Abstract
PI3k-Akt and p53 pathways are known to play anti- and pro-apoptotic roles in cell death, respectively. Whether these pathways are recruited in influenza virus infection in highly productive monkey (CV-1) and canine (MDCK) kidney cells was studied here. Phosphorylation of Akt (Akt-pho) was found to occur only early after infection (5–9 h.p.i). Nuclear accumulation and phosphorylation of p53 (p53-pho), and expression of its natural target p21/waf showed low constitutive levels at this period, whereas all three parameters were markedly elevated at the late apoptotic stage (17–20 h.p.i.). Up-regulation of Akt-pho and p53-pho was not induced by UV-inactivated virus suggesting that it required virus replication. Also, mRNAs of p53 and its natural antagonist mdm2 were not increased throughout infection indicating that p53-pho was up-regulated by posttranslational mechanisms. However, p53 activation did not seem to play a leading role in influenza-induced cell death: (i) infection of CV1 and MDCK cells with recombinant NS1-deficient virus provoked accelerated apoptotic death characterized by the lack of p53 activation; (ii) mixed apoptosis-necrosis death developed in influenza-infected human bronchial H1299 cells carrying a tetracycline-regulated p53 gene did not depend on p53 gene activation by tetracycline. Virus-induced apoptosis and signaling of Akt and p53 developed in IFN-deficient VERO cells with similar kinetics as in IFN-competent CV1-infected cells indicating that these processes were endocrine IFN-independent. Apoptosis in influenza-infected CV-1 and MDCK cells was Akt-dependent and was accelerated by Ly294002, a specific inhibitor of PI3k-Akt signaling, and down-regulated by the viral protein NS1, an inducer of host Akt. The obtained data suggest that influenza virus (i) initiates anti-apoptotic PI3k-Akt signaling at early and middle phases of infection to protect cells from fast apoptotic death and (ii) provokes both p53-dependent and alternative p53-independent apoptotic and/or necrotic (in some host systems) cell death at the late stage of infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza virus is a cytolytic virus that induces cell death in most cell types by apoptosis [1–3] and, only in certain cells, by necrosis [4–6]. Apoptosis kills infected cells by caspase-dependent mechanisms at late stages of infection [7–9]. Two types of virus-host interactions should be considered in the development of apoptosis/necrosis. First, apoptosis is induced only in infected cells by endocrine or intrinsic mechanisms. Second, infected cells may trigger paracrine or extrinsic apoptosis in non-infected neighboring cells through released signaling molecules. There are also two concepts with respect to the role of apoptosis in influenza virus replication. According to one of them cellular apoptotic factors, such as caspase 3 and NF-kB/Fas/Trail, promote influenza virus replication [10–12], whereas the other concept considers apoptosis as a host defense mechanism limiting virus replication [13, 14]. In either case, however, apoptosis is clearly regulated by virus and host cell factors.
Viral factors play quite different roles in apoptosis. Virus-specific RNAs serve as unique activators of host protein kinase R (PKR) [15–18] and TLR3/TLR7, RIG-1, MDA-5, and IPS-1 signaling that trigger NF-kB/IFN cascades recruited into host pro- and anti-apoptotic pathways [12, 19–21]. Viral neuraminidase (NA) was found to facilitate activation of TGF-β, a known inducer of apoptosis, at the plasma membrane of infected cells [22, 23]. The recently discovered influenza protein PB1-f2 appears to induce apoptosis in virus-infected cells by the formation of ionic channels in mitochondria and release of host proapoptotic intracellular mediators [24–26]. Influenza virus matrix protein M1 seems to be involved in the apoptotic process through interaction with caspase 8 [27, 28] and, when transiently expressed in cells from a DNA plasmid, both M1 and the ionic channel protein M2 were shown to provoke apoptosis [29]. The non-structural viral protein NS1 was found to down-regulate apoptosis in infected cells [9] probably through suppression of the PKR/NF-kB/IFN signaling pathway [12, 30]. Whether and how these viral factors interplay with each other in a coordinated fashion remains unclear.
Host cell factors involved in the apoptotic process include signaling molecules, such as Fas/FasL [17, 31, 32], Bcl-2 [33], TGF-β [23, 34], mitogen-activated Raf/MEK/ERK and AP-1/JNK [30, 35], NF-kB [12, 36], apoptosis signal-regulating kinase (ASK-1) [37, 38], TLRs 3, 7 and 8 [39–41], tumor suppressor p53 [42, 43], chemokine CCL5-CCR5 [44, 45], and nitric oxide [46], that are all up-regulated in influenza-infected cells. In general, these factors indicate which signaling pathways might be involved in apoptosis. Some of them, such as Raf/MEK/ERK [47], NF-kB/Fas/Trail [12, 48], and caspase 3 [11], were found to stimulate virus propagation, whereas others, such as Bcl-2 [33], NF-kB [36], and AP-1/JNK [30], were reported to impair or not influence it. Thus, apoptotic mechanisms may trigger pro- and anti-viral programs. Moreover, it is still unclear whether influenza virus initiates anti-apoptosis pathways to maintain its own replication or whether the host triggers pro-apoptotic ones as a programed anti-stress response to eradicate virus infection.
Here we investigated anti- and pro-apoptotic cell signaling pathways that could be recruited into influenza virus replication in infected cells. Specifically, we have analyzed the interaction of the cellular anti- and pro-apoptotic mediators Akt and p53, respectively, with the viral NS1 protein in monkey (CV-1) and canine (MDCK) kidney cells as well as the human bronchial cell line H1299 containing a tetracycline-regulated p53 promoter. The following observations have been made: (i) anti-apoptotic PI3k-Akt signaling was initiated at early and middle phases after influenza virus infection, and viral NS1 positively regulated this process; (ii) in contrast to NS1, selective inhibition of PI3k-Akt signaling with Ly294002 inhibitor accelerated development of apoptosis; (iii) the proapoptotic p53 was found to be activated only at the late apoptotic stage; (iv) influenza virus may provoke p53-dependent as well as alternative p53-independent cell death.
Materials and methods
Cells and viruses
Influenza strains A/PR/8/34 (H1N1), A/Aichi/2/68 (H3N2), A/Texas/96 (H3N2) (mouse lung-adapted), A/WSN/33 (H1N1), A/tern/South Africa/61 (H5N3), and A/Singapore/57 (H2N2) were grown in 10-day-old embryonated chicken eggs. Strains A/Moscow/328/03 (H3N2) and A/Moscow/343/03 (H3N2) were propagated in CACO-2 cells [6]. Influenza A/PR/8/34 (H1N1) wild type (wt) virus and its isogenic mutant lacking the NS1 gene [49] were propagated in 7-day-old embryonated chicken eggs. Virus titers were measured by virus focus assay in MDCK cells [6]. Madin Darby canine kidney cells (line MDCK-II) (collection of Institute of Virology, Marburg), monkey kidney cells (lines CV-1 and VERO) (European Collection of Cell Cultures; ECACC) were passaged in Dulbecco’s minimal essential medium (DMEM) containing 10% bovine fetal calf serum (FCS) (GIBCO-BRL, Karlsruhe, Germany). The human lung carcinoma cell line H1299, containing the p53 wild type gene under a tetracycline-regulated promoter [50], was propagated in DMEM supplemented with 10% FCS and doxycycline at 5 μg/ml. For infection, 2-day-old confluent cell monolayers were incubated with egg grown influenza virus (5 PFU/cell) for 1 h at 37°C. After infection, cells were washed and incubated with DMEM without serum at 37°C for different periods of time and were then prepared either for light microscope examination, protein gel electrophoresis, DNA fragmentation, or immunofluorescent analysis.
