Abstract
Peanut red spider mite, Tetranychus ogmophallos, exhibits a peculiar dispersal behavior using silk balls, which involves clustering of mites and spinning of webs at the top of plants. Such a dispersal mechanism has not been studied for this species yet. Therefore, this study aimed at using mathematical models to describe aerial dispersal and silk ball formation of peanut red spider mite on peanut plants. The influence of wind speed, generated by a wind tunnel, on the dispersal of mites was studied in two experiments, one with 500 mites per plant and one with 1000 mites per plant, and six wind speeds (5, 10, 15, 20, 25, and 30 km h−1) for each mite density. The proportion of displaced mites and the distance they were blown were measured. Another series of experiments considered the formation of silk balls to assess how fast balls were formed as a function of time and the number of mites present on a peanut plant. Data from the wind tunnel experiments were analyzed by logistic regression and multiple regression to assess the proportion of displaced mites and the distance moved, respectively, as functions of wind speed and the initial density of mites on the donor plant. The distribution of dispersal distances from the donor plant was fitted by a mathematical model proposed by Ricker (J Fish Res Board Can 11:559–623, 1954). The number of mites moving upwards on a plant to be involved in silk ball formation was modeled as a function of time based on the initial number of spider mites and their estimated birth, death and movement rates per capita. Logistic regression was used to analyze the presence of balls as a function of time elapsed since a plant was infested with spider mites. Finally, non-linear regression was applied to link ball size to the total number of mites occupying the ball. The data analyses revealed that wind speed had a significant positive effect on take-off probability and distance moved by individual mites, whereas mite density had little influence. Ricker’s model adequately described the distribution of dispersal distances. The models describing silk ball formation also described data very well. Ball size was found to increase almost linearly with the number of mites found in the ball. We expect that the knowledge provided by the present study will help to develop efficient management strategies against T. ogmophallos in peanut crops as dispersal seems to be a key factor in the species’ capability to become a serious pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main ways of dispersal in phytophagous mites is through wind currents (Boykin and Campbell 1984; Li and Margolies 1993; Lawson et al. 1996; Osakabe et al. 2008). Although apparently trivial, it is a complex behavior, especially for apterous arthropods such as mites. Studies show that some mite species expose themselves to wind currents voluntarily (Brandenburg and Kennedy 1982; Osakabe et al. 2008), with different behaviors according to age and gender (Li and Margolies 1993) and unlinked to food deprivation (Li and Margolies 1993; Azandámè-Hounmalon et al. 2014).
Among the Tetranychidae species, the peanut red spider mite Tetranychus ogmophallos Ferreira and Flechtmann is recognized for their vast colonies with thousands of individuals and the ability to weave large amounts of webs (Andrade et al. 2016). The silk, which is classified by Saito (1983) as produced by type CW-u spider mites, serves to protect colonies and helps the mites to disperse (Bell et al. 2005). Mites belonging to CW-u weave complex dense webs with an irregular, three-dimensional or layered structure. In the field, T. ogmophallos feeds on plants of Arachis species (Fabaceae) (Rivero 2018), and the mite is one of the key pests of peanut species [Arachis hypogaea L. and Arachis pintoi (Krap. and Greg.)] in Brazil (Bonato et al. 2000; Lourenção et al. 2001; Melville et al. 2019) (Fig. 1). In this country, T. ogmophallos occurring in peanut crops has been confused for decades with the tomato red spider mite, Tetranychus evansi Baker and Pritchard (Melville et al. 2018).
At high population levels, T. ogmophallos individuals move to the upper part of their food plants to form collective silk balls which serve to facilitate aerial dispersal (Clotuche et al. 2013). They produce a structure consisting of silk and mites, both dead and alive, which can be carried away by the wind (Weeks et al. 2000). Such a gregarious dispersal behavior confers many advantages to the population (Lioni and Deneubourg 2004), such as increased chances of mating (Hambäck 2010; Clotuche et al. 2011), and lower predation risk (Dittmann and Schausberger 2017). On the other hand, clustering also has disadvantages, such as higher competition for food and space (Clotuche et al. 2013).
Dispersal of mites, either as single individuals being carried away by the wind or as groups of individuals being collectively transported inside silk balls, is an essential factor for T. ogmophallos population dynamics. So, understanding dispersal behavior and the factors eliciting it may help to forecast outbreaks in peanut cropping areas and, thus, facilitate their management. Gregarious organization of a species is intrinsically related to its spatial and vertical distribution and time–space structure. Furthermore, some populations show a positive relationship between population density and growth rate, at least initially. This phenomenon is known as the Allee effect (Allee and Boven 1932; Wang and Kot 2001).
