Abstract
Ticks and fleas are essential vectors of pathogens that affect humans and animals, and among their hosts, synanthropic animals such as the black-eared opossum, Didelphis aurita, play a role in public health due to their ability to move between urban centers and forested areas in Brazil. This study aimed to assess the ectoparasite fauna of D. aurita, as well as the presence of pathogens and endosymbionts in ticks and fleas. Opossums (n = 58) captured in Tomahawk livetraps were examined for ectoparasites, and their blood sampled for further analysis. Additionally, spleen samples were collected in individuals found dead. Samples were PCR screened for Rickettsia spp., Borrelia spp., Anaplasmataceae, and Babesia spp. Two tick species were morphologically identified as Ixodes loricatus 24/58 (41.4%) and Amblyomma sculptum 1/58 (1.7%). For fleas, Ctenocephalides felis was detected in 60.3% (35/58) of the animals, and Xenopsylla cheopis in 5.2% (3/58). PCR analysis detected Anaplasmataceae DNA in 34% (16/47) of pooled samples of C. felis, and in 66.7% (2/3) pooled samples of X. cheopis. Sequence analysis revealed Wolbachia pipientis symbiont in all positive samples. Tick, blood and spleen samples were all negative for the microorganisms assessed. These findings suggest that these arthropods circulate among wildlife and urban environments, which may implicate in their participation in the cycle of zoonotic pathogens among opossums, humans and companion animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marsupials of the genus Didelphis are mammals geographically restricted to the American continent (Gardner 2008). In South America these animals are represented by five species divided in two groups, the D. marsupialis-group (D. marsupialis and D. aurita) and the D. albiventris-group (D. albiventris, D. pernigra, and D. imperfecta). The only Didelphis species that up to date is not found in this region is the D. virginiana, which occurs from Canada to Costa Rica (Gardner 2008). In Brazil, four species of this genus are reported, D. imperfecta (Guianan White-eared Opossum) and D. marsupialis (Common Opossum) in the Amazon rainforest, D. albiventris (White-eared Opossum) mostly in the Cerrado biome, and D. aurita (Black-eared Opossum) in the Atlantic forest (Faria and Melo 2017; Gardner 2008).
Species of the genus Didelphis are well adapted to the anthropogenic activity, being found on the roof of houses, hollows of trees and other shelters within urban centers (Jansen 2002). Due to this synanthropic behavior, the possibility of transmission of pathogens (i.e., Rickettssia spp. and Borrelia spp.) by ectoparasites to humans and domestic animals may occur (Muller et al. 2005; Cabrera et al. 2003).
It is known that ticks and fleas are commonly reported infesting Didelphis spp. (Linardi 2006; Muller et al. 2005). For instance, studies of the ectoparasite fauna of D. aurita have reported the occurrence of ticks of the genera Amblyomma and Ixodes (Luz et al. 2018, 2013; Gonzalez et al. 2017; Acosta et al. 2016; Oliveira et al. 2014; Saraiva et al. 2012; Dantas-Torres et al. 2012), and fleas of the genera Adoratopsylla, Polygenis, Leptopsylla, Xenopsylla and Ctenocephalides (Urdapilleta et al. 2019; Pinto et al. 2009; Salvador et al. 2007; Horta et al. 2007; Moraes et al. 2003). Interestingly, specimens of the genera Amblyomma and Ctenocephalides are commonly found on domestic animals (i.e., dogs, cats, horses) (Costa et al. 2017; Dantas-Torres and Otranto 2014), which may imply that these ectoparasites have major importance in the epidemiological life cycle of pathogens of medical and veterinary concern (De Sá et al. 2018; Muller et al. 2005). The aim of this study was to assess the ectoparasite fauna of D. aurita, as well as the presence of pathogens and endosymbionts in ticks and fleas.