Cell transfection experiments
pCAGGS vector containing combined chicken beta-actin/rabbit beta-globin hybrid promoter and human cytomegalovirus immediate early promoter [51] was used for expression of the NS1 protein. NS1 genes of A/PR/8/34 (H1N1) and A/Aichi/2/68 (H3N2) viruses were cloned into pCAGGS through the EcoRI site. Empty pCAGGS and pCAGGS/NS1 DNAs were propagated in E. coli strain XL1-blue and purified by Endotox-free kit (Promega). CV1 and 293T cells were grown on 30-mm dishes to 50–80% confluency and transfected with 2.5 μg pCAGGS or pCAGGS/NS1 using the superfect transfection reagent (Qiagen) (7 μl) according to the manufacturers protocol. DNA-superfect mixtures were prepared in opti-M medium (GIBCO). Transfected cells were incubated in DMEM containing 0.3% BSA, 0.1% FCS and ampicillin/streptomycin for 25–48 h. After incubation, cells were subjected to polypeptide analysis by WB-PAGE.
Polyacrylamide gel electrophoresis (PAGE)
Polypeptides were electrophoresed in 12% polyacrylamide gels using Tris-glycine-SDS buffer followed by autoradiography as previously described [52]. For gel staining with Coomassie Brilliant Blue R-350, the protocol of Pharmacia was applied. For electrophoresis, cellular polypeptides were treated in dissociation buffer (2% SDS, 0.01 M dithiotreitol, 0.02 M Tris-HCl, pH 6.8) for 5 min at 96°C. To quantitatively normalize samples in a one set of PAGE-western blot analysis the same amounts of protein were loaded into gel wells.
Western Blot analysis (WB)
After SDS-PAGE the polypeptides were transferred from the gel onto Protran-nitrocellulose membranes (pore-size 0.45 μm) (Schleicher & Schuell) by semidry electro-blotting with Tris-glycine buffer (pH ∼ 9.8) containing 15% ethanol and 0.005% SDS. Membranes were washed with 150 mM phosphate-buffered saline (PBS) and incubated for 1 h at 20°C in 5% dried milk in PBS. After washing with PBS, membranes were incubated overnight at 4°C in PBS containing 1% BSA, and specific antisera for Akt, Akt-Ser476-pho (Cell Signaling), p53 (Calbiochem), p53-Ser15-pho (Cell Signaling), p53-Ser46-pho (Cell Signaling), WAF/p21 (Biosourse), H3 and H1 influenza hemagglutinins (collection of the Institute of Virology, Marburg, Germany), and caspase 3 (Pharmingen). Mouse monoclonal antibodies against influenza virus NP (clones A1 and A3; CDC, Atlanta), and M1 (Serotec) were also used. After incubation with antibodies, membranes were exposed to horseradish peroxidase (HRP)-conjugated secondary anti-species antibodies (Dako), followed by visualization of positive bands by ECL on Kodak BioMax film. Protein bands were scanned and WB membrane images were calculated with TINA program, version 2.09.
p53 and Mdm2 transcription assay
Total RNA was isolated from CV-1 infected cells with a DNA-ase treatment step using the high pure RNA isolation kit (Roche), and differences in intracellular mRNA levels were determined by reverse transcription (RT) using Omni script RT kit (Roche) and semiquantitative PCR, as widely used previously [53]. For each gene assay, transcription was started with the first (RT) gene-specific primer, whereas PCR was carried out using the second gene-specific primer set and was performed using an initial 2-min denaturation at 94°C, followed by 22 and 30 cycles. These double-cycle programs were used to avoid comparison of mRNA levels at saturated PCR conditions [53]. One PCR cycle consisted of 20 s at 94°C, 30 s at 55°C, and 60 s at 72°C. Mdm2 specific primers were employed for RT (AAC ATC TGT TGC AAT GTG ATG G) and for PCR Fo (TCA GGA TTC AGT TTC AGA TCA G) and Re (CAT TTC CAA TAG TCA GCT AAG G). p53-specific primers were: RT (TCA CAA CCT CCG TCA TGT GCT GTG), and for PCR Fo (CTT GCC GTC CCA AGC AAT GGA TGA) and Re (TGT GGA ATC AAC CCA CAG CTG CAC).
DNA fragmentation analysis
For the isolation of cellular low molecular weight DNA, the SDS-high salt extraction method has been used [9]. Mock-infected and influenza-infected cells were suspended in PBS and gently mixed with 4 volumes of buffer containing 10 mM Tris-HCl (pH 7.6), 10 mM EDTA, and 0.6% SDS. Cell lysates were incubated overnight at 4°C in 1 M NaCl, and centrifuged at 14,000 rpm for 20 min at 4°C. Supernatants were treated sequentially with RNAase A (1 mg/ml) and proteinase K (0.2 mg/ml) for 20 min at 37°C, mixed with two volumes of ethanol, and left overnight at −20°C. Precipitated DNA was resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.6), electrophoresed in 1.5% agarose-TBE buffer, and stained with ethidium bromide (EB).
Immune fluorescent staining of cells
CV-1 cells were grown on 1 cm2 glass coverslips and then infected with WT and delNS1 A/PR/8/34 viruses (MOI 1). At different intervals after infection coverslips were fixed at 4°C for 20 min with 2% paraformaldehyde prepared in PBS and then cells were permeabilized for 10 min in 0.15% NP-40 in PBS. Permeabilized cells were incubated with mouse monoclonal anti-p53 antibody (Ab-3; Calbiochem, USA) for 1 h at room temperature, washed 4 times with PBS and incubated for 1.5 h in the dark with anti-mouse secondary Fab2 conjugated with TRITC (Jackson Immuno Research Inc., USA). Stained cells were washed with PBS, covered with moviol mount medium and observed in a fluorescent microscope at magnification 400.
Propidium iodide (PI) staining of cells
PI staining of infected cells was used to estimate the level of cell plasma membrane leakage as one of the hallmarks differentiating necrosis from apoptosis [54]. Uninfected and influenza-infected H1299 cells grown on 1 cm2 coverslips with and without doxycycline were incubated in darkness with DMEM containing 2 μg/ml of PI for 15 min and fixed with 2% paraformaldehyde prepared in PBS at 4°C for 20 min. Fixed cells were twice washed with PBS, covered with Moviol mount medium and observed in a fluorescence microscope with a 600-EFLP filter.