Dispersal is a mechanism that has a direct influence on the ability of a pest species to develop resistance against pesticides through the migration of individuals among populations (Fragoso et al. 2003; Alves et al. 2005). Therefore, an effective management strategy considering migration patterns of a species may reduce environmental impacts if fewer treatments of the crop with acaricides will be needed (Jeger 1999). In this context, mathematical models are important for quantifying information on ecological factors related to herbivorous mites and insects in crop areas, including dispersal mechanisms (Hiebeler 2004; Garcia et al. 2014). As far as we know, this is the first study on the dispersal mechanisms of T. ogmophallos. Thus, the purpose of this paper was to model dispersal and silk ball formation of the peanut red spider mite.
Materials and methods
Tetranychus ogmophallos colony and plants used in experiments
To establish a colony, we collected T. ogmophallos mites in a field of forage peanut (A. pintoi) located in Jaboticabal city, São Paulo state (Brazil). They were then reared on plants of A. hypogaea cv. Granoleico in 10-l pots filled with soil, sand, and bovine manure (1:1:1). Some specimens of adult males and females were mounted in Hoyer’s medium on microscope slides to confirm the species using a phase-contrast microscope, according to Ferreira and Flechtmann (1997). The pots were kept in a greenhouse (18–30 °C and 48–58% RH), and plants were replaced by new ones whenever needed, and mites were transferred to the new plants by leaning them against the old ones.
In all experiments, peanut plants (A. hypogaea cv. Granoleico) were about 25 cm high (50 days after seeding) and were grown under the same conditions as used to maintain the mite colony.
Influence of wind speed on aerial dispersal
The influence of wind speed on aerial dispersal was assessed by two experiments performed from August through November 2016 in a non-climatized room of 6 × 4 m. An experiment was initiated by infesting a plant with fertilized T. ogmophallos females of unknown age (either 500 or 1000 mites per plant). Initially, the mites were transferred to a peanut leaflet kept in a Petri dish (60 mm diameter, 15 mm high). Then, in order to ensure the contact of mites with plants at the same time, each leaflet was attached to the respective experimental plant.
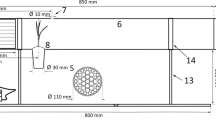
One day after infestation, plants were exposed to wind, using an experimental set-up adapted from Gamliel-Atinsky et al. (2009) (Fig. 2). The equipment consisted of a tube (1 m long, 30 cm diameter) coupled to another tube (1 m long, 40 cm diameter). A 20-cm-wide PVC board was mounted inside the larger diameter tube, where A4 transparent sheets, coated with industrial vaseline, were placed side-by-side in a 2-m-long row. The sticky sheets served to intercept mites blown-off the donor plant and were removed at the end of an experiment. An electric fan with a three-speed switch was positioned at the opening of the smaller diameter tube. The distance between the fan and plants was adjusted to obtain the desired wind speed. Wind speed was measured by a digital anemometer at the plant position (Boykin and Campbell 1984). Zinc discs (40 and 30 cm diameter) were placed in front of the fan to avoid turbulence from the movement of the fan propeller. The increase in the diameter of a disc reduced the wind speed in the tunnel.
Wind tunnel adapted from Gamliel-Atinsky et al. (2009) used to study the influence of wind speed on the aerial dispersion of Tetranychus ogmophallos. ZD1 and ZD2 are zinc discs of 400 and 300 mm in diameter, respectively, to prevent turbulence of the wind flow produced by the fan. (Color figure online)
The rate and distance at which mites were blown away from a plant were assessed at six airflow speeds (5, 10, 15, 20, 25, and 30 km h−1). Each speed was repeated 20 × and with an exposure time of 10 min per repetition. The distance of displacement was measured utilizing marks placed for each 10 cm inside the tube. Mites landing in each demarcated distance interval were counted, and the distance moved by an individual was assigned to the mid-point of the interval.
Modeling the effect of wind speed on aerial dispersal
The model proposed by Ricker (1954) was used to model the distribution of distances moved by the mites recovered inside the wind tunnel. For a given mite density and wind speed, the proportion of mites found in the distance interval between x and x + dx is predicted to be
where α and γ are the model’s shape parameters. In principle, x could take any value between 0 and infinity, but due to the length of the wind tunnel, x only took values between 0 and 200 cm, which means that mites blown more than 2 m away from the donor plant (x > 200 cm) were not recorded. Equation 1 was fitted to data by replacing dx with Δx (10 cm) and dy with Δy, defined as the proportion of recovered mites found in the interval between x and x + Δx cm where x = 0, 10, 20, …, 190 cm. Finally, x in Eq. 1 was replaced by the midpoint of the interval between x and x + Δx. Prior to statistical analysis, the 20 replicates for each experimental set-up were combined, so that Δy was calculated as the total number of recovered mites in the xth distance interval divided by the total number of mites found in all 20 intervals. The model was fitted to data by means of Proc NLIN (SAS Institute 2020), yielding estimates of α and γ, as well as their asymptotic standard errors, for each combination of initial mite density and wind speed.