Material and methods
Study area
The study was conducted in the municipality of Viçosa (20°45′14″S, 42°52′54″W), located in the State of Minas Gerais, Southeastern Brazil. The climate in this region is classified as Cwa (Köppen climate classification), mesothermic, with hot and rainy summers and cold and dry winters. The area is 650 m above sea level and presents an annual average temperature varying from 20 to 22 °C.
Animals and sampling
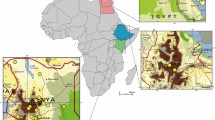
Animals were captured through Tomahawk live traps (0.45 × 0.21 × 0.21 m), which were armed and checked daily (5PM and 7AM, respectively), from January to June 2019, totaling a sampling effort of 516 trap-nights in urban environments of the study area (Fig. 1). A mix of corn flour, canned fish and banana were used as bait. After capture, the animals were mechanically restrained and classified by sex, age group (pups, subadults and adults), and marked with a small V cut at the right ear to identify recaptures (Morrant et al. 2010; Pestell and Petit 2007). Additionally, animals found dead (n = 9) on roads and streets of the study area were necropsied and fragments of spleen collected.
Captured opossums were individually inspected for a period of approximately 10 min for the presence of ectoparasites. Specimens collected were stored in plastic vials (ticks in empty tubes and fleas in tubes containing 70% alcohol) for morphological identification and molecular procedures. In addition, blood samples were collected via venipuncture of the caudal ventral or jugular vein and stored at − 20 °C until molecular processing.
Laboratorial procedures
Ectoparasites were quantified, separated according to the stage and sex, and morphologically identified using dichotomy keys (Barros-Battesti et al. 2006; Aragão and Fonseca 1961; Linardi and Guimarães 2000; Bicho and Ribeiro 1998). Afterwards, 58 pools of ticks (up to 3 specimens) and 50 pools of fleas (up to 4 specimens) were formed for DNA extraction.
For molecular procedures, genomic DNA of blood and spleen were extracted using a commercial kit for blood and spleen (Illustra tissue and cells genomicPrep Mini Spin Kit, GE Healthcare Life Sciences) following the manufacturer’s instructions. On the other hand, DNA extraction of ectoparasites were performed following a protocol previously described (Ramos et al. 2015).
Each DNA sample was screened for the gltA gene of Rickettsia spp., the flagellin gene of Borrelia spp., the 16S rRNA gene for Anaplasmataceae, and for the 18S gene for Babesia spp. (Table 1). Amplifications were performed using Taq Pol Master Mix Green 2 × following the manufacturer’s recommendations, 400 nmol of each primer, 5 µL of DNA sample and nuclease free water until complete 25 µL volume. DNA of Rickettsia vini (Preventive Veterinary Medicine and Animal Health Department of the University of São Paulo), Borrelia garini (Epidemiology and Public Health Department of the Federal Rural University of Rio de Janeiro), Ehrlichia canis and Babesia canis (Veterinary Medicine Department of the Federal University of Viçosa) were used as positive control, and nuclease free water as negative control.
All amplicons obtained were purified using PCR Purification Kit (Cellco Biotec) according to manufacturer’s recommendations. Sanger’s method was performed for sequencing amplicons in both directions (Sanger et al. 1977) in an automated sequencer AB 3500 Genetic Analyzer. DNA sequences were aligned using Mega7 (Kumar et al. 2016) and compared with sequences from GenBank using the BLAST search tool (Altschul et al. 1990).
Data analysis
Descriptive statistical analysis was performed to calculate the relative and absolute frequencies, as well as mean intensity and abundance of infestation by ticks and fleas. The normality of data was checked using the Lilliefors test. Additionally, the χ2 test with Yates correction was used to compare the occurrence of these ectoparasites according to sex and age of the animals. The significance level was set at 5%. All analyzes were carried out using the BioEstat 5.3 software.
