mg/l, adenine hemisulfate 20 mg/l, uracil 20 mg/l], and deficient in histidine (SD-Glc/His−). Cells were then transformed using the U373MG cDNA library and the LiOAc method, as previously described [23]. Bax-resistant transformants were selected directly on galactose-based SD medium supplemented with amino acids and deficient in histidine and leucine (SD-Gal/His−Leu−).
Spot assay
Yeast cells were grown in SD-Glc/His− medium for 1 day, and then diluted to an optical density at 600 nm (OD600) of 0.1. An aliquot of the cells was used to inoculate SD-Gal/His− media and then the cultures were incubated until they reached an OD600 of 0.3. The cells were diluted to the indicated concentrations, and equal aliquots were spotted onto SD-Glc/His− and SD-Gal/His− plates. Plates were then incubated for 2 or 3 days at 30°C.
Mammalian cell culture and generation of stable transfectants
Human glioblastoma U373MG cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin G and 100 μg/ml streptomycin at 37°C and 5% CO2. Stable U373MG transfectants that expressed either the Flag epitope alone, or a Flag epitope fusion protein of Ran (Flag-Ran), were generated by transfection with Flag-pcDNA3.1 or Flag-pcDNA3.1-Ran, respectively. Flag-pcDNA3.1-Ran was constructed by amplification of the Ran cDNA by PCR, followed by restriction enzyme digestion with BamHI/XbaI and ligation into the corresponding sites of Flag-pcDNA3.1. Following transfection, cells were cultured in medium containing 100 μg/ml hygromycin. Several independent clones were isolated, and positive clones were identified by Western blot analysis of cell lysates using an anti-Flag antibody.
MTT assay
Cells that expressed Flag or Flag-Ran were seeded into 24-well plates at a density of 1 × 104 cells per well. After incubation overnight, the cells were treated with the indicated concentration of paclitaxel for 48 h, and then cell viability was analyzed by MTT assay, as described previously [24].
Apoptosis assay
Apoptosis was measured by co-staining of cells with annexin V and PI. Following treatment with paclitaxel, cells were removed from the tissue culture plate by trypsinization. The translocation phosphatidylserine and the extent of apoptotic cell death were measured using an annexin V and PI apoptosis kit (Roche Applied Science, Penzberg, Germany), according to the manufacturer’s instructions. DNA fragmentation was also determined by gel electrophoresis, as follows. Briefly, following treatment with paclitaxel, genomic DNA was purified using a genomic DNA purification kit (Promega, Madison, WI), according to the manufacturer’s instructions. The genomic DNA (10 μg) was fractionated by 2% agarose gel electrophoresis, stained with ethidium bromide, and then photographed under UV illumination.
Cell cycle analysis
Paclitaxel-treated cells were collected and fixed in 80% ethanol overnight at −70°C. Cells were incubated in a solution of PI [10 mM Tris (pH 8.0), 1 mM NaCl, 0.1% NP40, 0.7 μg/ml RNase A, 0.05 mg/ml propidium iodide] for 30 min, and then analyzed using a FACScan flow cytometer (Becton Dickinson Bioscience, San Jose, CA).
Transient transfection
Mammalian expression vector for dominant negative JNK (DN-JNK) were kindly provided by Dr. A.-K. Yi (University of Tennessee Health Science Center, Memphis, Tennessee). Cells were seeded into 60 mm tissue culture dish at 18–24 h prior to transfection, and then transfected with DN-JNK expression plasmid and pcDNA 3 (Invitrogen, Carlsbad, CA) using SuperFect reagent (Qiagen, Valencia, CA). After treatment with 50 nM paclitaxel for 48 h, apoptotic cell death was analyzed as described above.