As the parameters of Eq. 1 are likely to depend on wind speed and probably also on mite density, Eq. 1 was generalized by including wind speed (W) and initial mite density (N) as covariates in the model. This was done by replacing the parameters α and γ in Eq. 1 with \(\alpha ={\alpha }_{0}{N}^{{\alpha }_{1}}{W}^{{\alpha }_{2}}\) and \(\gamma ={\gamma }_{0}{N}^{{\gamma }_{1}}{W}^{{\gamma }_{2}}\), respectively, where α0, α1, α2, γ0, γ1, and γ3 denote parameters. The model was fitted simultaneously to data for all combinations of wind speed and mite densities in order to estimate the parameters.
Take-off probability is the likelihood that an individual on an infested plant is blown off during an experiment. To estimate take-off probabilities (p) we used recovery probabilities (p′), defined as the number of mites found in the wind tunnel (R) at the end of an experiment divided by the initial number of mites on the donor plant (N), i.e., p′ = R/N. Recovery probability is a proxy for take-off probability because not all blown-off mites may have been recovered, especially at high wind speeds, implying that p′ ≤ p. Recovery probability was modeled by means of logistic regression as
where y may depend on the initial density of mites (N) and wind speed (W). The relationship is modeled as
where the βs denote the model’s parameters. The parameters of Eq. 3 were estimated by fitting the model to the observed values of R/N obtained for each experiment, using Proc GENMOD (SAS Institute 2020). To reduce the risk of Type I errors due to overdispersion in data, the DSCALE option was used. Distribution was set to BINOMIAL and Link to LOGIT.
To investigate whether wind speed (W) and/or mite density (N) affected the mean displacement distances (D) of recovered mites, we used the model
where a, b, and c represent the model’s parameters. The empirical values of D were computed as
where Δx is the length of an interval (10 cm) and nx the number of mites recovered from the interval between x and x + Δx. Equation 4 was log transformed before the model was fitted to data by means of Proc GLM (SAS Institute 2020).
Collective silk ball formation and their contents
The studies of Clotuche et al. (2011, 2013) on two-spotted spider mites, Tetranychus urticae Koch, were used as a model for our experiments. A total of 40 potted peanut plants were used, each plant constituting a replicate. Before an experiment, the plants were pruned, leaving only two compound leaves per plant (eight leaflets), and plant height was standardized to 20 cm. A black-painted bamboo stick (30 cm high, 2 cm wide) was tied with plastic tape to the stem of each plant. The stick was pressed into the soil, leaving 22 cm above the soil level (Fig. 3a). A piece of graph paper (5 × 5 mm) was glued to the top of the stick (Fig. 3b). The paper served to calibrate the software (ImageJ) used for measuring the size of the silk ball attached to the upper part of the stick.
Fifty adult female mites were transferred from the culture to each experimental plant with the aid of a fine brush. Then, the plants were kept in a climate room at 25 ± 2 °C, 65 ± 10% RH and L14:D10 h photoperiod. Over the next 12 days, mites were allowed to climb up on the sticks and to weave web tangles. Over this period, the graph papers were photographed twice a day (8 am and 8 pm) to record the formation and growth of the silk balls, using a Nikon Coolpix P510 camera equipped with a close-up macro lens. The flow of mites moving up to the top of the sticks was measured for 2 min twice daily (8 am and 8 pm).
Twelve days after the plants had been infested with mites, the collective silk balls were carefully removed from the tip of the sticks and deposited on Petri dishes (15 × 9 cm) for approximately 10 min. This allowed the mites on the surface of a silk ball to climb down onto the dish and then walk around, which enabled us to count them. The examined ball was then transferred to a new Petri dish, where it was dissected so that the number of dead and trapped mites inside could be counted.
Modeling silk ball formation
After 50 adult female spider mites had been introduced onto a plant, they gradually appeared on the sticks above the plants, where they started to form silk balls. The size of a silk ball is assumed to increase with the number of mites present on the stick and the time the mites had spent there. Therefore, modeling silk ball formation should take the rate at which mites move away from an inoculated plant into consideration. The per capita emigration rate is likely to increase as the plant deteriorates due to exploitation by the introduced mites. A dynamic model linking plant condition and emigration rate to silk ball formation is proposed in Supplementary Material.
The volume of a silk ball at the end of an experiment is likely to be correlated with the number of mites occupying it. To test this assumption, we used the model given as
to quantify the relationship between the volume of a silk ball (V) and the number of mites (y) occupying it. a and b are two positive parameters, which were estimated by fitting the line
to data, where y was either the number of mites inside or outside the balls, or the sum of the two numbers. If b = 1, the volume increases proportionally with the number of mites, whereas b < 1 means that the volume gradually levels off with increasing mite numbers.