The sites of capture of opossums were geoprocessed with the geographic information system program QGIS 3.4.12 (qgis.org). Digital maps of the Brazil, the State of Minas Gerais and the municipality of Viçosa were used as cartographic basis, and the SIRGAS2000 and the UTM coordinates system as ellipsoid of reference. The layers and points were automatically converted by the system (extension on the fly). The complement QuickMapServices was used to obtain the satellite images (Google Satellite).
Results
Fifty-eight animals were captured during the whole study, being 50% (29/58) males and 50% (29/58) females. In particular, 3.5% (2/58) were classified as pups, 46.6% (27/58) as subadults and 50% (29/58) as adults. A total of 69 ticks and 121 fleas were collected on the animals, which correspond to frequencies of infestation of 41.4% (24/58) and 60.3% (35/58), respectively (Table 2). No significant difference was observed for tick and flea infestation according to sex and age of the opossums (p > 0.05).
The PCR analysis detected Anaplasmataceae DNA in 34.04% (16/47) pool samples of C. felis felis, and in 66.66% (2/3) pool samples of X. cheopis. The sequence analysis of these products and comparisons in the GenBank database revealed Wolbachia pipientis with query Cover of 100% and identity of 98% as compared with BLAST results (Accession numbers: KP114101.1, KP165047.1, MF944223.1, EF121345.1, and AY026912.1). Tick, blood and spleen samples scored negative for the microorganisms assessed.
Discussion
This study assessed the occurrence of ectoparasites as well as the detection of microorganisms infecting ticks and fleas of D. aurita from Southeastern Brazil. I. loricatus was the most frequent tick species found, infesting 41.38% of the animals. In fact, most studies on ectoparasites of Didelphis spp. found the adult stage of I. loricatus to be the most predominant tick species in these marsupials (Luz et al. 2018; Gonzalez et al. 2017; Oliveira et al. 2014; Saraiva et al. 2012; Dantas-Torres et al. 2012; Muller et al. 2005; Barros-Battesti et al. 2000). In contrast with these studies, when compared with I. loricatus, A. auricularium was reported with a higher frequency on D. albiventris in a study performed in Northeastern Brazil (Lopes et al. 2018). Additionally, Acosta et al. (2016) have reported only immature stages of Amblyomma spp. in D. aurita from the State of Espirito Santo, Brazil; however, few opossums of this species were inspected in that study.
Amblyomma sculptum was detected with a very low frequency in the animals analyzed in the present study. This tick species presents great public health importance, as it is the main vector of Rickettsia rickettsii, the etiological agent of the Brazilian Spotted Fever (Parola et al. 2013). The presence of Amblyomma spp. in D. aurita captured in urban areas has a great epidemiological importance, since this opossum act as an amplifier host for R. rickettsii infection to A. sculptum ticks (Horta et al. 2009). In our study, a single animal was found parasitized by an A. sculptum nymph. Indeed, most studies reported that larvae and nymphs of Amblyomma are the most common life stages retrieved in opossums (De Sá et al. 2018; Lopes et al. 2018; Acosta et al. 2016).
Regarding the flea species found in our study, C. felis felis was predominantly identified in D. aurita, with a frequency of 60.34%. This species, known as the cat flea, has been reported with high prevalence rates in Didelphis spp. (Horta et al. 2007; Boostrom et al. 2002; Barros-Battesti and Arzua 1997). However, Salvador et al. (2007) studying ectoparasites of D. aurita in the state of Santa Catarina, Brazil, found only Adoratopsylla intermedia, Leptopsylla segnis and Xenopsylla cheopis. The authors of that study suggested that the absence of C. felis felis in the studied population was due to the lack of human habitations in the studied area, which consequently reduce the contact of the wildlife with domestic animals. In fact, it is known that C. felis felis is the most prevalent flea species in dogs and cats worldwide (Durden and Hinkle 2019). In our study, the animals were captured in urban areas, and the high frequency of C. felis felis may suggest the exchange of this ectoparasite species between companion animals and D. aurita.