Western blot analysis
Yeast were grown in SD-galactose medium to induce the expression of Bax. Cells were suspended in SDS-PAGE sample buffer [2% SDS, 0.06 M Tris–HCl (pH 6.8), 10% glycerol, 0.0025% bromophenol blue, 5% 2-mercaptoethanol] and then heated at 95°C for 5 min. Cells were subjected to centrifugation at 13,000 rpm for 5 min, and supernatants were collected. For mammalian cells, the cells were treated with paclitaxel for the indicated periods of time, and then total cellular extracts were prepared using the PRO-PREP protein extraction solution (iNtRON Biotechonology, Seoul, Korea). Proteins were separated by 10% SDS-PAGE and then transferred to a Hybond-P+ PVDF membrane (Amersham Biosciences, Piscataway, NJ). Membranes were incubated with the indicated primary and secondary antibodies, and immunoreactive proteins were visualized using WEST-ZOL PLUS (iNtRON Biotechnology, Seoul, Korea).
Measurement of intracellular reactive oxygen species (ROS)
Cells were incubated with 10 μM H2DCF-DA at 37°C for 30 min. After washing twice with ice-cold PBS, cell fluorescence was immediately analyzed using a FACScan flow cytometer (Becton Dickinson Bioscience, San Jose, CA).
Subcellular fractionation
Cells that overexpressed Flag or Flag-Ran were treated with paclitaxel, and mitochondrial and cytosolic fractions were isolated, as follows. Cells were collected by trypsinization, washed with ice-cold PBS, and then subjected to centrifugation for 5 min at 3,000 rpm at 4°C. Cells were washed twice in ice-cold PBS and then resuspended in S-100 buffer [20 mM HEPES (pH 7.5), 10 mM KCl, 1.9 mM MgCl2, 1 mM EGTA, 1 mM EDTA, and a mixture of protease inhibitors]. After incubation on ice for 20 min, the cells were homogenized with a glass dounce homogenizer. The homogenate was subjected to centrifugation at 1,000g for 5 min to remove intact cells, cell nuclei, and heavy membranes. The resulting supernatant was then subjected to centrifugation at 14,000g for 30 min to collect the mitochondria-rich and cytosolic fractions. The mitochondrial fraction was washed once with S-100 buffer and resuspended in lysis buffer [150 mM NaCl, 50 mM Tris–Cl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, and 1 mM EGTA] containing protease inhibitors.
Results
Overexpression of Ran inhibits Bax-induced cell death in yeast
Yeast strain W303-1a/Bax carries an inducible Bax expression plasmid, pGilda-Bax, which encodes full-length mouse Bax under the control of the galactose-inducible yeast GAL1 promoter [5]. W303-1a/Bax was transformed with a U373MG human glioblastoma cDNA library, and Bax-resistant transformants were selected on SD-Gal/His−Leu− medium. We screened a total of 105 transformants, and identified 24 clones that were resistant to Bax-induced apoptosis. Sequence analysis and a search of the GenBank/EMBL nucleotide sequence database identified Ran as an inhibitor of Bax-induced yeast cell apoptosis. The inhibitory effect of Ran was equivalent to that of Bcl-2 (Fig 1a). Bax protein was undetectable in cells grown in glucose-based medium, but accumulated in cells that were cultured in galactose-based medium, which suggested that the expression of Ran does not interfere with Bax protein expression in yeast (Fig. 1b).
