Abstract
Histone deacetylase (HDAC) inhibitors represent a promising group of anticancer agents. This paper shows that the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) stimulated at 5–10 μM apoptosis in human hepatoma HepG2 and Huh6 cells, but was ineffective in primary human hepatocytes (PHH). In HepG2 cells SAHA induced the extrinsic apoptotic pathway, increasing the expression of both FasL and FasL receptor and causing the activation of caspase-8. Moreover, SAHA enhanced the level of Bim proteins, stimulated alternative splicing of the Bcl-X transcript with the expression of the proapoptotic Bcl-Xs isoform, induced degradation of Bid into the apoptotic factor t-Bid and dephosphorylation and inactivation of the anti-apoptotic factor Akt. Consequently, SAHA caused loss of mitochondrial transmembrane potential, release of cytochrome c from mitochondria, activation of caspase-3 and degradation of PARP.
Interestingly, a combination of suboptimal doses of SAHA (1 μM) and bortezomib (5–10 nM), a potent inhibitor of 26S proteasome, synergistically induced apoptosis in both HepG2 and Huh6 cells, but was ineffective in PHH. Combined treatment increased with synergistic effects the expression levels of c-Jun, phospho-c-Jun and FasL and the production of Bcl-Xs. These effects were accompanied by activation of Bid, caspase-8 and 3.
In conclusion, SAHA stimulated apoptosis in hepatoma cells and exerted a synergistic apoptotic effect when combined with bortezomib. In contrast, these treatments were quite ineffective in inducing apoptosis in PHH. Thus, our results suggest the potential application of the SAHA/bortezomib combination in clinical trials for liver cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histone deacetylase inhibitors (HDACIs) constitute a group of compounds favouring histone acetylation and chromatin relaxation [1, 2], thereby allowing the transcription of genes [3] involved in the control of cell proliferation, differentiation and apoptosis. The first HDACIs employed as inducers of apoptosis [1] were of natural origin, such as sodium butyrate, a short chain fatty acid derived from the bacterial fermentation of fibres in the colon, and trichostatin A (TSA), isolated from Streptomyces. Our previous papers showed that butyrate efficaciously induced apoptosis in human retinoblastoma [4] and hepatoma cell lines [5]. However, butyrate displays a low clinical potential due to its instability when administered in vivo [6].
More recently, the hydroxamic acid class of HDACIs has attracted the attention of researchers. Specifically, suberoylanilide hydroxamic acid (SAHA) is currently considered with particular interest because of its marked apoptotic effect on a number of tumor cell types, whereas normal cells appear to be relatively resistant. Moreover, clinical investigations have provided evidence that SAHA exhibited a high therapeutic potential for different forms of tumor at doses that were well tolerated by patients [7, 8]. In addition, SAHA seemed to exert an antitumor effect in a synergistic manner with various compounds such as anticancer drugs [9], topoisomerase inibitors [10, 11], proteasome inhibitors [12–14], TRAIL [15] and perifosine [16].
Proteasome inhibitors are currently considered to be promising anticancer drugs. Preclinical evidence indicated that bortezomib (VELCADE, formerly known as PS-341), the only proteasome inhibitor that has undergone clinical trials, was effective in the treatment of haematological and solid malignancies by stimulating apoptosis [17]. Moreover, bortezomib has recently been approved for the treatment of multiple myeloma [18, 19] and has been shown to sensitize myeloma cells that are refractory to conventional radiotherapy [19]. In a previous study we have shown that bortezomib induced apoptosis in human hepatoma HepG2 cells and that JNK and AP-1 exerted a crucial role in the control of these mechanisms [20].
The present paper shows that SAHA strongly induced apoptosis in human hepatoma cells. In addition, we demonstrated for the first time in cells derived from a liver tumor that combinations of SAHA and bortezomib at suboptimal doses stimulated apoptosis with a synergistic effect in both HepG2 and Huh6 cells. Differently, SAHA alone or in combination with bortezomib was found to be ineffective in inducing apoptosis in primary human hepatocytes (PHH). This paper also analyses the mechanism of the effects observed in hepatoma cells.
Materials and methods
Cell cultures and reagents
Hepatoma HepG2 and Huh6 cell lines were grown as monolayers as reported [5, 20]. Primary human hepatocytes (PHH) were obtained from Cambrex Bio Science (Walkersville, MD) and grown as monolayer on collagen I coated plates according to the manufacture's instructions. Cells, plated either in 96-well or 24-well plates or in 100 mm culture dishes, were allowed to adhere overnight and then treated with chemicals or vehicle only. Cell viability was determined using the tetrazolium reduction assay EZ4U [21] gently provided by Pantec s.r.l, Torino, Italy (Biomedica, Vienna, Austria).
Co-cultures of PHH and HepG2 cells were performed in BD Biocoat collagen I-24 multiwells, supplied with 0.4 μM pore size PET-track-etched membrane inserts (Falcon, Bedford, MA). Cells were plated in the Biocoat-wells (PHH) or in the inserts (HepG2), incubated overnight and then treated with chemicals. After incubation, the inserts containing HepG2 cells were transferred into another plate containing fresh medium and the viability of both cell lines was determined separately by EZ4U assay.