Cell viability MTT test
The principle of the assay is the oxidation of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to purple formazan that takes place only when mitochondrial reductase is active. Colour formation is therefore directly related to the number of viable cells [55]. H1299 cells were grown on 24-well plates in the presence of doxycycline (5 μg/ml) to suppress p53 expression. To induce p53, doxycycline was removed and confluent cell monolayers were incubated overnight in DMEM. Then cells were infected either with influenza viruses (MOI about 5) and incubated for 24 and 48 h.p.i. At these times, cells in separate wells were incubated with MTT (final conc. 0.5 μg/ml) prepared in DMEM, for 2 h at 37°C to allow the intracellular oxidation of MTT, washed with DMEM, and then dissolved overnight in 25 mM solution of HCl containing 5% of SDS (0.3 ml per well). Oxidized formazan was measured spectrophotometrically at 570 nm
Results
Up-regulation of Akt in influenza virus-infected cells
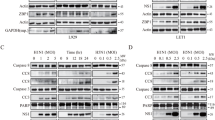
Akt and p53 signaling was monitored in influenza-infected MDCK and CV-1 cells. These cell lines are sensitive to influenza virus infection and maintain high virus replication to titers of 106–108 p.f.u. per ml of medium. Cells were infected with A/PR/8/34 (H1N1), A/Aichi/68 (H3N2) and A/tern/South Africa/61 (H5N3) viruses, and intracellular levels of Akt, its Ser473 phosphorylated form (Akt-pho), p53 and its Ser15 phosphorylated form (p53-pho), as well as p21/waf were monitored by WB technique at different times after infection. The results are shown in Fig. 1. The phosphorylated form of activated Akt was clearly detected in infected CV1 cells, and there was a transient up-regulation of Akt-pho at the early-middle phase of infection (5–9 h.p.i) (Fig. 1A). Akt-pho up-regulation was clearly seen in CV-1 cells infected with all virus strains studied. Some variations were observed in amounts and transient duration of Akt-pho up-regulation in infected cells. However, these virus-dependent variations seemed to be not so dramatic and were probably connected with well-known differences in levels of virus protein synthesis and rates of virus strain replication. It is important to mention that the up-regulation of intracellular Akt-pho developed without an increase of the total amount of Akt during infection (Fig. 1B). Also, the dynamics of Akt-pho up-regulation in infected MDCK cells were similar to those in CV-1 cells also showing transient stimulation of Akt-pho in the early-middle phase (5–9 h.p.i.) (Fig. 1B). Notably, Akt and Akt-pho were controlled in parallel and in all experiments only elevation of Akt-pho and a constant constitutive level of Akt were observed indicating that Akt activation occurred at the posttranslational level. It is worth to mention that the time course of Akt stimulation in infected cells correlated with the kinetics of virus growth (Fig. 1B and C). This suggests that Akt protects cells from apoptotic death during the early phases of productive virus replication.
Kinetics of virus production and patterns of Akt, Akt-pho, p53, p53-pho, p21/waf and caspase 3 in CV-1, MDCK , and VERO cells infected with influenza virus. CV-1 (A, D), MDCK (B, C), and interferon-deficient VERO (E) cells were inflected with A/WSN/33 (A), A/tern/South Africa/61 (A), Aichi/68 (A–E) viruses at MOI 5 and incubated for different times after infection. At these intervals the same amounts of cells were analyzed with PAGE-WB using antibodies specific for Akt, Akt-pho, p53, p53-pho, p21/waf, caspase 3 and its activated form 3a. Positive bands were visualized with secondary anti-species antibodies conjugated with HRP by ECL procedure. Ponseau S-stained Actin protein in WB membrane (panel 1B) was outlined as a quantity reference. Virus titers in culture fluids were measured by hemagglutination test at different times after infection (C)
Up-regulation of p53 in influenza virus-infected cells
Next, we have studied the dynamics of p53 phosphorylation in influenza virus-infected CV1 cells. To evaluate the level of p53, WB analysis using antibody specific for this protein was applied. To estimate the activation of p53, p53 phosphorylated on serine 15 (p53-pho) and p21/waf, a natural reporter of p53 intracellular transcriptional activity, were tested by WB using Ser15pho- and p21-specific antibodies (Fig. 1D). The background level of p53 was not markedly increased during the course of infection, whereas p53-pho was drastically up-regulated at the late stage of infection around 17–20 h.p.i. At this time, the cells had gone into apoptosis as indicated by activation of caspase 3 and appearance of its cleaved 11 kD subunit (caspase 3a; Fig. 1D bottom segment) and DNA degradation (Fig. 5E). p21/waf was also significantly up-regulated at the late stage in parallel with p53 phosphorylation supporting the concept that p53 was activated. It was also noticed that p53 displayed a double band, and only the upper component representing the phosphorylated form was dominant at the late stage and recognized as phosphorylated form (Fig. 1D; p53 and p53-pho segments). The significance of this phenomenon will be discussed below. Taken together, these data show that up-regulation and activation of cellular transactivator p53 occurred only at the late apoptosis stage in influenza virus-infected cells.
Up-regulation of Akt-pho and p53-pho requires virus replication
Up-regulation of Akt-pho and p53-pho raised the question of whether these processes were induced by virus adsorption to target cells alone or whether they required virus replication. To discriminate between these possibilities experiments with inactivated virus were done. Aichi/68 virus grown in chicken eggs was irradiated with a UV dose that fully retained HA activity and totally destroyed infectivity (2 min irradiation by a 15WT UV lamp at 18 cm distance). Incubation of CV-1 and MDCK cells with UV-treated virus (equivalent to MOI 15) for 2 h followed by incubation in DMEM at 37°C either for 7 or 15 h did neither induce synthesis of viral polypeptides nor Akt-pho and p53-pho stimulation in both cases (data not shown). These data clearly showed that up-regulation of Akt-pho and p53-pho required virus replication in infected cells.
Dynamics of up-regulation of Akt in IFN-deficient VERO cells
Recently it was found that interferon of type I (IFN-I) may be involved in stimulation of p53 in cells infected with different viruses, such as Newcastle disease virus, vesicular stomatitis virus, and herpesvirus [56]. We have therefore analyzed whether endocrine IFN-I had an effect on the up-regulation of Akt-pho and p53-pho in influenza-infected cells. For this purpose IFN-deficient VERO cells [57], were examined for the above markers at different intervals after infection with influenza A/Aichi/68 virus. The results obtained are shown in Fig. 1E. Transient Akt phosphorylation and late activation of p53 coupled with stimulation of p21/waf were similar to those seen in IFN-competent CV-1 cells. Notably, kinetic profile of p53-pho and coupled p21/waf were characterized by earlier up-regulation than in CV-1 and MDCK cells, p53-pho and p21/waf were seen in VERO cells already at 6–10 h.p.i. as compared to 15–20 h.p.i. in CV 1 cells (Fig. 1D and E). The earlier p53 activation provoked by virus in the absence of mature IFN in this cell line could be connected with the easier amplification of virus components in IFN-deficient host. These data suggest that early transient recruitment of Akt and following p53 signaling in cells infected with influenza virus does not significantly depend on IFN-I. Specifically, as in the case of CV-1 cells, p53 formed a double band of which the lower band appeared earlier (6–10 h.p.i) than the upper one (20 h.p.i.) (Fig. 1E). These differences in the phosphorylation patterns of p53 in VERO and CV1 cells might reflect multisite phosphorylation of p53 [58, 59] with host-specific variations in IFN-competent (CV1) and IFN-deficient (VERO) cells after influenza virus infection.
Up-regulation of p53 in virus-infected cells is not transcriptional
It is well known that p53 and mdm2 are antagonistically interlinked and regulated reciprocally both at the transcriptional and posttranslational levels through feedback loop [60]. Thus, the next question was whether p53 was up-regulated at the transcriptional level and whether this up-regulation was linked to a positive transcriptional regulation of mdm2. To determine p53 and mdm2 mRNAs at different stages after virus infection, a semiquantitative method using gene-specific primers and double-cycle regimen to exclude comparison of mRNA levels at saturated PCR conditions was applied. The results obtained in both PCR regimens are shown in Fig. 2. It could be seen that mRNAs of both p53 and mdm2 stayed virtually at the same constitutive level during infection. These data suggest that (i) p53 was not transcriptionally stimulated, and (ii) the observed up-regulation of p53 late in infection did not result from transactivation of mdm2, but was due to post-translational modification(s).