The detection of X. cheopis in the present study is a relevant finding. Salvador et al. (2007) found a higher rate of D. aurita infested by this flea species in the State of Santa Catarina, Brazil, with a frequency of 15.1%. Rodents are the main hosts of X. cheopis, and this species is involved in the transmission of important zoonotic pathogens such as the bacteria Yersinia pestis and Rickettsia typhi, the causative agents of Plague and Murine Typhus, respectively (Durden and Hinkle 2019; Civen and Ngo 2008). In addition, the presence of this flea in Didelphis spp. may have important implications in public health, as it is known that these opossums are probably involved in the zoonotic cycles of pathogens such as R. typhi and R. felis (Brown and Macaluso 2016; Boostrom et al. 2002).
Interestingly, DNA of W. pipientis was detected in C. felis felis and X. cheopis. It is known that this bacterium is an endosymbiont that infects a great number of insect species worldwide, which includes various flea species (Gorham et al. 2003; Werren and Windsor 2000) such as C. felis, C. canis, Tunga penetrans, Polygenis gwyni, Orchopeas howardi, Pulex irritans, P. simulans, Echidnophaga gallinacea, Stenoponia tripectinata tripectinata, and X. cheopis (Onder et al. 2019; Cevidanes et al. 2018; Zurita et al. 2016; Heukelbach et al. 2004; Dittmar and Whiting 2004; Rolain et al. 2003; Gorham et al. 2003). In fact, Dittmar and Whiting (2004) claim that there is a potentially widespread association between these bacterial symbionts with fleas in general. In addition, W. pipientis has influence on reproduction, sex determination, speciation and behavior of arthropods, being a potential candidate to biological control of insect vectors (LePage and Bordenstein 2013).
Conclusion
This study reports the occurrence of ticks and fleas in D. aurita opossums, as well as the infection by W. pipientis in siphonaptera of the species C. felis felis and X. cheopis collected in these animals in Southeastern Brazil. Data herein obtained demonstrates the parasitism in D. aurita by some species of ectoparasites, including specimens commonly found in domestic animals (C. felis felis and A. sculptum). These findings suggest that these arthropods circulate among wildlife and urban environments, which may implicate that they have important role in the cycle of zoonotic pathogens among opossums, humans and companion animals.
References
Acosta ICL, Martins TF, Marcili A, Soares HS, Krawczak FS, Vieira FT, Labruna MB (2016) Ticks (Acari: Ixodidae, Argasidae) from humans, domestic and wild animals in the state of Espírito Santo, Brazil, with notes on rickettsial infection. Vet Parasitol Reg Stud Rep 3(4):66–69
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Aragão H, Fonseca F (1961) Notas de Ixodologia VII Lista e Chave Para os Representantes da Fauna Ixodológica Brasileira. Mem Inst Oswaldo Cruz 59(2):115–129
Barros-Battesti D, Arzua M (1997) Geographical distribution by biomes of some Marsupial Siphonaptera from the State of Paraná Brazil. Mem Inst Oswaldo Cruz 92(4):485–486
Barros-Battesti D, Yoshinari NH, Bonoldi VLN, Gomes AC (2000) Parasitism by Ixodes didelphidis and I. loricatus (Acari: Ixodidae) on small wild mammals from an Atlantic Forest in the State of Sao Paulo Brazil. J Med Entomol 37(6):820–827
Barros-battesti DM, Arzua M, Bechara GH (2006) Carrapatos de Importância Médico-Veterinária da Região Tropical: Um guia ilustrado para identificação de espécies. USP/Fapesp, São Paulo