Suberoylanilide hydroxamic acid (SAHA) was a kind gift by Italfarmaco S.p.a. (Milan, Italy). Bortezomib was obtained from Millennium Pharmaceuticals (London, UK), benzyloxy-carbonyl-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) from Promega (Italy), carbobenzoxy-Ile-Glu-Thr-Asp-fluoromethylketone (z-IETD-fmk) from Calbiochem (La Jolla, Ca), Fas blocking antibody (ZB4) from Immunotech (Marseille Cedex, France), and chemicals were supplied from Sigma (St. Louis, Mo). Stock solutions of SAHA or bortezomib were prepared in dimethylsulfoxide (DMSO) and diluted to final concentrations in the culture medium. The final concentration of DMSO never exceeded 0.04%.
Assessment of apoptosis and cell cycle analysis
Apoptotic morphology was studied in cells stained with a combination of the fluorescent DNA-binding dyes acridine orange and ethidium bromide (AO/EB) (100 μg/ml each in phosphate buffer saline) [20]. Viable cells display nuclei with bright green fluorescence and homogeneous chromatin whereas apoptotic cells show condensed and fragmented chromatin with bright orange fluorescence. Necrotic cells display a diffuse orange staining pattern.
Both hepatoma cells and PHH (106 per condition) were subjected to flow cytometric analysis after propidium iodide staining of DNA using a Beckman Coulter Epics XL cytometer as reported [5]. The proportion of cells giving fluorescence in the hypodiploid sub G0/G1 peak of cell cycle was taken as a measure of apoptosis. Data were analysed using Expo32 software.
Measurement of mitochondrial transmembrane potential (Δψm)
Cells were incubated with 40 nM 3,3-dihexyloxacarbo-cyanine (DiOC6, Molecular Probes, Eugene, OR) for 20 min at 37°C, washed twice with PBS, and analysed by flow cytometry on a Beckman Coulter Epics XL flow cytometer with excitation and emission settings at 488 and 525 nm, respectively.
The percentage of cells showing a lower fluorescence reflectes loss of Δψm. Carbonylcyanide m-chlorophenylhydrozone (CCCP; 50 μM) was used as a positive control for maximum Δψm disruption (not shown).
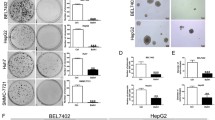
SAHA induced apoptosis in HepG2 and Huh6 cells, but not in primary human hepatocytes (PHH). Dose-dependence (A) and time-dependence (B) of the effect of SAHA on the viability of HepG2 and Huh6 cells in comparison with PHH. Cells were treated for 36 h with various concentrations of SAHA (A) or for various times with 5 μM SAHA (B). Cell viability was measured by EZ4U assay. (C) Flow cytometric analysis of propidium iodide-stained HepG2 cells or PHH treated for 36 h with 5 μM SAHA. x-axis indicates fluorescence intensity on linear scale and y−axis the number of events. (D) Morphological analysis performed with fluorescence microscopy. HepG2 cells or PHH were cultured with 5 μM SAHA for 36 h and then stained with acridine orange/ethidium bromide, as reported in materials and methods. The results were representative of three independent experiments
Western blotting analysis
Cell lysates were prepared as reported [20, 22]. Protein concentration was determined by Lowry assay [23]. Equal amounts of proteins (60 μg/lane) were resolved by SDS-PAGE and electroblotted to nitrocellulose for detection with specific antibodies conjugated with alkaline phosphatase. Immunoblots were detected as reported [24]. Bands were quantified by densitometric analysis using SMX Image software. Antibodies were obtained from Santa Cruz Biotechnology (St. Cruz, Ca), except for phospho-c-Jun and phospho-Akt, which were obtained from Cell Signaling (Beverly, MA). For each experiment, actin blots were included to show equal protein loading for all samples.
RNA extraction and RT-PCR analysis
Total RNA was extracted from cells (5–7×106 cells/sample) by guanidine isothiocyanate method [25] and RNA integrity was confirmed as previously reported [20]. Total RNA (1 μg) was reverse-transcribed using the GeneAmp Kit for RT-PCR (Applied Biosystems, Foster City, Ca) according to the manufacturer's instructions. PCR experiments were performed as described previously [5, 20]. Specific primers were used to amplify cDNA for Bcl-X (forward, 5′-TTGGACAATGGACTGGTTGA-3′; reverse, 5′-GTAGAGTGGATGGTCAGTG-3′) and for FasL (forward, 5′-ATGTTTCAGCTCTTCCACCTACAGA-3′; reverse, 5′-TGCATAGACTCGAGAGAGACCA-3′). As a control for normalizing samples GAPDH gene was amplified using the forward primer: 5′-TGACATCAAGAAGGTGGTGA-3′ and the reverse primer: 5′-TCCACCACCCTGTTGCTGTA-3′. The number of PCR cycles was decreased to obtain a semi-quantitative result.
PCR amplification products were resolved by agarose gel electrophoresis (1% agarose-0.5 μg/ml ethidium bromide), photographed and scanned into Adobe Photoshop. Bands were quantified by densitometric analysis using SXM Image software.
Results
The apoptotic effect induced by SAHA in hepatoma cells and the synergistic interaction of SAHA and bortezomib
EZ4U assay showed that SAHA treatment markedly reduced both HepG2 and Huh6 cell viability in a dose-dependent (Fig. 1(A)) and time-dependent (Fig. 1(B)) manner. A modest reduction was seen in the first 16 h of treatment with 5 μM SAHA, the effect then increased and at 36–48 h cell number decreased to about 25 and 30% of control in HepG2 and Huh6 cells, respectively. In contrast, SAHA treatment did not induce any decrease in cell viability when tested in PHH (Fig. 1(A) and (B)).