Transcriptional levels of p53 and mdm2 in influenza virus-infected cells. MDCK cells were infected with A/Aichi/2/68 virus at MOI 5 and incubated for 10 and 20 h.p.i. Intracellular RNA was isolated and used as template in RT-PCR, followed by semi-quantitative PCR in two regimens with 22 (A) and 30 (B) cycles using in both reactions two different sets of Mdm2- and p53-specific primers, as described in methods. The levels of Mdm2- and p53-specific fragments synthesized on the corresponding intracellular mRNAs are shown in the top panel, the bottom panel outlines the reference amounts of intracellular ribosome RNAs taken into the RT-PCR reactions
Apoptosis development and dynamics of Akt and p53 in cells infected with delNS1 virus
Previously we have shown that protein NS1 down-regulates apoptosis in cells infected with influenza virus [9]. This implied that NS1 may interact with anti-apoptotic Akt and/or pro-apoptotic p53 signaling in infected cells. To test this hypothesis we have studied recombinant A/PR/8/34 virus lacking the NS1 gene (delNS1 virus). DelNS1 virus was compared with its wild type (WT virus). In agreement with our earlier data [9] we observed first that delNS1 virus induced apoptosis more rapidly than WT virus. This was clearly indicated by the earlier development of apoptotic changes such as cell rounding and blebbing, host caspase activation, DNA laddering, anexin V exposition, and caspase-dependent cleavage of virus protein NP in delNS1 virus-infected cells [6, 9]. We have then estimated the levels of the main viral proteins in infected cells. As expected, NS1 was absent in delNS1 infected cells. The late viral proteins M1 and HA0 were reduced, whereas synthesis of NP was normal when compared to WT-infected cells (Fig. 3A). Cells infected with delNS1 showed earlier and more apoptotic cleavage of the nucleocapsid protein NP (Fig. 3A; lanes 12 h.p.i) and of caspase 3 (Fig. 3B). Finally, the patterns of Akt-pho and p53-pho were found to be significantly different in WT- and delNS1- infected cells (Fig. 3B). (i) Phosphorylation of Akt was very distinct and clear transient up-regulation of Akt-pho was achieved in CV-1 cells infected with WT virus, whereas only background levels of Akt-pho were seen in delNS1-infected cells (Fig. 3B, C). These data suggest that NS1 plays a role in stimulation of anti-apoptotic Akt signaling in infected cells that leads to a delay of apoptosis in comparison to the delNS1-infected cells characterized by the lack of Akt activation and accelerated apoptosis. (ii) Only minor levels of p53/Ser15 and p53/Ser46 phosphorylation (Fig. 3B, C) were observed in delNS1 virus-infected cells, contrary to WT virus infected cells where both phosphorylation processes were found to be clearly up-regulated. Additionally, the level of p21/waf, a natural reporter of p53 activation, was tested in infected cells. This protein was found to be markedly up-regulated late after infection with WT virus, whereas this was not the case in delNS1-infected cells (Fig. 3C). Finally, to further estimate the levels of p53 activation, it’s nuclear accumulation was studied by immune fluorescence at 15 h.p.i., when apoptosis was clearly achieved in delNS1-infected cells and already started to develop WT-infected cells. In both cases the major part of cells was still attached to coverslips (Fig. 3D; upper slides). Most of nuclei (∼70%) of WT-infected cells showed intensive accumulation of p53. In contrast, in delNS1-infected cells as in mock-infected a minor fraction of nuclei (∼25%) showed distinct p53-specific staining indicating only background activation of p53 (Fig. 3D; bottom slides). The number of p53-positive nuclei at 7 h.p.i. both in WT- and delNS1-infected cells were similar to background in mock-infected sample (not shown). The negligible level of p53 activation and the development of rapid apoptosis in delNS1 infected cells indicate that there is an alternative pathway of p53-independent apoptosis in this virus-host system.
Viral and host protein profiles in cells infected with wt and delNS1 influenza viruses. Two sets of CV-1 cells were infected with WT and delNS1 variants of A/PR/8/34 virus with MOI ∼1 for both viruses. At different intervals after infection cellular polypeptides of these cell sets were analyzed by PAGE-WB using antibodies either specific for viral proteins HA, NP, M1, NS1 (A), or host cell proteins Akt-pho, p53-pho(S15), p53-pho(S46), p21/waf, caspase 3/3a (B, C). Positive bands were visualized with secondary anti-species antibodies conjugated with HRP by ECL procedure. Actin in Ponceau-stained western-blot membrane is shown as a quantity reference. In parallel (D), CV-1 cells seeded on glass coversplips and infected with WT and delNS1 viruses (MOI 1) were fixed at 15 h.p.i. and examined either by light microscopy (×250; upper slides) or by immune fluorescent analysis in an AxioVert 200M microscope using mouse anti-p53 monoclonal antibody and secondary TRITC-conjugated anti-mouse antibody Fab2 (×400; bottom slides). The percentage of bright nuclei (p53-positive nuclei) was calculated in 10 random microscopy fields (×200) in relation to the overall amounts of nuclei in the field (100%) and shown at the bottom
Up-regulation of Akt in cells transfected with a DNA vector expressing NS1
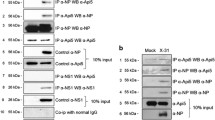
The above data showing the absence of Akt-pho up-regulation in delNS1-infected cells suggested that influenza NS1 protein could be responsible for this Akt stimulated signaling in infected cells. This concept was further tested in NS1 transfection experiments. CV1 and 293T cells were transfected either with empty pCAGGS or pCAGGS/NS1 plasmid DNAs, and intracellular levels of Akt-pho were evaluated by WB technique. Transfection efficiency measured by immunostaining as the percentage of NS1 positive cells in the monolayer at 25 h post transfection was found to vary at the level 15–25% in different experiments. Accumulation of Akt-pho appeared to be significantly higher in cells transfected with the pCAGGS/NS1 vector than with the empty one (Fig. 4). Interestingly, pCAGGS constructs expressing viral HA and NP proteins, unlike plasmids expressing NS1, did not induce Akt phosphorylation in CV1 transfected cells (not shown). Negligible stimulation of Akt-pho in pCAGGS-transfected cells was most probably provoked by lipophylic transfecting reagent used in these experiments. The overall amounts of Akt identified by anti-Akt antibody were equal in pCAGGS- and pCAGGS/NS1-transfected cells (Akt lanes in Fig. 4), but there was posttranslational up-regulation of Akt-pho when NS1 protein was expressed. Importantly, there was no apoptosis in cells transfected both with pCAGGS alone and pCAGGS/NS1 (not shown). A similar up-regulation of Akt-pho was observed in Cos7 and 293T cells expressing NS1 of A/Aichi/2/68 and A/PR/8/34 viruses (not shown). Interestingly, the Akt-stimulating activity of NS1, calculated as a Akt-pho/NS1 ratio (Fig. 4, bottom), was about 10 times higher in pCAGGS/NS1-transfected cells than in virus-infected cells. Thus, there are redundant levels of synthesis of NS1 and its Akt-stimulating potential in infected cells to induce Akt phosphorylation, and low amounts of the protein seem to be sufficient to saturate intracellular Akt-signaling. However this redundancy may be illusive, and big amounts of NS1 are necessary to maintain its multiple functions in infected cells. All of these data indicate that the NS1 protein has the ability to induce activation of Akt both individually and in the context of virus replication.