Bicho CL, Ribeiro PB (1998) Chave pictórica para as principais espécies de Siphonaptera de importância médica e veterinária, no Brasil. Rev Bras Parasitol Vet 7(1):47–51
Boostrom A, Beier MS, Macaluso JA, Macaluso KR, Sprenger D, Hayes J, Radulovic S, Azad AF (2002) Geographic association of Rickettsia felis-Infected opossums with human murine typhus, Texas. Emerg Infect Dis 8(6):549–554
Brown DL, Macaluso KR (2016) Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep 3:27–39
Cabrera AAC, Paula AA, Camacho LAB, Marzochi MCA, Xavier SC, Silva VM, Jansen AM (2003) Canine visceral leishmaniasis in Barra de Guaratiba, Rio de Janeiro, Brazil: assessment of risk factors. Rev Inst Med Trop São Paulo 45(2):79–83
Casati S, Sager H, Gern L, Piffaretti JC (2006) Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med 13:65–70
Cevidanes A, Cataldo S, Vera F, Lillo P, Millan J (2018) Molecular detection of vector-borne pathogens in rural dogs and associated Ctenophalides felis fleas (Siphonaptera: Pulicidae) in Easter Island (Chile). J Med Entomol 55(6):1659–1663
Civen R, Ngo V (2008) Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis 46:913–918
Costa FB, Costa AP, Moraes-Filho J, Martins TF, Soares HS, Ramirez DG, Dias RA, Labruna MB (2017) Rickettsia amblyommatis infecting ticks and exposure of domestic dogs to Rickettsia spp. in an Amazon-Cerrado transition region of northeastern Brazil. PLoS ONE 12(6):e0179163
Dantas-Torres F, Otranto D (2014) Dogs, cats, parasites, and humans in Brazil: opening the black box. Parasit Vectors 7:22
Dantas-Torres F, Aléssio FM, Siqueira DB, Mauffrey JF, Marvulo MFV, Martins TF, Moraes-Filho J, Camargo MCGO, Dauria SRN, Labruna MB, Silva JCR (2012) Exposure of small mammals to ticks and rickettsiae in Atlantic Forest patches in the metropolitan area of Recife, North-Eastern Brazil. Parasitology 139:83–91
De Sá EFGG, Rodrigues VS, Garcia MV, Zimmermann NP, Ramos VN, Blecha IMZ, Duarte PO, Martins TF, Bordignon MO, Andreotti R (2018) Ticks on Didelphis albiventris from a Cerrado area in the Midwestern Brazil. Syst Appl Acarol 23(5):935–945
Dittmar K, Whiting MF (2004) New Wolbachia Endosymbionts from nearctic and neotropical fleas (Siphonaptera). J Parasitol 90(5):953–957
Durden LA, Hinkle NC (2019) Fleas (Siphonaptera). In: Mullen GR, Durden LA (Eds.) Medical and veterinary entomology (3 Ed). Academic Press, Cambridge
Faria BF, Melo FR (2017) Didelphis imperfecta, Didelphimorphia, Didelphidae (Mondolfi & Pérez-Hernández, 1984): a new record in the Brazilian Amazon. Bol Soc Bras Mastozool 79:44–46
Gardner AL (2008) Mammals of South America, Volume I. Marsupials, xenarthrans, shrews, and bats. The University of Chicago Press, Chicago
Gonzalez IHL, Labruna MB, Chagas CRF, Salgado PAB, Monticelli C, Morais LH, Moraes AA, Antunes TC, Ramos PL, Martins TF (2017) Ticks infesting captive and free-roaming wild animal species at the São Paulo Zoo, São Paulo Brazil. Braz J Vet Parasitol 26(4):496–499
Gorham CH, Fang QQ, Durnen LA (2003) Wolbachia endosymbionts in fleas (Siphonaptera). J Parasitol 89(2):283–289
Heukelbach J, Bonow I, Witt L, Feidmeier H, Fischer P (2004) High infection rate of Wolbachia endobacteria in the sand flea Tunga penetrans from Brazil. Acta Trop 92:225–230
Horta MC, Labruna MB, Pinter A, Linardi PM, Schumaker TTS (2007) Rickettsia infection in five areas of the state of São Paulo, Brazil. Mem Inst Oswaldo Cruz 102(7):793–801