The loss in cell viability was caused by apoptosis, as revealed by flow cytometric analysis showing a significant proportion of treated HepG2 cells in the subG0/G1 phase (Fig. 1(C)), corresponding to subdiploid DNA. Moreover, fluorescence microscopy after AO/EB staining (Fig. 1(D)) revealed clear signs of apoptosis in treated HepG2 cells. Similar apoptotic effects were observed in Huh6 cells (not shown). In contrast, 5 μM SAHA did not modify either the flow cytometric profile (Fig. 1(C), Fig. 3(A)) or the morphology after AO/EB staining in PHH (Fig. 1(D)).
The apoptotic effect of combinations of bortezomib/SAHA in HepG2 cells. (A) Cells were treated for 36 h with various doses of SAHA, bortezomib or combinations of bortezomib/SAHA at a fixed ratio (1:200). Percentages of cells in the subdiploid region, evaluated by flow cytometric analysis of propidium iodide stained cells, were assumed as index of apoptosis. (B) Values of combination index (CI) for apoptosis were calculated in relation to the fraction affected. Values of CI less than 1.0 indicate synergistic interactions between the two drugs
To demonstrate that SAHA can synergistically interact with bortezomib, HepG2 cells were treated for various times with combinations of suboptimal doses of the two compounds at a fixed ratio (1:200 bortezomib: SAHA) (Fig. 2(A)). This ratio was chosen on the basis of the effects shown for SAHA in this study and for bortezomib in a previous paper [20]. Analysis of the combination index (CI) [26] with respect to the proportion of apoptotic cells showed synergistic interaction between SAHA and bortezomib with values of CI considerably less than 1.0 (Fig. 2(B)). Co-treatment with 1 μM SAHA and 10 nM bortezomib induced a time dependent effect with the maximum observed at 36 h when the subdiploid population increased to 78% (Fig. 3(B)). Comparable results were obtained when apoptosis was monitored using AO/EB staining (not shown).
In some experiments we explored the possibility of sensitizing HepG2 cells to SAHA or to bortezomib by pre-exposing these cells to suboptimal doses of the other drug. Therefore, HepG2 cells were pretreated with 1 μM SAHA or 5 nM bortezomib alone. The other agent was added after 8–12 h and the incubation protracted for the established times. Evaluation of apoptosis by flow cytometric analysis at the different times did not indicate any significant modification with respect to the condition in which the two agents were added together (not shown). Thus, the observed synergistic effect can be considered the result of the contemporaneous action of the two agents.
Combinations of SAHA (1–2 μM) with bortezomib (5–10 nM) were ineffective at inducing any apoptotic effect in PHH (not shown). In addition, co-culture experiments were performed in order to evaluate the influence of soluble proapoptotic factors produced and released by tumours cells on PHH viability. Our results showed that treatment for 24 and 36 h of co-cultures of PHH and HepG2 cells with 5 μM SAHA or combinations of suboptimal doses of SAHA and bortezomib induced apoptotic effect only in hepatoma cells, but not in PHH (not shown).
As shown in Fig. 3, the apoptotic effect induced by SAHA (panel A) or combinations of SAHA/bortezomib (panel B) was wholly prevented by the general caspase inhibitor z-VAD-fmk and partially reduced by the caspase-8 inhibitor z-IETD-fmk, suggesting that SAHA-induced apoptosis was dependent on caspase activities. Also, the Fas inhibiting antibody ZB4 (4 μg/ml) caused a partial inhibition of the apoptotic effect induced by SAHA. These results were confirmed by fluorescence microscopy after staining the cells with AO/EB (not shown). Interestingly, 10 mM N-acetylcysteine (NAC) did not modify the SAHA effect, suggesting that oxidative stress was not involved in SAHA-induced apoptosis (Fig. 3(A)). On the other hand, NAC was capable of attenuating the effect of the combination SAHA/bortezomib (Fig. 3(B)). This result was in line with our previous evidence that oxidative stress plays a role in bortezomib-induced apoptosis in HepG2 cells [20].
The effects of caspase inhibitors, N-acetylcysteine (NAC) and Fas-inhibiting antibody, ZB4, on apoptosis induced in PHH or HepG2 or Huh6 cells by SAHA alone or in combination with bortezomib. PHH or HepG2 or Huh6 cells were treated with 5 μM SAHA (A) or combinations of 1 μM SAHA with 10 nM bortezomib (B) in the absence or presence of 100 μM z-VAD-fmk, 100 μM z-IETD-fmk, 10 mM NAC or 4 μg/ml ZB4. After 36 h of treatment cell cycle distribution was analysed. Percentages of cells in the subdiploid region were assumed as index of apoptosis. The results are the mean of three separate experiments ± SE
The effect of SAHA on Fas, FasL and caspase-8
Western blotting analysis of HepG2 cells (Fig. 4(A)) showed that 36 h treatment with 5 μM SAHA markedly increased the level of FasL visible at 37 kDa. Another band at 68 kDa was also seen, which could be identified as a dimeric form of FasL. SAHA also increased the level of Fas ligand receptor (Fas) in either monomeric (45 kDa) or aggregate form (150 kDa). In treated cells, another band at 97 kDa was detected, which most likely represented a processed form of the high molecular weight aggregates [27]. Finally, also the presence of FLIP (short) was observed in HepG2 cells, but its level decreased with SAHA treatment.