Influenza protein NS1 induces up-regulation of Akt-pho in mammalian cells. CV1 cells were transfected with empty pCAGGS and pCAGGS/NS1 vectors using Superfect transfection reagent. Transfected cells were incubated for 27 h and host Akt-pho and viral NS1 protein were analyzed by WB-PAGE using Akt-pho(Ser473)- and NS1-specific antibodies and ECL procedure. As a reference, CV1 cells infected with Aichi/68 virus at MOI 2 (8 h.p.i.) were analyzed in the same manner. The equal protein amounts of different samples loaded in the gel were equilibrated to evaluate Akt-pho and NS1 in parallel in one gel. Akt-pho and NS1 have been quantitated as described in materials and methods, and the ratios are shown. Ponseau S-stained host actin has been used as standard
p53-independent death of human lung cells H1299 infected with influenza virus
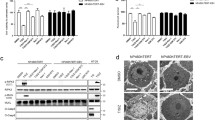
Next, we addressed the question whether p53 activation plays a role in the initiation of influenza-provoked cell death or whether it is a secondary signaling in already dying cells. For this purpose, a p53 null human lung carcinoma cell line H1299 carrying an exogenous wild type p53 gene expressed under the control of a tetracycline regulated promoter [50] was employed. This cell line was found to be permissive for influenza viruses and maintain virus replication to titers of about 105 p.f.u. per ml of medium. These cells incubated with and without doxycycline were infected with different influenza viruses, and cell death was monitored by (i) cell viability with an MTT test, (ii) plasma membrane integrity with Propidium iodide test (PI) , (iii) DNA ladder formation, and (iv) WB analysis of the activated form (3a) of caspase 3 and the apoptotic form (aNP) of NP reflecting the intracellular level of caspase activity (Fig. 5).
Cell death development and dynamics of p53 up-regulation in H1299 cells infected with influenza virus. H1299 cells grown in the presence of doxycycline to suppress p53 expression were incubated overnight in medium lacking doxycycline to initiate p53 synthesis. p53(+) and p53(−) cells were infected with PR/8/34 (wt), PR/8/34(delNS1) and Aichi/68 viruses (MOI 5) and incubated for different intervals in medium with and without doxycycline, respectively. At these intervals, p53 (A), caspase 3/3a (F), NP/aNP (G) were identified by PAGE-WB using specific antibodies and ECL technique. The levels of cell death in monolayers infected with different viruses were evaluated by MTT test (B), examination of cell morphology (C) and PI inclusion test (D). The amount of viable cells in the infected cultures as determined by the MTT test was calculated in comparison to mock-infected cell samples (100%) manipulated in parallel. (E) DNA ladder analysis of H1299 cells was performed at 35 h.p.i. in 1% agarose gel stained with EB, and CV1 cells infected with Aichi virus (23 h.p.i.) were used as a control
First, it can be seen that withdrawal of doxycycline from culture medium initiated p53 expression and accumulation in H1299 cells, whereas in the presence of doxycycline only a trace leakage level of p53 was detected (Fig. 5A). Second, the viability data clearly showed that the dynamics of death development of H1299 cells infected with different influenza viruses was independent of p53 expression. The results of a typical experiment are shown in Fig. 5B. Third, morphological examination and PI inclusion tests revealed plasma membrane damage (Fig. 5C, D), and there was no DNA ladder formation when chromatin degradation was analyzed (Fig. 5E). These parameters developed similarly in doxycycline-treated (p53−) and non-treated (p53+) H1299 cell cultures. These data implied that influenza-infected H1299 cells recruited necrotic mechanisms and their death developed in a p53-independent fashion. Fourth, caspase-dependent cleavages of caspase 3 (Fig. 5F) and NP (Fig. 5G) were similar in influenza-infected H1299 cells treated and non-treated with doxycycline. In these cells infected with delNS1 virus caspase-dependent cleavages of NP and caspase 3 were significantly higher than in cells infected with WT virus, but also these cleavages were principally on the same levels in p53- and p53+ cell samples (Fig. 5G). The observed caspase cleavage suggested that death in H1299 infected cells was not only necrotic but had also apoptotic characteristics which were more prominent in delNS1-infected cells. These results are also consistent with the concept that p53 does not play a leading role in death of influenza-infected cells and in caspase activation, one of the major mechanisms of cell killing.
Interplay of apoptosis and PI3k-Akt signaling in influenza virus-infected cells
Akt is known to be activated by PI3k, and PI3k-Akt signaling initiates an antiapoptotic program in cells [61]. Stimulation of this program after influenza virus infection may increase cell resistance to virus stress and prevents early apoptosis. On the other hand, suppression of the PI3k-Akt pathway might promote earlier apoptosis and probably interfere with virus replication. These hypotheses were tested in an experiment where PI3k-Akt signaling was suppressed by Ly294002, a cell permeable inhibitor [62]. Cell cultures were infected with influenza virus and incubated with and without this inhibitor, and apoptosis development and virus reproduction were examined (Fig. 6). First, we have found that phosphoactivation of Akt was effectively suppressed by Ly-294002 in influenza-infected cells (Fig. 6A). Infected cells incubated with Ly294002 failed to display Akt-pho indicating the role of PI3K signaling in Akt activation in influenza-infected cells. Second, apoptosis in infected CV-1 and MDCK cells treated with Ly294002 developed markedly earlier than in non-treated control cells. Apoptosis markers, such as cell rounding and damage (Fig. 6B), reactivity with cyto-death antibody (clone M30) (Fig. 6C), caspase-dependent cleavage of viral nucleocapsid protein NP (Fig. 6D), caspase 3 activating cleavage, and chromatin DNA laddering (not shown), developed earlier in Ly-treated cells than in non-treated control cells. Apoptosis markers appeared in Ly-treated CV-1 cells as early as 10–12 h.p.i., whereas in non-treated cells these markers were detectable only at 14–16 h.p.i. Ly294002 did not induce apoptotic signs by itself in mock-infected cells indicating the provoking role of virus replication in apoptosis enhancement when the host Akt pathway was inhibited. A similar increase of apoptosis was observed in influenza-infected MDCK cells treated with Ly204002 (Fig. 6B; top segment). These data support the concept that up-regulation of PI3k-Akt signaling counteracts early apoptosis in influenza-infected cells.