Effect of Ran expression on Bax-induced cell death in yeast. (a) Yeast were grown in SD-Glc medium for 1 day, cultures were adjusted to an OD600 of 0.1, and then diluted to the indicated concentrations (OD600 = 1, 0.1, 0.01, 0.001, and 0.0001). An aliquot (5 μl) of each dilution was spotted onto SD-Glc or SD-Gal plates. (b) Yeasts were incubated in SD-Glc or SD-Gal medium for 12 h and cellular protein extracts were prepared. An aliquot of total protein (30 μg per lane) was subjected to Western blot analysis, as described in Section "Materials and methods". The results are representative of three or four independent experiments
Ran inhibits paclitaxel-induced apoptosis in human glioblastoma cells
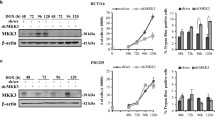
U373MG glioblastoma cells were transfected with Flag-pcDNA3.1-Ran, or Flag-pcDNA3.1 as a control, and stable transfectants were selected using hygromycin resistance. Flag-pcDNA3.1-Ran transfectants expressed a high level of Ran protein, which indicated that the plasmid-based Ran cDNA used in these studies encoded a functional protein in U373MG cells (Fig. 2a, insert). When we treated cells with paclitaxel, we observed a dose-dependent decline in the cell viability of Flag transfectants, but not Flag-Ran transfectants (Fig. 2a). Thus, paclitaxel-mediated cytotoxicity was significantly attenuated by the overexpression of Flag-Ran in U373MG cells. To characterize the type of cell death that was induced by paclitaxel, we carried out an annexin V staining and DNA fragmentation assay (Fig. 2b and c). In the presence of paclitaxel, the number of annexin V-positive cells increased in a dose-dependent manner in cells that expressed Flag alone or Flag-Ran, but the increase was significantly attenuated in Flag-Ran transfectants. The attenuation of annexin V-positive staining correlated with the level of DNA fragmentation in cells. DNA fragmentation was readily detectable in Flag transfectants 48 h after paclitaxel treatment, and fragmentation was markedly reduced in Flag-Ran transfectants. These results indicated that Ran partially inhibits paclitaxel-induced apoptosis in glioblastoma cells.
Effect of Ran on paclitaxel-induced cell death. (a) Cell viability was determined by MTT assay in cells incubated for 48 h. (b) Externalization of phosphatidylserine was measured by annexin V and PI staining. Cells that expressed Flag or Flag-Ran (Ran) were treated with the indicated concentrations of paclitaxel for 48 h. Data represents the means ± standard error (SE) (n = 3 or 4), *P < 0.05, **P < 0.01. (c) Cells were treated with 50 nM paclitaxel for the indicated time periods, and DNA fragmentation was measured by gel electrophoresis. The results are representative of three independent experiments
Ran suppresses the paclitaxel-induced accumulation of cells in sub-G1 phase
Paclitaxel has been reported to induce cell-cycle arrest during mitosis, after which cells can progress to apoptosis [11]. To determine whether Ran affected paclitaxel-induced mitotic arrest, we carried out a cell cycle analysis of U373MG stable transfectants (Fig. 3). In stable Flag transfectants, the accumulation of cells in sub-G1 phase was dramatically increased 48 h after treatment with paclitaxel, and there was a corresponding reduction in the number of cells in G2/M phase. In contrast, in Flag-Ran transfectants in the presence of paclitaxel, there was a sustained G2/M arrest, with less accumulation of cells in sub-G1 than Flag transfectants. These results indicated that Ran does not abrogate paclitaxel-induced cell cycle arrest in G2/M, but it suppresses the accumulation of cells in sub-G1 phase.
Effect of Ran on paclitaxel-induced cell cycle arrest. Cells that expressed Flag or Flag-Ran (Ran) were treated with 50 nM paclitaxel for the indicated time periods. Cells were incubated with PI for 30 min, and cell cycle was analyzed using a FACScan flow cytometer. (a) The histograms are representative of three separate determinations. (b) Data represents the means ± SE (n = 3), *P < 0.05, **P < 0.01 compared with Flag transfectant
Ran abolishes the generation of ROS in response to paclitaxel
To determine whether the generation of ROS was involved in paclitaxel-induced apoptosis in U373MG cells, we examined the level of ROS in Flag and Flag-Ran stable transfectants in the presence and absence of paclitaxel. Although paclitaxel significantly promoted the generation of ROS in cells that overexpressed Flag alone, the generation of ROS in response to paclitaxel treatment was markedly attenuated in cells that overexpressed Flag-Ran (Fig. 4a and b). To clarify whether ROS were directly involved in paclitaxel-induced apoptosis, cells were treated with paclitaxel in the presence or absence of N-acetyl-l-cysteine (NAC), a free radical scavenger. In the presence of NAC, paclitaxel-induced apoptosis was not affected in Flag and Flag-Ran transfectants, which suggested that ROS are not directly involved in paclitaxel-induced cell death (Fig. 4c).