Horta MC, Moraes-Filho J, Casagrande RA, Saito TB, Rosa SC, Ogrzewalska M, Matushima ER, Labruna MB (2009) Experimental infection of opossums Didelphis aurita by Rickettsia rickettsii and evaluation of the transmission of the infection to ticks Amblyomma cajennense. Vector Borne Zoonotic Dis 9(1):109–117
Jansen AM (2002) Marsupiais Didelfídeos: gambás e cuícas. In: Andrade A, Pinto SC, Oliveira RS (eds) Animais de Laboratório: criação e experimentação. FIOCRUZ, Rio de Janeiro, pp 167–173
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
LePage D, Bordenstein S (2013) Wolbachia: can we save lives with a great pandemic? Trends Parasitol 29(8):385–393
Linardi PM (2006) Os ectoparasitos de marsupiais brasileiros. In: Cáceres NC, Monteiro-Filho ELA (eds) Os marsupiais do Brasil: biologia, ecologia e evolução. Editora da Universidade Federal do Mato Grosso do Sul, Campo Grande, pp 37–52
Linardi PM, Guimarães LR (2000) Sifonápteros do Brasil. Museu de Zoologia USP/Fapesp, São Paulo
Lopes MG, Muñoz-Leal S, De Lima JTR, Fournier GFSR, Acosta ICL, Martins TF, Ramirez DG, Gennari SM, Labruna MB (2018) Ticks, rickettsial and erlichial infection in small mammals from Atlantic forest remnants in Northeastern Brazil. Int J Parasitol Parasites Wildl 7:380–385
Luz HR, Faccini JLH, Landulfo GA, Sampaio JS, Neto SFC, Famadas KM, Onofrio VC, Barros-Battesti DM (2013) New host records of Ixodes luciae (Acari: Ixodidae) in the State of Pará Brazil. Braz J Vet Parasitol 22(1):152–154
Luz HR, Neto SFC, Weksler M, Gentile R, Faccini JLH (2018) Ticks parasitizing wild mammals in Atlantic Forest areas in the state of Rio de Janeiro, Brazil. Braz J Vet Parasitol 27(3):409–414
Martin AR, Brown GK, Dunstan RH, Roberts TK (2005) Anaplasma platys: an improved PCR for its detection in dogs. Exp Parasitol 109:176–180
Moraes LB, Bossi DEP, Linhares AX (2003) Siphonaptera parasites of wild rodents and marsupials trapped in three mountain ranges of the Atlantic Forest in Southeastern Brazil. Mem Inst Oswaldo Cruz 98(8):1071–1076
Morrant DS, Petit S, Schumann R (2010) Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercartetus concinnus, at Innes National Park, South Australia. Ecol Res 25:579–589
Muller G, Brum JGW, Langone PQ, Michels GH, Sinkoe AL, Ruas JL, Berne MEA (2005) Didelphis albiventris Lund, 1841, parasitado por Ixodes loricatus Neuman, 1899, e Amblyomma aureolatum (Pallas, 1772) (Acari: Ixodidae) no Rio Grande do Sul. Arq Inst Biol 72:319–324
Oliveira HH, Gomes V, Amorim M, Gazeta GS, Serra-Freire NM, Quinelato IPF, Morelli-Amaral VF, Almeida AB, Carvalho RW, Carvalho AG (2014) Diversidade de ixodida em roedores e marsupiais capturados no Parque Estadual da Pedra Branca, Rio de Janeiro, Brasil. Arq Bras Med Vet Zootec 66(4):1097–1104
Onder Z, Ciloglu A, Duzlu O, Yildirim A, Okur M, Yetismis G, Inci A (2019) Molecular detection and identification of Wolbachia endosymbiont in fleas (Insecta: Siphonaptera). Folia Microbiol 64(6):789–796. https://doi.org/10.1007/s12223-019-00692-5
Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, Abdad MY, Stenos J, Bitam I, Fournier PE, Raoult D (2013) Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 26:657–702
Pestell AJL, Petit S (2007) Methods and ethical considerations of pitfall trapping for the western pygmy possum Cercartetus concinnus Gould (Marsupialia: Burramyidae), with observations on capture patterns and nest sites. Wildl Res 34:296–305