Results
Modeling the influence of wind speed on aerial dispersal
Figure 4 shows that, as the wind speed increased, a higher percentage of mites was blown-off the plant to be recovered inside the wind tunnel, and the mites were found at longer distances from the donor plant.
When Eq. 2 was used to model recovery probabilities, the full model explained 82.0% of the null model’s deviance. All terms were significantly different from 0, indicating that both wind speed (W), mite numbers (N), and their interaction (NW) contributed significantly to explain the proportion of mites found inside the wind tunnel. The full model was parameterized as (with standard errors in parentheses):
Although the initial number of mites on a plant seemingly affected the probability of a take-off, the most important factor was by far wind speed. Thus, wind speed alone could account for 71.4% of the null model’s deviance, which was highly significant (Wald’s \({\chi }_{1}^{2}\) = 476.73, P < 0.0001). Figure 5 shows the fit of the reduced model to the combined data set.
The proportion of adult female Tetranychus ogmophallos introduced on a plant and recovered in the wind tunnel at the end of an experiment (points). Data for experiments with 500 and 1000 mites per plant were combined. The red line shows the predicted relationship between wind-speed and take-off rate, based on Eq. 3, with 95% confidence limits for the predicted relationship shown as black lines. (Color figure online)
Figure 6 shows the fit of Eq. 1 to the proportion of mites recovered at different distances from the donor plants for each combination of initial mite abundance (N) and wind speed (W). As α decreased with wind speed, whereas the opposite applied to γ (Table 1), the two parameters were replaced with \(\alpha ={\alpha }_{0}{N}^{{\alpha }_{1}}{W}^{{\alpha }_{2}}\) and \(\gamma ={\gamma }_{0}{N}^{{\gamma }_{1}}{W}^{{\gamma }_{2}}\) and the general model was fitted to all data. The full model explained 88.8% of the total variation in recovery rates (F6,234 = 308.6, P < 0.0001) (Fig. 7). As wind speed was by far the most important factor, mite density was omitted from the model which then explained 87.3% (F4,236 = 403.9, P < 0.0001). The parameters of the reduced model were estimated as α0 = 0.5666 (SE = 0.0982), α1 = − 2.2154 (SE = 0.0753), γ0 = 1.1475 (SE = 0.1213) and γ1 = 1.1619 (SE = 0.0457).
Distribution of adult female T. ogmophallos recovered in the wind tunnel at different distances from the donor plant shown for each combination of number of introduced mites and wind speed (points). The red lines show the fit of Ricker’s (1954) model (Eq. 1) to data (red line: predicted distribution; blue lines: 95% confidence limits for the predicted relationship. (Color figure online)
When the mean displacement distances (D) were modeled by Eq. 4, the model’s parameters were estimated as ln a = − 1.0279 (SE = 0.4736), b = 0.2794 (SE = 0.0679) and c = 1.1245 (SE = 0.0413). The model explained 76.8% of the total variation in the observed values of D (F2,224 = 371.4, P < 0.0001). Though the results indicate that displacement distances tended to increase with the density of mites on the donor plant, it was wind speed that was the most important factor responsible for how far a mite was blown away. Thus, omitting mite density (N) from the model yielded ln a = 0.8606 (SE = 0.1199) and c = 1.1066 (SE = 0.0425) (Fig. 8). The reduced model explained 75.1% of the total variation (F1,225 = 677.9, P < 0.0001). As c was not significantly different from 1 (t225 = 0.251, P = 0.80), it seems justified to assume that displacement distances increase proportionally with wind speed. Thus, if c is set to unity, the resulting model was found as D = aW = 3.174 W, implying that the mean displacement distance is expected to increase with 3.174 cm each time wind speed increases with 1 km h−1.
Average distances at which adult females of Tetranychus ogmophallos were recovered in the wind tunnel after having been exposed to different wind speeds (points). Data from all experiments were combined. The lines are based on fitting the model (Eq. 4) D = aW = 3.174 W to data [red line: predicted relationship between wind speed (W) and mean displacement distance (D); blue lines: 95% confidence limits for the predicted relationship; black lines: 95% confidence limits for individual observations]. (Color figure online)
Modeling silk ball formation
The total number of mites found in the 40 silk balls 12 days after the mites had been introduced onto the plants was 1505 of which 1045 (69.4%) were found inside and 460 (30.6%) outside the balls. Adults constituted 21.8% of the mites found inside and 67.3% of the mites outside the balls, equivalent to 35.7% of all mites occupying the silk balls.