Effect of Ran on the generation of ROS by paclitaxel. (a and b) Cells were treated with 50 nM paclitaxel for 90 min (a) or for the indicated time periods (b), then treated with the oxidant-sensitive probe H2DCF-DA (10 μM). Relative levels of ROS were quantified by FACScan flow cytometry. Data represents the means ± SE (n = 3), *P < 0.05, **P < 0.01. (c) Cells that expressed Flag or Flag-Ran (Ran) were treated with 50 nM paclitaxel in the presence or absence of NAC. After 48 h, the cells were stained with PI, and the sub-G1 population was analyzed using a FACScan flow cytometer. The results are representative of three independent experiments
Ran inhibits the activation of JNK by paclitaxel
To further clarify the role of Ran in paclitaxel-induced apoptosis, we examined the activation of MAP kinases and PI3 K/Akt in response to paclitaxel in U373MG stable transfectants. As shown in Fig. 5a, paclitaxel treatment resulted in the activation of JNK, ERK and p38, but not PI3 K/Akt, in Flag transfectants. In contrast, the overexpression of Ran markedly reduced the paclitaxel-induced phosphorylation of JNK1/2, which indicated that JNK-mediated signaling pathways are involved in the suppression of paclitaxel-induced apoptotic cell death by Ran. To further confirm that JNK is involved in the Ran-mediated inhibition of apoptosis in a process mediated by paclitaxel, Flag and Flag-Ran transfectants were transiently transfected with dominant negative JNK (DN-JNK) in the presence or absence of paclitaxel. Paclitaxel-induced apoptosis was significantly inhibited in the presence of DN-JNK in both transfectants (Fig. 5b), indicating that JNK plays a pivotal role in the Ran-mediated suppression of apoptotic cell death by paclitaxel.
Effect of Ran on the activation of MAP kinases and PI3 K/Akt by paclitaxel. (a) Cells that expressed Flag or Flag-Ran (Ran) were treated with 50 nM paclitaxel for the indicated time periods. Total cell lysate (30 μg of protein per lane) was analyzed by Western blot using the indicated activation-specific antibodies. Total kinase levels were analyzed in parallel by Western blot. Data is representative of three independent experiments. (b) Cells that expressed Flag or Flag-Ran (Ran) transfected with DN-JNK or control vector treated with 50 nM paclitaxel for 48 h and analyzed using a FACScan flow cytometer. Data represents the means ± SE (n = 3), *P < 0.05, **P < 0.01
Ran regulates the expression of apoptosis-related proteins
To determine whether Ran was involved in the regulation of Bcl-2 family proteins during paclitaxel-induced apoptosis, we examined the expression of the pro-apoptotic and anti-apoptotic proteins Bax and Bcl-2, respectively, by Western blot (Fig. 6a). Although the expression of Bax did not appear to be affected in Flag or Flag-Ran transfectants that were treated with paclitaxel, the phosphorylation of Bcl-2 by paclitaxel was markedly reduced in cells that expressed Flag-Ran as compared to cells that expressed Flag alone. In addition, the activation of caspase-3, the translocation of Bax, and release of mitochondrial cytochrome c were markedly attenuated in cells that expressed Flag-Ran, which indicated that Ran regulates apoptosis-related proteins (Fig. 6).