Development of apoptosis in influenza-infected cells treated with Ly294002. CV-1 and MDCK cells were infected with Aichi/68(Ai) virus (MOI 2) and incubated in DMEM alone or for 13 h (MDCK cells) and 19 (CV-1 cells) h in DMEM containing 10 μM of freshly prepared Ly294002. To maintain the Ly294002 concentration the culture media were changed in 6 h intervals. Growing cells were photographed to visualize cell morphology (A) and then fixed with 2% paraformaldehyde and stained with cytodeath kit (Roche) using apoptosis-specific M-30 antibody [70] and HRP-conjugated secondary anti-species antibodies, followed by staining of apoptotic cells with water-insoluble substrate “True Blue” (KPL) (B). M30-stained CV-1 cultures were photographed, and the average percentage of M30-positive apoptotic cells in 10 microscopic fields was determined (outlined at the bottom). Canine (MDCK) apoptotic cells were found to be non-susceptible to M30 antibody. Apoptotic NP, Akt and Akt-pho (D) in MDCK-infected cells were analyzed at 8 and 13 h.p.i., respectively, by PAGE-WB using antibody specific for NP, Akt, and Akt-pho. The aNP/NP ratio was calculated from scanned WB membrane images using the TINA program
Discussion
Influenza virus is a cytolytic virus that kills cells either through apoptosis [1–3] or necrosis [4–6]. Accordingly, this virus was found to recruit in infected cells a number of host proapoptotic signaling cascades, such as Fas/FasL, FADD/caspase 8, PKR/INF, TLR/IRF3/NF-kB, Mapk/Erk, Jun/AP-1, and p53 pathways (for review see [63]). As pointed out in the introduction initiation of these pathways leads to cell death late in infection when significant amounts of progeny virus have already been produced. Apoptosis and necrosis are determined by a complex interplay of these pathways and the virus and host factors involved. The data presented here show that PI3k-Akt signaling is activated in the early and middle phases of infection. Activation of the PI3k-Akt pathway through CCL5-CCR5 chemokine signaling was recently found in macrophages infected with Sendai or influenza viruses and believed to have an anti-apoptotic role in these cells [45]. The CCL5-CCR5 chemokine tandem was reported to be enhanced in influenza-infected cells at the late stage of infection [44]. This implies that infected cells secreting CCL5 might potentiate Akt signaling in neighboring epithelial cells and macrophages in an infection locus in a paracrine fashion. The Akt signaling pathway is known to be one of the prominent anti-apoptotic programs in mammalian cells that leads to activation or inactivation of numerous anti- and pro-apoptotic stimuli, respectively, including inactivation of pro-apoptotic caspase 9, Bad, Forkhead, p21/waf, and IkB, and activation of anti-apoptotic NF-kB, Mdm2, and XIAP (summarized in Fig. 7). Our observation that Ly294002, a specific inhibitor of PI3k, inhibited Akt phosphorylation and accelerated apoptosis in influenza-infected cells is in a full agreement with the concept that Akt has an anti-apoptotic function. Activation of Akt at the beginning phase of the infection process seems to permit influenza virus replication by preventing apoptosis at this stage. However, we cannot exclude that phosphorylated Akt is also involved in virus assembly. The temporal coincidence of Akt stimulation with the logarithmic phase of virus production in infected cells is consistent with this concept.
Anti- and pro-apoptotic stages during influenza virus infection. Serine/threonine kinase Akt is activated by phosphatidylinositol-3-kinase (PI3k)-dependent signaling that can be artificially blocked by Ly294002. In mock-infected cells, PI3k initiated through a receptor tyrosine kinase (RTK) generates phosphatidylinositol-triphospate (pip3), whereas phosphotase PTEN reverses this reaction. Pip3 mediates the translocation of Akt to plasma membrane where it is activated by phosphoinositide-dependent kinase (PDK) 1 and 2 at Thr309 and Ser473 [61]. In infected cells, influenza virus induces synthesis of early proteins: NS1, NP, and polymerase proteins PB1, PB2, PA [66, 67], so called the early phase of infection (0–5 h.p.i.). At this period the Akt kinase signaling is initiated through the action of host PI3k and the viral protein NS1. This direct Akt-stimulating action of NS1 can be dominant at the early stage of infection and then other viral proteins amplified by NS1 may be additionally involved in the Akt pathway (shown by arrow). Akt signaling can promote a numerous anti-apoptotic pathways, such as activation of anti-apoptotic Mdm2, XIAP, and NF-kB, and down-regulate pro-apoptotic Bad, Caspase 9, Forkehad, p21/waf, YAP, etc [61]. During the next phase (5–10 h.p.i.) synthesis of late viral proteins (HA, NA, M1, NEP, M2 and PB1-f2) is amplified and the logarithmic phase of virus growth develops. Virus production ceases at 12–13 h.p.i. Pro-apoptotic signaling then begins to overbalance anti-apoptotic signaling, and at the late stage (15–20 h.p.i.) apoptosis develops either through p53-dependent or alternative p53-independent pathways. Time-periods in Figure are shown for highly sensitive for influenza viruses MDCK cells. Symbols (↑) and (⊤) show up- and down-regulatory effects, respectively
In contrast to the antiapoptotic pattern, the proapoptotic pattern was observed late in infection. At this stage many apoptotic markers appeared, such as rounding and blebbing of cells, caspase activation, chromatin DNA laddering, as well as caspase-specific cleavage of viral and host proteins. Only at this apoptotic stage the activating phosphorylation of p53 and the coupled up-regulation of its natural transactivated target p21/waf were seen. This observation indicated that p53, a well-known apoptotic mediator [64], was predominantly activated in influenza virus-infected cells late in infection. Our findings are consistent in part with previous observations showing up-regulation and activation of p53 in influenza virus-infected human epithelial cells (line A549), mouse embryo fibroblasts [43], and in mouse lungs [42]. These earlier data were interpreted to show that p53 plays an essential role in death of influenza-infected cells [43]. In the light of our data this interpretation of the p53 role in influenza infection process cannot be considered as universal and obligatory for all virus-host systems. First, our data are compatible with the observed nuclear accumulation of p53 only in a part of infected cells in mouse lungs [42], since the absence of p53 in some nuclei might reflect a pre-apoptotic stage and the presence of p53 in other nuclei may indicate an apoptotic stage. Second, in CV-1 and MDCK cells infected with delNS1 virus accelerated apoptosis developed without remarkable activation of p53. Moreover, our experiments in human H1299 cells carrying a regulated p53 gene have shown that infection mediated death characterized by a mixed necrosis-apoptosis mechanism also developed independently of whether p53 gene expression was switched on or off. The p53 activation observed at the late infection stage in our experiments unlikely played a leading role in cell death. It rather seemed to be a secondary product of the apoptotic process triggered by the alternative p53-independent pathway. Thus, the role of p53 activation in apoptosis induced by influenza virus may be distinct in different host-virus systems. Notably, accelerated p53-independent apoptosis induced in cells by delNS1 virus may be considered with respect to specific killing of tumor cells [65]. Many of these cells are deficient of p53, and their death requires p53-independent apoptosis. In this context delNS1 virus seems to be a useful tool as an oncolytic agent.
It was reported earlier that activation of p53 enhanced apoptosis in cells infected with vesicular stomatitis virus and some other including influenza viruses in an IFN-alpha/beta-depending fashion [56]. We therefore compared the profiles of Akt-pho, p53-pho, and p21/waf in IFN-α/β-deficient VERO and IFN-α/β-competent CV-1 cells after infection with influenza virus. We did not observe substantial differences in Akt-pho and p53-pho patterns between these host systems, and only phosphorylation of p53 was found to develop slightly earlier in IFN-deficient VERO cells than in IFN-competent CV1 cells. This suggests that endocrine IFN-α/β does not play a significant pro-apoptotic role in Akt and p53 signaling pathways in influenza-infected cells. The different roles of IFN in virus-induced apoptosis observed here and by Takaoka et al [56] suggest that there is no universal host pathway recruited by various viruses in different host systems. It rather appears that various viruses may recruit sufficiently distinct and alternative patterns of host signaling pathways in infected cells. Finally, it is important to mention that in our experimental design specifically the role of endocrine IFN was studied, whereas in the experiments of Takaoka et al. the mixed action of endo- and exocrine IFN was analyzed.