Figure 9 shows the volume (V) of each of the 40 silk balls plotted against the total number of mites found inside and outside a ball (y). The relationship between ln V and ln y was adequately described by Eq. 7 (\({R}_{adj}^{2}\) = 0.768; F1,38 = 130.3, P < 0.0001), yielding (with standard errors in parentheses)
Volumes of silk balls plotted against the total number of Tetranychus ogmophallos mites occupying them 12 days after 50 adult female mites had been introduced onto each of the 20 plants. The lines are based on fitting the model (Eq. 6) \(V=a{y}^{b}\) = 0.4091 \({y}^{0.8397}\) to data [red line: the predicted relationship between a number of mites (y) and ball volume (V); blue lines: 95% confidence limits for the predicted relationship; black lines: 95% confidence limits for individual observations]. (Color figure online)
corresponding to
As b was significantly less than unity (t38 = 2.178, P = 0.036), it indicates that the volume of silk balls increased sub-proportionately with mite abundance (both dead and alive). 69% of the mites were found inside the silk balls, of which 22% were adults. In contrast, 67% of the mites outside the balls were adults.
Figure 10a shows the growth of silk balls from day 0 (when the mites were introduced) and until day 12 when the balls were removed, and the mites counted. Figure 10b shows the number of mites on the sticks observed during 2 min of observation periods. The predicted values in Fig. 10 are based on the dynamic model described in Supplementary Material. The model uses plant damage (D) as an auxiliary state variable. D was not measured experimentally, but Fig. 10c shows the predicted values of this hidden state variable. The number of mites on a plant (x) was only known at the time of introduction, whereas the number of mites occupying the silk ball (y) was only assessed on day 12 (Fig. 10d). The eight parameters of the model were estimated by simultaneously fitting the model to 37 data points, using the sum of squared deviations to minimize the difference between the observed and predicted values. The model was capable of explaining 93.7% of the total variation in data. The model indicates that the major part of the second generation of adult mites (i.e., the recruitment rate exceeded the loss rate) occurred 9.9 days after the first generation had been introduced and that the majority of newly emerged adults quickly left the plant to enter the silk ball.
a Observed (points with 95% confidence limits) and predicted (line) volume of silk balls (V); b Observed (points with 95% confidence limits) and predicted (line) number of mites on sticks; c Predicted plant damage index; d Observed (points with 95% confidence limits) and predicted number of mites occupying the plant (red line), the silk ball (blue line) and the total number of mites (black line). The predicted values were obtained from the model presented in Supplementary Material with λ = 0.1082 day−1, s = 0.00174 day−1, a0 = 0.0624 min−1, w = 2.728 mm3 day−1, μ0 = 0.1210 h−1, m = 4.902, ρ = 0.0364 day−1 and τ = 9.9 days. (Color figure online)
Discussion
Spider mites live in large groups comprising several thousand individuals. When experiencing poor conditions, such as food scarcity, competition or presence of predators, they strive to occupy new areas (Dittmann and Schausberger 2017). For these wingless arthropods, aerial dispersal is a key factor for their persistence in a given environment (Hoelscher 1967; Osakabe et al. 2008). In this context, this is the first study on the dispersal response of T. ogmophallos and its ability to form collective silk balls, which may contribute to make the species an important emerging pest in peanut crops (Melville et al. 2019).
In this study, we could demonstrate the influence of wind speed and population density on the dispersal behavior of T. ogmophallos. Especially wind speed was important for the proportion of mites taking off from an infested plant and how far they were displaced. Thus, it was found that ca. 30–60% of the introduced mites were recovered in the wind tunnel after exposing the plant to a wind speed of 30 km h−1 (Fig. 5) and at an average distance of about 100 cm from the plant. However, as indicated from the cut-off of the right tails of the displacement distributions (Figs. 6 and 7), an increasing proportion of the displaced mites seems to have been blown more than 200 cm away from the infested plant and were therefore not recovered in the wind tunnel, as the wind speed increased. Consequently, the experiments may have underestimated take-off rates and displacement distances, especially at high wind speeds, implying that wind may be an even more important factor for mite dispersal than actually revealed from the current experiments. The circumstance that Ricker’s dispersal model (Ricker 1954) fitted recovery data quite well indicates that the model may serve as a tool for estimating the proportion of mites that will be blown to any distance from the donor plant. Especially the generalized version of Ricker’s model may prove useful as it allows for the incorporation of both wind speed and mite density to predict their combined influence on displacement distances outside the range for which empirical data are available. For instance, at a wind speed of 23 km h−1 and a density of 1200 mites/plant, the model predicts that 12.9 and 5.6% of the displaced mites will land more than 2 and 3 m from the donor plant, respectively. At a wind speed of 35 km h−1, the corresponding values are 29.7 and 12.5%.