Effect of Ran on the activation of apoptosis-related proteins by paclitaxel. Cells that expressed Flag (F) or Flag-Ran (Ran; R) were treated with 50 nM paclitaxel for the indicated time periods. An aliquot of total cell lysate (30 μg of protein per lane) was analyzed by Western blot to determine the levels of Bax and Bcl-2 (Bcl, a), or activated caspase-3 (b) using the indicated specific antibodies. Cells were treated as for (a), and then fractionated into mitochondrial (c) and cytosolic fractions (d). Fractions were analyzed by Western blot using the indicated antibodies to assess the translocation of Bax and release of cytochrome c (cyt). Data is representative of three independent experiments. The density of each band was quantified by densitometry and plotted as a percentage of protein indicated to β-actin or Hsp60 ratio relative to the time zero
Discussion
In the current study, we identified Ran as a suppressor of Bax-induced cell death in yeast, and showed that Ran partially inhibits paclitaxel-induced cell death in a human glioblastoma-derived cell line, U373MG. The accumulation of cells in sub-G1 phase in response to paclitaxel was partially suppressed by the overexpression of Ran, which suggests that Ran inhibits JNK alone but not other signal cascades including p38, ERK, and Akt stimulated by paclitaxel. In line with above speculation, the overexpression of Ran suppressed the activation of JNK and resulted in a reduction in the apoptosis-related protein Bcl-2, which suggests that the mechanism of anti-apoptotic activity of Ran involves the activation of JNK-mediated signaling pathways and the regulation of apoptotic proteins.
To our knowledge, this is the first report demonstrating that Ran suppresses Bax- or paclitaxel-induced apoptosis. A number of recent studies have demonstrated the pivotal role of yeast-based functional screening in the identification of anti-apoptotic proteins, including as BI-1, Ku70, sphingomyelin synthase 1, ascorbate peroxidase, and chromosomal high-mobility group box-1 [5–8, 25]. Our study identified Ran as a suppressor of Bax-induced apoptosis in yeast, however, little is known about the anti-apoptotic activity of Ran in mammalian cells. Ran is a member of the Ras superfamily of small G-proteins, and plays an important role in nucleo-cytoplasmic transport and other cellular processes, including mitotic spindle assembly and post-mitotic nuclear envelope assembly [20]. Although Ran has been shown to be involved in the regulation of cell cycle transitions, a recent report demonstrated that Ran is overexpressed in most cancer cell lines and cancer tissues [22]. Our results raise the intriguing possibility that during the development and treatment of cancer, Ran may function as an inhibitor of cell death induced by Bax or paclitaxel.
The overexpression of Ran inhibited many of the cellular responses that are evoked by exogenous apoptotic stimuli, such as paclitaxel. Paclitaxel activates the JNK signaling pathway, and JNK activation appears to be a common point of regulatory control in paclitaxel-induced gene expression and cell death in ovarian cancer cells [26]. Previous studies have also shown that the activation of JNK enhances the phosphorylation of apoptosis regulatory proteins, such as Bax and Bcl-2 [27, 28]. In the current study, we showed that the phosphorylation of JNK, but not ERK and p38 MAPK, is markedly suppressed by the overexpression of Ran, indicating the partial effect of Ran in the paclitaxel-induced apoptosis. When we examined Bcl-2 family proteins in paclitaxel-treated cells that overexpressed Ran, we found that the phosphorylation of Bcl-2 by paclitaxel was markedly suppressed in cells that overexpressed Ran. Moreover, the paclitaxel-induced mitochondrial translocation of Bax, release of cytochrome c and the activation of caspase-3 were also inhibited by the overexpression of Ran. These results suggest that Ran inhibits paclitaxel-induced apoptosis through the inhibition of JNK activation. Our results are also consistent with previous reports that Bcl-2 protein is significantly decreased by paclitaxel treatment in many cancer cells [28, 29].
Although it has been reported that paclitaxel-induced apoptotic cell death is mediated by mitochondrial ROS [30], there did not appear to be a relationship between ROS production and cell death induced by paclitaxel in our study. In cells that expressed either Flag alone or Flag-Ran, NAC had no effect on paclitaxel-induced cell death. This observation is in agreement with previous findings that paclitaxel induces ROS-independent apoptosis in human leukemia cells [31]. Therefore, the possibility remains that Ran participates in paclitaxel-induced apoptosis through a ROS-independent mechanism. Additional studies are needed to clarify the precise molecular mechanism(s) of suppression of paclitaxel-induced apoptotic cell death by Ran.