(A) The effect induced by SAHA alone or in combination with bortezomib on Fas/FasL pathway in HepG2 cells and PHH. Cells were treated for 36 h with various concentrations of SAHA or bortezomib or combination SAHA/bortezomib. (B) Curcumin suppressed the increasing effect exerted by combined treatment SAHA/bortezomib on c-Jun and FasL level. HepG2 cells were treated for 36 h with combination of SAHA and bortezomib in the absence or presence of curcumin. Cell lysates were prepared and analysed in duplicate samples by western blotting using antibodies which recognize FasL, FasR, FLIPs (A) or FasL, c-Jun (B). Actin blot was included to show equal protein loading for all the samples. The intensity of the bands was determined by densitometry in two separate experiments with similar results. (C) Treatment with SAHA alone or in combination with bortezomib induced FasL and Bcl-Xs mRNA expression in HepG2 cells. Cells were treated for 36 h with 1 or 5 μM SAHA or 10 nM bortezomib or combination 1 μM SAHA/10 nM bortezomib. FasL, Bcl-Xl and Bcl-Xs mRNAs were determined by RT-PCR using specific primers as indicated in materials and methods. Glyceraldehide-3-phosphate-deydrogenase (GAPDH) was employed as a loading control to check the quality of cDNA. The experiments was repeated three times with similar results
Modest levels of FasL, Fas and FLIPs were found in PHH (Fig. 4(A)). In these cells treatment with SAHA alone or in combination with bortezomib did not modify FasL and Fas levels, but significantly increased FLIPs levels in a dose-dependent manner.
In HepG2 cells, Fas and FasL markedly increased after co-treatment with 1 μM SAHA/10 nM bortezomib (Fig. 4(A)), while very modest effects were seen after treatments with the single compounds. The effect provoked by co-treatment on FasL level was attenuated by curcumin (Fig. 4(B)) [28], a compound that prevents AP-1 function in bortezomib-induced apoptosis [20]. Finally, treatment with SAHA or combination SAHA/bortezomib markedly increased FasL mRNA expression in HepG2 cells (Fig. 4(C)).
The effect of SAHA on the members of Bcl-2 family
Western blotting analysis (Fig. 5(A)) demonstrated that a modest level of Bcl-2 is present in HepG2 cells and that SAHA treatment further decreased the intensity of this band. SAHA also caused a modest reduction in Bcl-Xl level, while it markedly increased the level of Bcl-Xs, a factor which stimulates apoptosis by antagonizing the pro-survival properties of Bcl-Xl. The appearance of Bcl-Xs occurred at 16 h of treatment and the intensity of the band increased with time until 36 h (not shown). Moreover, semi-quantitative RT-PCR showed that treatment with 5 μM SAHA for 36 h increased the mRNA level of Bcl-Xs (591 bp) while that of Bcl-Xl (780 bp) remained unchanged (Fig. 4(C)).
The effects of SAHA alone or in combination with bortezomib on apoptosis regulatory factors. HepG2 cells were treated for 36 h with various doses of SAHA or bortezomib or combinations of the two drugs. Cell lysates were prepared and analysed in duplicate samples by western blotting using antibodies against (A) members of Bcl-2 family, (B) factors involved in apoptosis or cell survival or (C) caspase and PARP activities. Actin blot was included to show equal protein loading for all the samples. The intensity of the bands was determined by densitometry in two separate experiments with similar results
In addition, SAHA induced significant modifications in the levels of several BH3-only proteins in HepG2 cells (Fig. 5(A)). In particular SAHA increased the level of the three members of Bim family. However the effect was modest in the case of BimEL level (27 kDa) and remarkable for BimS (16 kDa), which is considered the most potent inducer of cell death among Bim isoforms [29]. Finally SAHA induced the appearance of another unknown form of 34 kDa.
Moreover, SAHA decreased the level of the native Bid protein (23 kDa), with the appearance of a 15 kDa band corresponding to the active form t-Bid, which can translocate to mitochondria where induces Bax and Bak to oligomerize [30]. Finally, SAHA reduced the level of the phosphorylated form of Bad, increasing its dephosphorylated form, which can stimulate apoptosis by heterodimerising with Bcl-Xl or Bcl-2 [31].
Suboptimal doses of both SAHA (1 μM) and bortezomib (10 nM) induced only modest effects on the levels of Bcl-2 family members (Fig. 5(A)), when tested separately. Co-treatment markedly increased the production of t-Bid, as well as the expression of Bcl-Xs protein (Fig. 5(A)) and that of the related mRNA (Fig. 4(C)). Differently co-treatment did not modify the level of Bcl-Xl, the level of Bim isoforms and that of phospho-Bad (Fig. 5(A)).
Treatments for 36–48 h with SAHA alone or in combination with bortezomib were ineffective at modifying the level of the apoptotic factor Bax in HepG2 cells (not shown). Finally, treatment with SAHA or combinations of drugs did not induce any appreciable changes in the levels of the members of Bcl-2 family in PHH (not shown).
The effect of SAHA on factors that control the fate of cells
Figure 5(B) shows that SAHA (1–5 μM) markedly increased p53 levels in HepG2 cells. An increasing effect on p53 level was also observed after treatment with combinations of suboptimal doses of SAHA and bortezomib, but in this case an additive effect rather than a synergistic interaction was seen. In addition, SAHA also caused a modest increase in the level of p21, a transcriptional target of p53. Interestingly, treatment with SAHA lowered the level of phospho-Akt, the active form of Akt, a kinase that plays a role in the promotion of cell survival.