Our results indicate that such behavior can be voluntary (i.e., non-enforced) up to certain wind speeds. A similar outcome had previously been reported for other spider mite species, such as T. urticae and Oligonychus pratensis (Banks) (Smitley and Kennedy 1985; Margolies 1987). Moreover, we assume that T. ogmophallos exhibits a similar aerial dispersal behavior as that of T. urticae, which was studied by Boykin and Campbell (1984) in peanut crops located in North Carolina (USA). These authors verified by laboratory experiments that T. urticae disperses by passive transport when wind speed is in the range of 8 to 24 km h−1. They also highlighted that the majority of mites would disperse at a wind speed of 24 km h−1, mainly when they occur at high infestation levels. Besides, in the presence of wind, light, and scarce food, T. urticae displays an aerial take-off behavior involving the lifting of the first legs upright and raising the forebody (Li and Margolies 1993; Osakabe et al. 2008). Following these findings, Smitley and Kennedy (1985) observed that T. urticae only exhibit aerial dispersal behavior when exposed to wind. Moreover, Bell et al. (2005) found that wind-borne dispersal by T. urticae involves ballooning by means of silk threads. Such ballooning behavior has been observed in many spider mites, including citrus red mite Panonychus citri (McGregor), avocado brown mite Oligonychus punicae (Hirst), tea red spider mite Oligonychus coffeae (Nietner) and six-spotted mite Eotetranychus sexmaculatus (Riley) (Osakabe et al. 2008). Another interesting fact of relevance for understanding aerial dispersal is that T. urticae adult females, which developed under crowded conditions, have their weight reduced to nearly half the weight of females developed under uncrowded conditions, and disperse without feeding (Mitchell 1973). Hence, qua their lower weight starved individuals are likely to be blown further away than well-fed conspecifics.
In general, we consider that the dispersal capacity of T. ogmophallos through wind currents contributes significantly to why mite outbreaks may occur almost simultaneously over extensive areas. Thus, in years of climatic conditions favorable for the development of spider mite populations (dry and hot), T. ogmophallos can quickly spread over wide croplands, which may require an immediate intervention strategy. Azandámè-Hounmalon et al. (2014) reported that T. evansi exhibits a more gregarious behavior than T. urticae in tomato plants, dispersing mainly at high population levels compared with the latter species. According to these authors, the dispersal behavior of T. evansi facilitates discovering infested tomato plants, thereby allowing early control measures and, consequently, reducing the risk of outbreaks. Therefore, rapid localization of mite outbreaks also seems to be a key factor for successful management of T. ogmophallos in peanut crops.
Studies on collective silk balls are fundamental for understanding how these structures are build and their importance for T. ogmophallos dispersal. The formation of collective silk balls is closely linked to the cumulated flow of mites reaching the top of plants. However, their formation is conditioned to a minimum number of individuals (Clotuche et al. 2011).
To understand the complex dynamics underlying between mite abundance and ball formation, we developed a dynamic model based on how mites respond to plant exploitation. The model is conceptual in the sense that we lack sufficient experimental data that can substantiate the underlying assumptions, first of all, how leaf damage developed during a mite infestation. Instead, we used leaf damage as an auxiliary variable that is assumed to increase with the number of mites present on the plant and the time they stay there. We further assumed that declining leaf quality affects mite activity, causing mites to escape from the plant by moving to the stick above the plant where they contribute to ball formation. As an experiment lasted 12 days, the model also had to take into account that some of the introduced adult female mites may die during an experiment but also that they lay eggs, which eventually develop into adults. The model was fitted to the available data on mite activity (expressed as the number of mites counted on the sticks), the size of silk balls, and the number of mites found on the silk balls at the end of an experiment. It has to be emphasized that the circumstance that the model described the existing data very well does not in itself prove that its underlying assumptions are true or that its estimated parameter values are realistic. On the other hand, we have not been able to falsify the model when it was confronted with the available data. Proper validation of the model would require that its predictions regarding, e.g., leaf injury and the final number of mites found on the infested plant, are in agreement with actual observations. Consequently, future experiments should include measurements of these missing variables.
We found that T. ogmophallos silk balls were occupied mainly by immature mites. Likewise, Clotuche et al. (2011, 2013) found that immatures were more abundant than adults in T. urticae silk balls. According to these authors, a higher number of immature individuals will increase the chance of mating with non-genetically related conspecifics, thereby reducing the risk of inbreeding. This also increases the probability of selecting the best adapted individuals. Moreover, Clotuche et al. (2013) inferred that group dispersal by means of silk-balls is a key factor for the success of T. urticae to escape adverse conditions because it reduces the energy costs otherwise involved in individual dispersal.
During silk-ball formation, T. ogmophallos presented a recruitment behavior similar to that reported by Pralavorio et al. (1989) and Azandámè-Hounmalon et al. (2014) for other spider mites. Tetranychus ogmophallos mites climbed up and down the sticks frantically, leaving threads that connected the plant to the top of sticks, as if they were recruiting other mites to climb up to the top of sticks (Fig. 3b). This might be related to the circadian rhythm of T. urticae (Pralavorio et al. 1989; Azandámè-Hounmalon et al. 2014). Previous studies have shown that tetranychid mites use to leave silk threads along their way, allowing other individuals to follow them along the same trail (Yano 2008). As mites climbed up and down, a substantial increase in web production was observed at the top of sticks and around sticks and plants, besides the attempt of building web bridges between pots.