Initiation of PI3k-Akt signaling was found to depend on the viral NS1 protein and was not observed in cells infected with delNS1 virus. This observation is in a good agreement with our previous findings that NS1 down-regulates apoptosis in infected cells [9]. Taken together, these observations indicate that NS1 down-regulates apoptosis in infected cells acting through activation of the Akt antiapoptotic program. It seems logical that NS1, synthesized as one of the early viral proteins [66,67], initiates the Akt-dependent anti-apoptotic program at the early-middle phase of virus infection to protect cells from fast death and promote virus replication at this period. The Akt-stimulating function of NS1 was not coupled with its IFN-antagonistic pathway, since it was also observed in IFN-deficient VERO cells. The mechanism of how NS1 activates Akt-signaling in infected cells is not sufficiently clear. Only recent data, published when our manuscript was in preparation, shed light on this process [68, 69]. (i) It was shown that NS1 activates PI3k [68] and acts through the binding to p85-beta, a regulatory subunit of PI3k, causing the phosphorylation of Akt. (ii) Furthermore, there is evidence that the highly conserved tyrosine at residue 89 of the NS1 plays a role in the interaction with p85beta and the activation of PI3K [69]. Thus, our data that acceleration of apoptosis and activation of Akt require PI3K signaling and NS1 expression are in a full agreement with these results. On the other hand, it is not excluded that in infected cells the role of NS1 is more complicated. Thus, it may stimulate Akt indirectly by amplifying the synthesis of late viral genes, such as M1 and HA, which in turn may cooperate with the Akt-stimulating function of the NS1 protein. This notion is supported by the at least partial temporal overlap of Akt activation and the up-regulation of these late viral proteins (Fig. 7).
Conclusion
Taken together our data support the concept that the apoptotic process has two phases in influenza virus infection both of which play important, yet different roles in the interplay between the virus and the host. By activating the antiapoptotic Akt program the virus eliminates early in infection an important defense mechanism of the host and, thus, provides conditions for efficient replication. Once this has been accomplished, apoptosis is up-regulated at the final stage of the viral life cycle in both a p53-dependent and an alternative p53-independent manner, thereby promoting clearance of infected cells and initiating immune response mechanisms.
References
Fesq H, Bacher M, Nain M, Gemsa D (1994) Programmed cell death (apoptosis) in human monocytes infected by influenza A virus. Immunobiology 190:175–182
Hinshaw VG, Olsen CW, Dybdahi-Sissoko N, Evans D (1994) Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol 68:3667–3673
Takizawa T, Matsukawa S, Higuchi Y, Nakamura S, Nakanishi Y, Fukuda R (1993) Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol 74:2347–2355
Arndt U, Wennemuth G, Barth P, Nain M, Al-Abed Y, Meinhardt A, Gemsa D, Bacher M (2002) Release of macrophage migration inhibitory factor and CXCL8/interleukin-8 from lung epithelial cells rendered necrotic by influenza A virus infection. J Virol 76:9298–9306
Seo SH, Webby R, Webster RG (2004) No apoptotic deaths and different levels of inductions of inflammatory cytokines in alveolar macrophages infected with influenza viruses. Virology 329:270–279
Zhirnov OP, Klenk HD (2003) Human influenza viruses are proteolytically activated and do not induce apoptosis in CACO-2 cells. Virology 313:198–212
Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN (2000) Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J Virol 74:1513–1523
Takizawa T, Tatematsu C, Ohashi K, Nakanishi Y (1999) Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol Immunol 43:245–252
Zhirnov OP, Konakova TE, Wolff T, Klenk HD (2002) NS1 protein of influenza A virus down-regulates apoptosis. J Virol 76:1617–1625
Stray SJ, Air GM (2001) Apoptosis by influenza viruses correlates with efficiency of viral mRNA synthesis. Virus Res 77:3–17
Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzohn T, Pleschka S, Ludwig S (2003) Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J 22:2717–2718
Wurzer WJ, Ehrhardt C, Pleschka S, Berberich-Siebelt F, Wolff T, Walczak H, Planz O, Ludwig S (2004) NF-kB dependent induction of TRAIL and Fas/FasL is crucial for efficient influenza virus propagation. J Biol Chem 279:30931–30937
Barber GN (2001) Host defense, viruses and apoptosis. Cell Death Dif 8:113–126
Kurokawa M, Koyama AH, Yasuoka S, Adachi A (1999) Influenza virus overcomes apoptosis by rapid multiplication. Int J Mol Med 3:527–530
Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN (1998) Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signalling. EMBO J 17:6888–6902
Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S, Busse WW (2003) Double-stranded RNAinduces the synthesis of specific chemokines by bronchial epithelial cells. Amer J Resp Cell Mol Biol 28:731–737
Takizawa T, Ohashi K, Nakanishi Y (1996) Possible involvement of dsRNA-activated protein kinase in cell death by influenza virus infection. J Virol 70:8128–8132
Uetani K, Der SD, Zamanian-Daryoush M, de La Motte C, Lieberman BY, Williams BR, Erzurum SC (2000) Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J Immunol 165:988–996
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reise Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441(7089):101–105
Guo Z, Chen LM, Zeng H, Gomez JA, Plowden J, Fujita T, Katz JM, Donis RO, Sambhara S (2006) NS1 Protein of Influenza A Virus Inhibits the Function of Intracytoplasmic Pathogen Sensor, RIG-I. Am J Respir Cell Mol Biol Oct 19; [Epub ahead of print]
Hiscott J, Kwon H, Génin P (2001) Hostile takeovers: viral appropriation of the NF-κB pathway. Jclin Invest 107:143–151
Morris SJ, Price GE, Barnett JM, Hiscox SA, Smith H, Sweet C (1999) Role of neuraminidase in influenza virus-induced apoptosis. J Gen Virol 80:137–146
Schultz-Cherry S, Hinshaw VS (1996) Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol 70:8624–8629
Chanturiya AN, Basanez G, Schubert U, Henklein P, Yewdell JW, Zimmerberg J (2004) PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes. J Virol 78:6304–6312
Chen WS, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, OTNeill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW (2001) A novel influenza A virus mitochondrial protein that induces cell death. Nat Med 7:1306–1312
Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW (2003) The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J Virol 77:7214–7224
Timofeeva TA, Klenk HD, Zhirnov OP (2001) Identification of the protease-binding domain in the N-terminal region of influenza virus A matrix protein M1. Mol Biol (Russian) 35:411–416
Zhirnov OP, Ksenofontov AL, Kuzmina SG, Klenk HD (2002). Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry (Russian) 67:534–539
Morris SJ, Smith H, Sweet C (2002) Exploitation of the Herpes simplex virus translocating protein VP22 to carry influenza virus proteins into cells for studies of apoptosis: direct confirmation that neuraminidase induces apoptosis and indications that other proteins may have a role. Arch Virol 147:961–979
Ludwig S, Wang X, Ehrhardt C, Zheng H, Donelan N, Planz O, Pleschka S, Garcia-Sastre A, Heins G, Wolff T (2002) The influenza A virus NS1 protein inhibits activation of the jun N-terminal kinase and AP-1 transcription factors. J Virol 76:11166–11171