Differently from bortezomib, SAHA modified neither the level of HSP70 nor those of phospho-c-Jun and phospho-JNK, inducing only a modest increase in c-Jun level. Finally, SAHA (1–5 μM) did not change DNA-binding activity of AP-1 (not shown), as ascertained by electrophoretic mobility shift assay [20].
Suboptimal doses of SAHA and bortezomib produced modest effects on phospho-Akt level. Interestingly, when the cells were treated with combinations of the two compounds the decreasing effect exerted by SAHA prevailed over the stimulatory action of bortezomib. Finally, combined treatment was effective in inducing a marked increase in the content of c-Jun, phospho-c-Jun and phospho-JNK (Fig. 5(B)). The increasing effect of c-Jun was attenuated by 50 μM Curcumin (Fig. 4(B)).
SAHA caused dissipation of the mitochondrial transmembrane potential (Δψm) and activation of caspases
Treatment of HepG2 cells with 5 μM SAHA or combinations of suboptimal doses of SAHA and bortezomib caused a pronounced decrease in Δψm evaluated using DiOC6, while separate treatments did not modify it (Fig. 6). In PHH, changes in Δψm were not observed after treatment for 36 h with SAHA either alone or in combination with bortezomib (not shown).
Treatment with SAHA alone or in combination with bortezomib induced loss of mitochondrial transmembrane potential in HepG2 cells. Cells were treated for 36 h with 1 or 5 μM SAHA or 10 nM bortezomib or combinations of 1 μM SAHA/10 nM bortezomib. Quantification of Δψm was performed by flow cytometry with the lipophilic dye DiOC6. The results were representative of three independent experiments. x-axis indicates DiOC6 fluorescence intensity on a logarithmic scale and y-axis indicates the number of events
Processing of caspase-8 in HepG2 cells was stimulated by SAHA. The intensity of the 57 kDa band, corresponding to pro-caspase-8, decreased with time of treatment, while the cleavage product (43 kDa), corresponding to caspase-8, concomitantly increased (Fig. 5(C)). Moreover, treatment with 5 μM SAHA for 36 h induced release of cytochrome c from mitochondria to cytosol (not shown), fragmentation of procaspase-3 with production of the active form of caspase-3 and finally, breakdown of PARP, a specific substrate of caspase-3 (Fig. 5(C)). Also, co-treatment with suboptimal doses of SAHA and bortezomib caused activation of both caspase-8 and caspase-3 and breakdown of PARP, while separate treatments were ineffective on caspase activities.
Discussion
Target genes are regulated by a mechanism consisting of acetylation of lysine residues in the N-terminal tails of core histones. The process depends on the enzymes histone acetyltransferase (HAT) and histone deacetylase (HDAC), which control the acetylation status of histones. Inhibition of HDAC results in histone acetylation and relaxation of chromatin structure [1], with increased transcription of a subpopulation of target genes required e.g. for induction of apoptosis.
SAHA, a powerful inhibitor of HDAC, induces apoptosis in many human tumor cells, such as multiple myeloma [32], leukemia [33], breast cancer [34] and thyroid cancer cells [35]. The drug seems to exert its effect by different mechanisms. In particular SAHA has been shown to induce up-regulation of p21 and p27 [34], attenuation of Bcl-2 and Bcl-Xl level [33, 34], enhancement in the level of apoptotic members of Bcl-2 family [33, 34], cleavage of Bid [36], down-regulation of Akt [32], and in some cases production of ROS [34]. Peart et al. [3], using DNA microarrays, showed that SAHA altered the expression of a large proportion of genes which can be involved in many apoptosis or cell survival mechanisms. While many studies have been carried out, the precise apoptotic mechanism induced by SAHA in different tumor cells has not been ascertained.
This paper demonstrates that SAHA, either alone or in combination with bortezomib, markedly reduced the viability of human hepatoma HepG2 and Huh6 cells by stimulating apoptosis mechanisms, as suggested by fluorescence microscopy after AO/EB staining and flow cytometric profiles of cell cycle distribution.
To ascertain the mechanism of SAHA-induced apoptosis in HepG2 cells we first focused on the extrinsic pathway of apoptosis, showing that SAHA markedly increased both Fas and FasL levels. As a consequence of the enhanced engagement of Fas by FasL, caspase-8 activation was observed.
Another important aspect of the effects of SAHA in HepG2 cells was the remarkable changes observed in the level of many Bcl-2 family members. SAHA decreased the content of both the survival factors Bcl-2 and Bcl-Xl. In concomitance, the drug stimulated the expression of the apoptotic factor Bcl-Xs, an alternative splicing isoform of Bcl-X gene, increasing the level of the protein as well as that of the specific transcript. We plan to study the way SAHA induces alternative splicing of Bcl-X gene. In this regard a correlation has already been shown between alternative splicing of Bcl-X gene and apoptosis [37]. Moreover a role for SR proteins dependent on their phosphorylation status has been demonstrated in the splicing mechanism [38]. We suggest that in our conditions SAHA may disrupt HDAC-protein phosphatase-1 (PP-1) complexes, as demonstrated by Chen et al. [39], with consequent dephosphorylation of SR proteins by PP-1 and induction of alternative splicing.