In conclusion, we have shown that JNK-mediated signaling pathways are involved in the inhibitory effect of Ran on paclitaxel-induced cell death in U373MG cells (Fig. 7). The down-regulation of JNK in cells that overexpressed Ran resulted in a reduction in the activation of multiple molecular targets of paclitaxel. The identification of Ran as a novel suppressor of Bax-induced apoptosis in cancer cells will aid in the development of new therapeutic strategies for cancer treatment.
Proposed scheme on the Ran-mediated inhibition of apoptosis induced by paclitaxel in U373MG cells. Ran inhibits activation of JNK, and this inhibition causes sequential suppression of downstream apoptosis-related proteins, suggesting that the main role of Ran in the paclitaxel-induced apoptosis is the regulation of JNK pathway
Abbreviations
- ROS:
-
Reactive oxygen species
- JNK:
-
c-Jun NH2-terminal kinase
- ERK:
-
Extracellular signal regulated kinase
- MAPK:
-
Mitogen-activated protein kinases
- NAC:
-
N-Acetyl-l-cysteine
References
Raff MC (1992) Social controls on cell survival and cell death. Nature 356:397–400. doi:10.1038/356397a0
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603. doi:10.1038/nrc1412
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. doi:10.1080/01926230701320337
Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-xL during apoptosis. Proc Natl Acad Sci USA 94:3668–3672. doi:10.1073/pnas.94.8.3668
Xu Q, Reed JC (1998) Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell 1:337–346. doi:10.1016/S1097-2765(00)80034-9
Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S (2003) Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol 5:320–329. doi:10.1038/ncb950
Yang Z, Khoury C, Jean-Baptiste G, Greenwood MT (2006) Identification of mouse sphingomyelin synthase 1 as a suppressor of Bax-mediated cell death in yeast. FEMS Yeast Res 6:751–762. doi:10.1111/j.1567-1364.2006.00052.x
Moon H, Baek D, Lee B, Prasad DT, Lee SY, Cho MJ et al (2002) Soybean ascorbate peroxidase suppresses Bax-induced apoptosis in yeast by inhibiting oxygen radical generation. Biochem Biophys Res Commun 290:457–462. doi:10.1006/bbrc.2001.6208
Kampranis SC, Damianova R, Atallah M, Toby G, Kondi G, Tsichlis PN et al (2000) A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J Biol Chem 275:29207–29216. doi:10.1074/jbc.M002359200
Eisenhauer EA, Vermorken JB (1998) The taxoids. Comparative clinical pharmacology and therapeutic potential. Drugs 55:5–30. doi:10.2165/00003495-199855010-00002
Bokemeyer C, Hartmann JT, Kuczyk MA, Truss MC, Kollmannsberger C, Beyer J et al (1998) Recent strategies for the use of paclitaxel in the treatment of urological malignancies. World J Urol 16:155–162. doi:10.1007/s003450050044
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubles assembly in vitro by taxol. Nature 277:665–667. doi:10.1038/277665a0
André N, Carré M, Brasseur G, Pourroy B, Kovacic H, Briand C et al (2002) Paclitaxel targets mitochondria upstream of caspase activation in intact human neuroblastoma cells. FEBS Lett 532:256–260. doi:10.1016/S0014-5793(02)03691-8
Wang YF, Chen CY, Chung SF, Chiou YH, Lo HR (2004) Involvement of oxidative stress and caspase activation in paclitaxel-induced apoptosis of primary effusion lymphoma cells. Cancer Chemother Pharmacol 54:322–330. doi:10.1007/s00280-004-0831-0