We noticed a significant part of the colony remained on plants without climbing up to the silk-balls, even after the plants has deteriorated. Li and Margolies (1993) noticed that mites from the same population of T. urticae may react differently to dispersal or feeding stimuli. Likewise, Le Goff et al. (2011) found that individuals respond differently depending on the level of competition or their physiological status. Li and Margolies (1993) also observed that the contribution of adult females to dispersal fell considerably with age. Although dispersal by collective silk balls is risky, as the landing site is uncertain, this mechanism may nevertheless prove to be advantageous. It seems to be an effective means for colonizing new suitable plants located in some distance from an over-exploited plant. For example, in addition to oviposition on plants, individuals of T. ogmophallos also lay large amounts of eggs on webs and in the clusters.
In short, knowledge of how T. ogmophallos disperse under field conditions is a key factor for preventing population outbreaks in peanut production. Thus, our results contribute to a better understanding of the main dispersal mechanisms of T. ogmophallos and may serve as guidance for adopting more effective control measures, thereby reducing use of acaricides to the benefit of the environment.
References
Allee WC, Boven E (1932) Studies in animal aggregations: mass protection against colloidal silver in goldfishes. J Exp Zool 61:185–207
Alves EB, Casarin NFB, Omoto C (2005) Dispersal mechanisms of Brevipalpus phoenicis (Geijskes) (Acari: Tenuipalpidae) in citrus groves. Neotrop Entomol 34:89–96
Andrade DJ, Melville CC, Michelotto MD (2016) Manual prático sobre ácaros do amendoim no Brasil. Funep, Jaboticabal, pp 1–14
Azandámè-Hounmalon GY, Fellous S, Kreiter S, Fiaboe KKM, Subramanian S, Kungu M, Martin T (2014) Dispersal behavior of Tetranychus evansi and T. urticae on tomato at several spatial scales and densities: implications for Integrated Pest Management. PLoS ONE 9:1–8
Bell JR, Bohan DA, Shaw EM, Weyman GS (2005) Ballooning dispersal using silk: world fauna, phylogenies, genetics and models. Bull Entomol Res 95:69–114
Bonato O, Santarosa PL, Ribeiro G, Lucchini F (2000) Suitability of three legumes for development of Tetranychus ogmophallos (Acari: Tetranychidae). Fla Entomol 83:203–205
Boykin LS, Campbell WV (1984) Wind dispersal of the twospotted spider mite (Acari: Tetranychidae) in North Carolina peanut fields. Environ Entomol 13:221–227
Brandenburg RL, Kennedy GG (1982) Intercrop relationships and spider mite dispersal in a Corn/Peanut Agro-Ecosystem. Entomol Exp Appl 32:269–276
Clotuche G, Mailleux AC, Fernández AA, Deneubourg JL, Detrain C, Hance T (2011) The formation of collective silk balls in the spider mite Tetranychus urticae Koch. PLoS ONE 6:e18854
Clotuche G, Navajas M, Mailleux AC, Hance T (2013) Reaching the ball or missing the flight? Collective dispersal in the two-spotted spider mite Tetranychus urticae. PLoS ONE 8:e77573
Dittmann L, Schausberger P (2017) Adaptive aggregation by spider mites under predation risk. Sci Rep 7:1–9
Ferreira DNM, Flechtmann CHW (1997) Two new phytophagous mites (Acari: Tetranychidae, Eriophyidae) from Arachis pintoi from Brazil. Syst Appl Acarol 2:181–188
Fragoso DB, Guedes RNC, Ladeira JA (2003) Seleção na evolução de resistência a organofosforados em Leucoptera coffeella (Guérin-Mèneville) (Lepidoptera: Lyonetiidae). Neotrop Entomol 32:329–334
Gamliel-Atinsky E, Freeman S, Sztejnberg A, Maymon M, Ochoa R, Belausov E, Palevsky E (2009) Interaction of the mite Aceria mangiferae with Fusarium mangiferae, the causal agent of mango malformation disease. Phytopathology 99:152–159
Garcia A, Cônsoli FL, Godoy WAC, Ferreira CP (2014) A mathematical approach to simulate spatio-temporal patterns of an insect-pest, the corn rootworm Diabrotica speciosa (Coleoptera: Chrysomelidae) in intercropping systems. Landsc Ecol 29:1531–1540
Hambäck PA (2010) Density-dependent processes in leaf beetles feeding on purple loosestrife: aggregative behaviour affecting individual growth rates. Bull Entomol Res 100:605–6011
Hiebeler D (2004) Spatially correlated disturbances in a locally dispersing population model. J Theor Biol 272:143–149