Our results show that SAHA increased the production of Bim proteins, in particular stimulating the expression of BimS isoform. In order to explain this effect, we hypothesize that SAHA can act as a modulator of the alternative splicing of Bim transcript. In particular, we suggest that Brm, the catalytic subunit of SWI/SNF complex, is involved in this process, since Brm has been shown to function as a regulator of alternative splicing of Bim transcript [40] and its activity seems to be regulated by acetylation [41].
Furthermore, SAHA induced cleavage of Bid with the production of its truncated and active form (t-Bid), an event that can be correlated with caspase-8 activation [42]. The drug also provoked dephosphorylation and activation of Bad, a substrate of the survival factor Akt. Activation of Bad was most likely a consequence of the remarkable decrease in the phosphorylated and active state of Akt observed in treated cells. In this regard we suggest that SAHA may bind to HDAC, preventing the association of HDAC with specific phosphatases [39], which can then interact with Akt inducing its dephosphorylation and inactivation.
The consistent changes in the level of many members of the Bcl-2 family may be responsible for the dissipation of Δψm induced by SAHA, with the consequent release of cytochrome c from mitochondria and activation of terminal caspase-3.
SAHA-induced apoptosis in HepG2 cells seems to be independent of c-Jun/JNK pathway, as the drug modified neither the level of c-Jun and phospho-c-Jun nor the activity of JNK. Moreover, SAHA was ineffective on HSP70, a stress-inducible protein that prevents apoptosis triggered by a number of cytotoxic agents [43].
Interestingly, in HepG2 cells SAHA provoked a marked increase in p53 level but did not modify the level of Bax and induced only a modest increase in the amount of p21. In order to explain these effects, we advance the hypothesis that the involvement of p53 in SAHA-induced apoptosis may be also correlated with the activation of acetylation cascade, as it has been observed in response to DNA-damage [44]. In particular acetylation of p53 on multiple lysine residues may result in profound differences in the formation of transcriptional complexes, which are differently recruited to the promoters of p53 responsive genes [45]. These considerations may explain why only modest effects were seen under our conditions on Bax and p21 levels, while a marked up-regulation induced by HDACIs was observed by other authors [34, 35]. Our results also show that oxidative stress was not involved in apoptosis, since the antioxidant N-acetylcysteine did not protect the cells from death.
In conclusion, our results demonstrate that SAHA induced apoptosis in HepG2 cells with a pleiotropic mechanism that involves several different events: (i) activation of the extrinsic apoptotic pathway, an event which is correlated with up-regulation of FasL and activation of caspase-8; (ii) loss of mitochondrial integrity resulting from changes in the content of many members of Bcl-2 family; (iii) inactivation of the survival factor Akt. SAHA-induced apoptosis can be considered as a result of the concomitant activation of both the extrinsic and the intrinsic pathways of apoptosis. This conclusion is suggested by the observation that z-IEDT-fmk, a specific inhibitor of caspase-8, and ZB4, a Fas blocking antibody, only partially inhibited the effect of SAHA, while z-VAD, a general inhibitor of caspase activities, wholly prevented SAHA-induced apoptosis.
We have previously shown that combinations of the inhibitor of histone deacetylase sodium butyrate and the proteasome inhibitor MG132 produced a synergistic effect in human retinoblastoma cells [4]. This was determined by the ability of MG132 to stabilise proteins involved in apoptosis, thereby accelerating the induction of cell death promoted by butyrate.
Many authors have shown that SAHA induces synergistic apoptotic effects by interacting with proteasome inhibitors. In particular, combinations of SAHA with bortezomib, an efficacious apoptotic agent, stimulated cell death in leukemia cells [12, 16] and in human multiple myeloma cells [13]. However, the molecular basis of this synergistic effect has been not clearly ascertained, although ROS generation [13], inactivation of NF-kB [9] or mitochondrial disfunction [13] have been correlated with this effect.
Recently, we have shown that bortezomib induced apoptosis in HepG2 cells associated with many modifications in the expression of survival or death factors [20]. In particular, bortezomib inhibited degradation of some regulatory factors and increased the activity of JNK and AP-1 with enhanced expression of AP-1 transcriptional targets, such as FasL and c-Jun [20]. In this paper we demonstrate that combinations of low and suboptimal doses of SAHA and bortezomib induced a synergistic apoptotic effect in HepG2 cells, which can be considered as a consequence of many synergistic interactions, leading to remarkable increases in the levels of Fas, FasL, c-Jun, t-Bid and Bcl-Xs and to activation of both caspases 8 and 3. Synergistic interactions determined by the combined treatment can be explained considering that SAHA may induce a more open transcriptional state of chromatin, while bortezomib may stabilize many factors involved in apoptosis. In particular, the improvement of DNA accessibility to the transcriptional factor AP-1, whose level is increased by bortezomib [20], could be responsible for the stimulation of the expression of FasL and c-Jun, observed in HepG2 cells treated with combinations of the two drugs. This role for AP-1 is supported by the finding that curcumin, a drug which prevents bortezomib action on AP-1 activity [20], reduced the effect of the combined treatment on FasL and c-Jun expression. In addition the increased level of both Fas and FasL, leading to stimulation of the extrinsic apoptotic pathway, seems to be the cause of the activation of caspase-8 with the consequent cleavage of Bid into t-Bid induced by the combined treatment. Finally, to explain the remarkable effect induced on Bcl-Xs by the combined treatment, we suggest that SAHA and bortezomib may activate alternative splicing of Bcl-X transcript, although with different mechanisms, thus favouring Bcl-Xs production. In particular, SAHA may modulate the activity of SR proteins [37–39], while bortezomib may induce their stabilization [46]. Since the combined treatment was ineffective at inducing synergistic effects as regards Bcl-2, Bcl-Xl, BimL, BimS and Bad, while markedly increased the level of Bcl-Xs, we suggest that this effect may assume particular relevance in the induction of the mitochondrial damage and the dramatic loss of Δψm, observed in HepG2 cells treated with combinations of the two drugs.