Pinto IS, Botelho JR, Costa LP, Leite YLR, Linardi PM (2009) Siphonaptera associated with wild mammals from the Central Atlantic forest biodiversity corridor in Southeastern Brazil. J Med Entomol 46(5):1146–1151
Ramos RAN, Campbell BE, Whittle A, Lia RP, Montarsi F, Parisi A, Danta-Torres F, Wall R, Otranto D (2015) Occurrence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus (Acari: Ixodidae) in Southern Italy. Ticks Tick-Borne Dis 6(3):234–236
Regnery RL, Spruill CL, Plikaytis BD (1991) Genotypic identification of rickettsiae and estimation of interspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 173:1576–1589
Rolain J, Franc M, Davoust B, Raoult D (2003) Molecular Detection of Bartonella quintana, B. koehlerae, B. henselae, B. clarridgeiae, Rickettsia felis, and Wolbachia pipientis in Cat Fleas, France. Emerg Infect Dis 9(3):338–342
Salvador CH, Carvalho-Pinto C, Carvalho R, Graipel ME, Simões-Lopes P (2007) Interação parasito-hospedeiro entre ectoparasitos (Ixodida & Siphonaptera) e gambás Didelphis aurita Wied-Neuwied, 1826 (Mammalia: Didelphimorphia), no continente e em ilhas do litoral de Santa Catarina, Sul do Brasil. Biotemas 20(4):81–90
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 74:5463–5467
Saraiva DG, Fournier GFSR, Martins TF, Leal KPG, Vieira FN, Câmara EMVC, Costa CG, Onofrio VC, Barros-Battesti DM, Guglielmone AA, Labruna MB (2012) Ticks (Acari: Ixodidae) associated with small terrestrial mammals in the state of Minas Gerais, Southeastern Brazil. Exp Appl Acarol 58:159–166
Skotarczak B, Wodecka B, Hermanowska-Szpakowicz T (2002) Sensitivity of PCR method for detection of DNA of Borrelia burgdorferi sensu lato in different isolates. Przeglad Epidemiol 56:73–79
Urdapilleta M, Linardi PM, Lareschi M (2019) Fleas associated with sigmodontine rodents and marsupials from the Paranaense Forest in Northeastern Argentina. Acta Trop 193:71–77
Werren JH, Windsor DM (2000) Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc Lond 267:1277–1285
Zurita A, Gutièrrez SG, Cutillas C (2016) Infection Rates of Wolbachia sp. and Bartonella sp. in Different Populations of Fleas. Curr Microbiol 73:704–713
Acknowledgements
Authors would like to thank the Professors Dr. Marcelo Bahia Labruna and Dr. Adivaldo Henrique da Fonseca who kindly donated the positive controls for Rickettsia and Borrelia spp., respectively. Secondly, authors acknowledge Professor Gisele Mendes Lessa del Giúdice for providing traps to capture the animals used in our study. Finally, the authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and the Graduate Program in Veterinary Medicine of the Universidade Federal de Viçosa (UFV) for the support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures herein performed were in accordance with the ethical standards of the institution or practice at which the study was conducted, as approved by the Ethics Committee for Animal Experimentation (ECAE) of the Universidade Federal de Viçosa (license number: 80/2018) and by the Biodiversity Information and Authorization System (SISBIO) of the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) under the license number 64930-1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bezerra-Santos, M.A., Nogueira, B.C.F., Yamatogi, R.S. et al. Ticks, fleas and endosymbionts in the ectoparasite fauna of the black-eared opossum Dipelphis aurita in Brazil. Exp Appl Acarol 80, 329–338 (2020). https://doi.org/10.1007/s10493-020-00468-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00468-4