Hoelscher CE (1967) Wind dispersal of brown soft scale crawlers, Coccus hesperidum (Homoptera: Coccidae), and Texas Citrus Mites, Eutetranychus banksi (Acarina: Tetranychidae) from Texas Citrus. Ann Entomol Soc Am 60:673–678
Jeger MJ (1999) Improved understanding of dispersal in crop pest and disease management: current status and future directions. Agric For Meteorol 97:331–349
Lawson DS, Nyrop JP, Dennehy TJ (1996) Aerial dispersal of European red mites (Acari: Tetranychidae) in commercial apple orchards. Exp Appl Acarol 20:193–202
Le Goff GJ, Hance T, Detrain C, Deneubourg JL, Clotuche G, Mailleux AC (2011) Impact of starvation on the silk attractiveness in a weaving mite, Tetranychus urticae (Acari: Tetranychidae). J Ethol 30:125–132
Li J, Margolies DC (1993) Effects of mite age, mite density, and host quality on aerial dispersal behaviour in the two-spotted spider mite. Entomol Exp Appl 68:79–86
Lioni A, Deneubourg JL (2004) Collective decision through self-assembling. Naturwissenschaften 91:237–241
Lourenção AL, Kasai FS, Návia D, Godoy IJ, Flechtmann CHW (2001) Ocorrência de Tetranychus ogmophallos Ferreira e Flechtmann (Acari: Tetranychidae) em amendoim no estado de São Paulo. Neotrop Entomol 30:495–496
Margolies DC (1987) Conditions eliciting aerial dispersal behavior banks grass mite, Oligonychus pratensis (Acari: Tetranychidae). Environ Entomol 16:928–932
Melville CC, Andrade SC, Oliveira NT, Andrade DJ (2018) Impact of Tetranychus ogmophallos (Acari: Tetranychidae) on different phenological stages of peanuts. Bragantia 77:116–123
Melville CC, Zampa SF, Savi PJ, Michelotto MD, Andrade DJ (2019) Peanut cultivars display susceptibility by triggering outbreaks of Tetranychus ogmophallos (Acari: Tetranychidae). Exp Appl Acarol 78:295–314
Mitchell R (1973) Growth and population dynamics of a spider mite (Tetranychus urticae K., Acarina: Tetranychidae). Ecology 54:1349–1355
Osakabe MH, Isobe H, Kasai A, Masuda R, Kubota S, Umeda M (2008) Aerodynamic advantages of upside down take-off for aerial dispersal in Tetranychus spider mites. Exp Appl Acarol 44:165–183
Pralavorio M, Fournier D, Millot P (1989) Activité migratoire des tétranyques: mise en évidence d'un rythme. Entomoph 34:129–134
Ricker WE (1954) Stock and recruitment. J Fish Res Board Can 11:559–623
Rivero YR (2018) Characterization of the rotation system peanut-sugarcane and evaluation of the intercropping peanut-sorghum-sunflower, aiming to the control of mite pests. Thesis (Master in Agricultural Entomology)—Faculdade de Ciências Agrárias e Veterinárias, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Jaboticabal
Saito Y (1983) The concept of ‘life types’ in Tetranychinae. An attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–391
SAS Institute (2020) SAS on Demand for Academics, SAS Studio, Cary, North Carolina. https://odamid-euw1.oda.sas.com/SASODAControlCenter/. Accessed 5 Oct 2019
Smitley DR, Kennedy GG (1985) Photo-oriented aerial-dispersal behavior of Tetranychus urticae (Acari: Tetranychidae) enhances escape from the leaf surface. Ann Entomol Soc Am 78:609–614
Wang MH, Kot M (2001) Speeds of invasion in a model with strong or weak Allee effects. Math Biosci 171:83–97
Weeks AR, Turelli M, Hoffmann AA (2000) Dispersal patterns of pest earth mites (Acari: Penthaleidae) in pastures and crops. J Econ Entomol 93:1415–1423
Yano S (2008) Collective and solitary behaviors of two spotted spider mite (Acari: Tetranychidae) are induced by trail following. Ann Entomol Soc Am 101:247–252
Acknowledgements
The authors want to thank Dr. Eric Palevsky from the Agricultural Research Organization (Israel) for reviewing this manuscript, and the reviewers for the suggestions and comments. This work was carried out with support from CAPES (Coordination of Superior Level Staff Improvement), Brazil. We thank Edvaldo Pereira dos Santos for providing the photo used in Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santos, F.A., Rolim, G.S., Nachman, G.S. et al. Using mathematical models to describe aerial dispersal and silk ball formation of peanut red spider mite, Tetranychus ogmophallos (Acari: Tetranychidae). Exp Appl Acarol 81, 85–102 (2020). https://doi.org/10.1007/s10493-020-00495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00495-1