All these considerations lead us to the conclusion that the synergistic apoptotic effect provoked by the combined treatment also depends on the concomitant activation of the extrinsic and intrinsic pathways.
We have previously shown that in HepG2 cells bortezomib displays potent proapoptotic properties at 30–50 nM [20]. However, this effect was counteracted, particularly in the first phase of treatment, by the increase in the levels of several survival factors such as Hsp70, Hsp27 [20], NF-kB [47] and phospho-Akt (Fig. 5(B)). Differently, when HepG2 cells were treated with combinations of SAHA (1–2 μM) and bortezomib (10 nM), stimulation of proapoptotic factors was not counteracted by increased levels of survival factors.
It has been reported that the apoptotic mechanism induced in HepG2 cells by HDACIs is independent of TRAIL activation [48]. However, it has been shown that TRAIL-resistance can be overcome by a combinatory application of soluble recombinant human TRAIL with HDACIs, such as valproic acid and ITF2357 [48]. Therefore, we suggest that SAHA can synergize with bortezomib and contemporaneously sensitize the cells to recombinant TRAIL. New research to test this hypothesis is planned.
Although it has been reported that PHH are susceptible to Fas-mediated apoptosis [49, 50], evidence has been provided that HDACIs, such as valproate and ITF2357, that induce cellular damage in HCC-derived cells, are not effective in PHH [51]. In agreement with this finding, we ascertained that SAHA alone or in combination with bortezomib did not induce any toxic effect on PHH. Rather, SAHA seemed to increase the viability of PHH, an event most likely resulting from up-regulation of a number of survival factors. In PHH SAHA did not modify the intracellular level of FasL, but enhanced the level of FLIPs, an inhibitor of FasL- or TRAIL- mediated apoptosis. This finding is in accordance with a previous observation by other authors [48], who showed an increasing effect exerted by HDACIs on the expression of FLIPs in PHH.
Since it has been shown that, under particular conditions, PHH can undergo apoptosis following Fas receptor stimulation [49], we have performed co-culture experiments to evaluate the effect of soluble FasL released by HepG2 cells on PHH cell viability. Our results, showing in co-culture experiments that SAHA or combinations of SAHA/bortezomib induced apoptotic effects in HepG2 cells, but not in PHH, suggest the conclusion that PHH became resistant to activators of Fas potentially produced by neighbouring cells, most likely owing to the increased expression of FLIP induced by SAHA.
Conclusion
Taken together the findings reported in this paper demonstrate that SAHA induced apoptosis in hepatoma cells, with a pleiotropic mechanism causing profound changes in the expression of Bcl-2 family members and stimulating both the extrinsic and the intrinsic pathways of apoptosis. Association of suboptimal doses of SAHA and bortezomib resulted in an apoptotic synergistic effect, while it was ineffective in PHH. Our results strongly suggest that combination SAHA/bortezomib may represent a new and attractive pharmacological tool for the treatment of liver tumors.
References
Kristeleit R, Fong P, Aherne GW, de Bono J (2004) Histone modification enzymes: novel targets for cancer drugs. Expert Opin Emerg Drugs 9:135–154
Somech R, Izraeli S, J Simon A (2004) Histone deacetylase inhibitors–a new tool to treat cancer. Cancer Treat Rev 30:461–472
Peart MJ, Smyth GK, van Laar RK et al (2005) Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc Natl Acad Sci USA 102:3697–3702
Giuliano M, Vento R, Tesoriere G et al (1999) The apoptotic effects and synergistic interaction of sodium butyrate and MG132 in human retinoblastoma Y79 cells. Cancer Res 59:5586–5595
Emanuele S, Vento R, Tesoriere G et al (2004) Sodium butyrate induces apoptosis in human hepatoma cells by a mitochondria/caspase pathway, associated with degradation of beta-catenin, pRb and Bcl-Xl. Eur J Cancer 40:1441–1452
Newmark HL, Young CW (1995) Butyrate and phenylacetate as differentiating agents: practical problems and opportunities. J Cell Biochem Suppl 22:247–253
Kelly WK, Marks PA (2005) Drug insight: Histone deacetylase inhibitors–development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol 3:150–157
Kelly WK, O’Connor OA, Krug LM et al (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F (2003) Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res 63:7291–7300
Ocker M, Alajati A, Ganslmayer M et al (2005) The histone-deacetylase inhibitor SAHA potentiates proapoptotic effects of 5-fluorouracil and irinotecan in hepatoma cells. J Cancer Res Clin Oncol 131:385–394
Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN (2004) Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem 92:223–237
Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S (2003) The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood 102:3765–3774
Pei XY, Dai Y, Grant S (2004) Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res 10:3839–3852
Drexler HC, Euler M (2005) Synergistic apoptosis induction by proteasome and histone deacetylase inhibitors is dependent on protein synthesis. Apoptosis 10:743–758
Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK (2005) Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia 7:646–657