Fujimoto I, Takizawa T, Ohba Y, Nakanishi Y (1998) Co-expression of Fas and Fas-ligand on the surface of influenza virus-infected cells. Cell Death Differ 5:426–431
Wada N, Matsumura M, Oba Y, Kobayashi N, Takizawa T, Nakanishi Y (1995) Transcription stimulation of the Fas-encoding gene by nuclear factor for interleukin-6 expression upon influenza virus infection. J Biol Chem 270:18007–18012
Olsen CW, Kehren JC, Dybdahl-Sissoko NR, Hinshaw VS (1996) bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J Virol 70(1):663–666
Oh S, McCaffery JM, Eichelberger MC (2000) Dose-dependent changes in influenza virus-infected dendritic cells result in increased allogeneic T-cell proliferation at low, but not high, doses of virus. J Virol 74(12):5460–5469
Lin C, Zimmer SG, Lu Z, Holland RE Jr, Dong Q, Chambers TM (2001) The involvement of a stress-activated pathway in equine influenza virus-mediated apoptosis. Virology 287:202–213
Bernasconi D, Amici C, La Frazia S, Ianaro A, Santoro MG (2005) The IkappaB kinase is a key factor in triggering influenza A virus-induced inflammatory cytokine production in airway epithelial cells. J Biol Chem 280:24127–24134
Maruoka S, Hashimoto S, Gon Y, Nishitoh H, Takeshita I, Asai Y, Mizumura K, Shimizu K, Ichijo H, Horie T 2003. ASK1 regulates influenza virus infection-induced apoptotic cell death. Biochem Biophys Res Commun 307(4):870–876
Takizawa T, Tatematsu C, Nakanishi Y (2002) Double-stranded RNA-activated protein kinase interacts with apoptosis signal-regulating kinase 1. Implications for apoptosis signaling pathways. Eur J Biochem 269(24):6126–6132
Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MBA (2004) Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231–238
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529
Lund JM, Alexpoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA (2004) Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA 101:5598–5603
Technau-Ihling K, Ihling C, Kromeir J, Brandner J (2001) Influenza A virus infection of mice induces nuclear accumulation of the tumor-suppressor protein p53 in the lung. Arch Virol 146:1655–1666
Turpin E, Luke K, Jones J, Tumpey T, Konan K, Schultz-Cherry S (2005) Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J Virol 79:8802–8811
Brydon EWA, Smith H, Sweet C (2003) Influenza A virus-induced apoptosis in bronchiolar epithelial (NCI-H292) cells limits proinflammatory cytokine release. J Gen Virol 84:2389–2400
Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ (2005) CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 11:1180–1187
McKinney LC, Galliger SJ, Lowy RJ (2003) Active and inactive influenza virus induction of tumor necrosis factor-alpha and nitric oxide in J774.1 murine macrophages: modulation by interferon-gamma and failure to induce apoptosis. Virus Res 97:117–126
Pleschka S, Wolff T, Ehrhardt C, Hobom G, Planz O, Rapp UR, Ludwig S (2001) Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol 3(3):301–305
Nimmerjahn F, Dudziak D, Dirmeier U, Hobom G, Riedel A, Schlee M, Staudt LM, Rosenwald A, Behrends U, Bornkamm GW, Mautner J (2004) Active NF-kappaB signalling is a prerequisite for influenza virus infection. J Gen Virol 85:2347–2356
Garcia-Sastre A, Egorov A, Matassov D, Brandt S, Levy ED, Durbin JE, Palese P, Muster T (1998) Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330
Chen X, Ko LJ, Jayaraman L, Prives C (1996) p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev 10:2438
Niwa H, Yamamura K, Miyazaki J (1991) Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108(2):193–199
Zhirnov OP, Konakova TE, Wgarten W, Klenk H-D (1999) Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J Virol 73:10158–10163
Contente A, Dittmer A, Koch MC, Roth J, Dobbelstein M (2002) A polymorphic microsatellite that mediates induction of PIG3 by p53. Nat Genet 30:315–320
McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR (1995) The end of the (cell) line - methods for the study of apoptosis in vitro. Methods Cell Biol 46:153–185
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T (2003) Integration of interferon-alpha/beta signaling to p53 responses in tumour suppression and antiviral defense. Nature 424(6948):516–523
Diaz MO, Zeimin S, Le Beau MM, Pitha P, Smith SD, Chilcote RR, Rowley JD (1988) Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci USA 85:5259–5263
Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis Nat Rev Cancer 4(10):793–805
Yang XJ (2005) Multisite protein modification and intramolecular signaling. Oncogene 24(10):1653–1662
Bond GL, Hu W, Levine AJ (2005) MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets 5:3–8
Downward J (2004) PI-3 kinase, Akt and cell survival. Cell Dev Biol 15: 177–182
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351(Pt 1):95–105
Brydon EW, Morris SJ, Sweet C (2005) Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol Rev 29:837–50
Yee KS, Vousden KH (2005) Complicating the complexity of p53. Carcinogenesis 26:1317–22
Muster T, Rajtarova J, Sachet M, Unger H, Fleischhacker R, Romirer I, Grassauer A, Url A, Garcia-Sastre A, Wolff K, Pehamberger H, Bergmann M (2004) Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int J Cancer 110(1):15–21
Inglis SC, Carroll AR, Lamb RA, Mahy BWJ (1976) Polypeptides specified by the influenza virus genome. 1. Evidence for eight distinct gene products specified by fowl plague virus. Virology 74:489–503
Skehel JJ (1973) Early polypeptide synthesis in influenza virus-infected cells. Virology 56:394–399
Ehrhardt C, Marjuki H, Wolff T, Nurnberg B, Planz O, Pleschka S, Ludwig S (2006) Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol 8(8):1336–48
Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE (2006) Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc Natl Acad Sci USA 103(38):14194–9
Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorklund P, Ramaekers FC, Bjorklund B, Nap M, Jornvall H, Schutte B (1999) mmunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol 187:567–572
Acknowledgements
The authors thank Dr. Peter Chumakov from the Engelhard Institute of Molecular Biology (Moscow) and Prof. Matthias Dobbelstein, Dr. Michael Schuemann, and Dr. Francoise Debierre-Grockiego from the Institute of Virology, Philipps University of Marburg, for useful discussions and help with the experiments. The authors acknowledge Dr. Irina Vorobjeva for assistance with the purification of DNA plasmids. This work was supported by SFB 593 program and Grant 04-48290 of Russian Foundation for Basic research.
Author information
Authors and Affiliations
Corresponding author
Additional information
These data have been partially presented at The 3rd Orthomyxovirus Research Conference (sponsored by ESWI and NIH). Abstr. p. 23 entitled: “Influenza virus-specific up-regulation of Akt and Mdm2 in infected cells” by Zhirnov O.P., and Klenk H.D., July 28–21, 2005. Queen’s College, Cambridge, United Kingdom; and at The Annual Meeting of Virology in Munich, March 15–18 (2006)—“Influenza virus-specific up-regulation of Akt, Mdm2, and p53 in infected cells” by O. P. Zhirnov and H. D. Klenk; Book of abstracts, p. 339
Rights and permissions
About this article
Cite this article
Zhirnov, O.P., Klenk, HD. Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis 12, 1419–1432 (2007). https://doi.org/10.1007/s10495-007-0071-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0071-y