Pushkarev VM, Starenki DV, Saenko VA, Namba H, Kurebayashi J, Tronko MD et al (2004) Molecular mechanisms of the effects of low concentrations of taxol in anaplastic thyroid cancer cells. Endocrinology 145:3143–3152. doi:10.1210/en.2004-0127
Bischoff FR, Ponstingl H (1991) Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci USA 88:10830–10834. doi:10.1073/pnas.88.23.10830
Sweet DJ, Gerace L (1996) A GTPase distinct from Ran is involved in nuclear protein import. J Cell Biol 133:971–983. doi:10.1083/jcb.133.5.971
Bischoff FR, Ponstingl H (1995) Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Meth Enzymol 257:135–144. doi:10.1016/S0076-6879(95)57019-5
Kalab P, Pu RT, Dasso M (1999) The Ran GTPase regulates mitotic spindle assembly. Curr Biol 9:481–484. doi:10.1016/S0960-9822(99)80213-9
Joseph J (2006) Ran at a glance. J Cell Sci 119:3481–3484. doi:10.1242/jcs.03071
Rush MG, Drivas G, D’Eustachio P (1996) The small nuclear GTPase Ran: how much does it run? Bioessays 18:103–112. doi:10.1002/bies.950180206
Azuma K, Sasada T, Takedatsu H, Shomura H, Koga M, Maeda Y et al (2004) Ran, a small GTPase gene, encodes cytotoxic T lymphocyte (CTL) epitopes capable of inducing HLA-A33 restricted and tumor-reactive CTLs in cancer patients. Clin Cancer Res 10:6695–6702. doi:10.1158/1078-0432.CCR-04-0818
Hill J, Ian KA, Donald G, Griffiths DE (1991) DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res 19:5791. doi:10.1093/nar/19.20.5791
Kang ES, Woo IS, Kim HJ, Eun SY, Paek KS, Kim HJ et al (2007) Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radic Biol Med 43:535–545. doi:10.1016/j.freeradbiomed.2007.05.006
Brezniceanu ML, Volp K, Bosser S, Solbach C, Lichter P, Joos S et al (2003) HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma. FASEB J 17:1295–1297
Lee LF, Li G, Templeton DJ, Ting JP (1998) Paclitaxel (Taxol)-induced gene expression and cell death are both mediated by the activation of c-Jun NH2-terminal kinase (JNK/SAPK). J Biol Chem 273:28253–28260. doi:10.1074/jbc.273.43.28253
Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto U et al (2004) JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J 23:1889–1899. doi:10.1038/sj.emboj.7600194
Briches L, Cazettes G, Valette A (2004) JNK is associated with Bcl-2 and PP1 in mitochondria. Cell Cycle 3:e47–e54
Tudor G, Aguilera A, Halverson DO, Laing ND, Sausville EA (2000) Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ 7:574–586. doi:10.1038/sj.cdd.4400688
Varbiro G, Veres B, Gallyas F Jr, Sumegi B (2001) Direct effect of taxol on free radical formation and mitochondrial permeability transition. Free Radic Biol Med 31:548–558. doi:10.1016/S0891-5849(01)00616-5
Park SJ, Wu CH, Gordon JD, Zhong X, Emami A, Safa AR (2004) Taxol induces caspase-10-dependent apoptosis. J Biol Chem 279:51057–51067. doi:10.1074/jbc.M406543200
Acknowledgements
This work was supported in part by Korean Science and Engineering Foundation grant R13-2005-012-02001-0, which is funded by the Korean government and a grant (2007040103403301) from BioGreen 21 Program, RDA, Republic of Korea. I.S.W. is a recipient of the Korea Research Foundation Grant (KRF-2006-005-J04202).
Author information
Authors and Affiliations
Corresponding author
Additional information
Im Sun Woo and Han-Su Jang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Woo, I.S., Jang, HS., Eun, S.Y. et al. Ran suppresses paclitaxel-induced apoptosis in human glioblastoma cells. Apoptosis 13, 1223–1231 (2008). https://doi.org/10.1007/s10495-008-0247-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0247-0