Rahmani M, Reese E, Dai Y et al (2005) Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res 65:2422–2432
Ludwig H, Khayat D, Giaccone G, Facon T (2005) Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104:1794–1807
San Miguel J, Blade J, Boccadoro M et al (2006) A practical update on the use of bortezomib in the management of multiple myeloma. Oncologist 11:51–61
Goel A, Dispenzieri A, Greipp PR, Witzig TE, Mesa RA, Russell SJ (2005) PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol 33:784–795
Lauricella M, Vento R, Tesoriere G et al (2006) JNK and AP-1 mediate apoptosis induced by bortezomib in HepG2 cells via FasL/caspase-8 and mitochondria-dependent pathways. Apoptosis 11:607–625
Perabo FG, Frossler C, Landwehrs G et al (2006) Indirubin-3′-monoxime, a CDK inhibitor induces growth inhibition and apoptosis-independent up-regulation of survivin in transitional cell cancer. Anticancer Res 26:2129–2135
Emanuele S, Vento R, Tesoriere G et al (2002) Apoptosis induced in hepatoblastoma HepG2 cells by the proteasome inhibitor MG132 is associated with hydrogen peroxide production, expression of Bcl-XS and activation of caspase-3. Int J Oncol 21:857–865
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lauricella M, Vento R, Tesoriere G et al (2001) pRb suppresses camptothecin-induced apoptosis in human osteosarcoma Saos-2 cells by inhibiting c-Jun N-terminal kinase. FEBS Lett 499:191–197
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Kamitani T, Nguyen HP, Yeh ET (1997) Activation-induced aggregation and processing of the human Fas antigen. Detection with cytoplasmic domain-specific antibodies. J Biol Chem 272:22307–22314
Shishodia S, Sethi G, Aggarwal BB (2005) Curcumin: getting back to the roots. Ann NY Acad Sci 1056:206–217
Marani M, Tenev T, Hancock D, Downward J, Lemoine N R (2005) Identification of novel isoforms of the BH3 domain protein bim which directly activate bax to trigger apoptosis. Mol Cell Biol 22:3577–3589
Yi X, Yin XM, Dong Z (2003) Inhibition of Bid-induced apoptosis by Bcl-2. tBid insertion, Bax translocation, and Bax/Bak oligomerization suppressed. J Biol Chem 278:16992–16999
She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8:287–297
Mitsiades CS, Mitsiades NS, McMullan CJ et al (2004) Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc Natl Acad Sci USA 101:540–545
Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK (2005) Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med 16:1125–1138
Bali P, Pranpat M, Swaby R et al (2005) Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of Her-2. Clin Cancer Res 11:6382–6389
Mitsiades CS, Poulaki V, McMullan C et al (2005) Novel Histone Deacetylase Inhibitors in the Treatment of Thyroid Cancer. Clin Cancer Res 11:3958–3965
Ruefli AA, Ausserlechner MJ, Bernhard D et al (2001) The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci USA 98:10833–10838
Chalfant CE, Rathman K, Pinkerman RL et al (2002) De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem 277:12587–12595
Tenenbaum SA, Aguirre-Ghiso J (2005) Dephosphorylation shows SR proteins the way out. Mol Cell 20:499–501
Chen CS, Weng SC, Tseng PH, Lin HP, Chen CS (2005) Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem 280:38879–38887
Batsche E, Yaniv M, Muchardt C (2006) The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol 13:22–29
Bourachot B, Yaniv M, Muchardt C (2003) Growth inhibition by the mammalian SWI-SNF subunit Brm is regulated by acetylation. EMBO J 22:6505–6515
Degli Esposti M (2002) The roles of Bid. Apoptosis 7:433–440
Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G (2003) HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2:579–584
Barlev NA, Liu L, Chehab NH et al (2001) Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 8:1243–1254
Roy S, Tenniswood M (2006) Site specific acetylation of p53 directs selective transcription complex assembly. J Biol Chem In press
Lai MC, Lin RI, Tarn WY (2003) Differential effects of hyperphosphorylation on splicing factor SRp55. Biochem J 1:937–945
Calvaruso G, Giuliano M, Portanova P, De Blasio A, Vento R, Tesoriere G (2006) Bortezomib induces in HepG2 cells IkBα degradation mediated by caspase-8. Mol Cellular Biol 287:13–19
Pathil A, Armeanu S, Venturelli S et al (2006) HDAC inhibitor treatment of hepatoma cells induces both TRAIL-independent apoptosis and restoration of sensitivity to TRAIL. Hepatology 43:425–434
Galle PR, Hofmann WJ, Walczak H et al (1995) Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med 182:1223–1230
Schulze-Bergkamen H, Untergasser A, Dax A et al (2003) Primary human hepatocytes---a valuable tool for investigation of apoptosis and hepatitis B virus infection. J Hepatol 38:736–744
Armeanu S, Pathil A, Venturelli S et al (2005) Apoptosis on hepatoma cells but not on primary hepatocytes by histone deacetylase inhibitors valproate and ITF2357. J Hepatol 42:210–217
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emanuele, S., Lauricella, M., Carlisi, D. et al. SAHA induces apoptosis in hepatoma cells and synergistically interacts with the proteasome inhibitor Bortezomib. Apoptosis 12, 1327–1338 (2007). https://doi.org/10.1007/s10495-007-0063-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0063-y