Abstract
Argas vespertilionis is a geographically widespread haematophagous ectoparasite species of bats in the Old World, with a suspected role in the transmission of Babesia vesperuginis. The aims of the present study were (1) to molecularly screen A. vespertilionis larvae (collected in Europe, Africa and Asia) for the presence of piroplasms, and (2) to analyze mitochondrial markers of A. vespertilionis larvae from Central Asia (Xinjiang Province, Northwestern China) in a phylogeographical context. Out of the 193 DNA extracts from 321 A. vespertilionis larvae, 12 contained piroplasm DNA (10 from Hungary, two from China). Sequencing showed the exclusive presence of B. vesperuginis, with 100% sequence identity between samples from Hungary and China. In addition, A. vespertilionis cytochrome oxidase c subunit 1 (cox1) and 16S rRNA gene sequences had 99.1–99.2 and 99.5–100% similarities, respectively, between Hungary and China. Accordingly, in the phylogenetic analyses A. vespertilionis from China clustered with haplotypes from Europe, and (with high support) outside the group formed by haplotypes from Southeast Asia. This is the first molecular evidence on the occurrence of B. vesperuginis in Asia. Bat ticks from hosts in Vespertilionidae contained only the DNA of B. vesperuginis (in contrast with what was reported on bat ticks from Rhinolophidae and Miniopteridae). Molecular taxonomic analyses of A. vespertilionis and B. vesperuginis suggest a genetic link of bat parasites between Central Europe and Central Asia, which is epidemiologically relevant in the context of any pathogens associated with bats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bats (Chiroptera) represent the second largest order of mammals, with nearly 20% of living mammalian species (Wilson and Reeder 2005). They can be found all over the globe, except the polar regions (Altringham 1996). In the past few decades the eco-epidemiological importance of bats has become increasingly recognized, because they can be natural reservoirs and host of viruses, bacteria and parasites (Klimpel and Mehlhorn 2014). In this context, particularly zoonotic agents deserved attention, owing to the high population density and roosting behavior of bats, as well as to their occurrence in urban habitats, even in man-made buildings. The migratory habit of certain bat species further contributes to their role in disease transmission, implicating them in the long distance transportation of relevant pathogens. Infections can be contracted from bats in several ways, including direct contact or through blood-sucking arthropod vector species. Considering the latter, one of the epidemiologically most important haematophagous ectoparasites of bats is the bat soft tick Argas vespertilionis, because it was also reported to infest humans and domestic animals (Hoogstraal 1956; Jaenson et al. 1994; Manzano-Román et al. 2012).
Several categories of vector-borne protozoa may occur in the blood of bats, including trypanosomes and piroplasms (Gardner et al. 1987). Babesia species (Apicomplexa: Piroplasmida) are intra-erythrocytic piroplasms with more than 100 species described in birds and mammals (Hunfeld et al. 2008), of which only B. vesperuginis is known to infect bats. Babesia vesperuginis was reported for the first time from Nyctalus noctula in Italy (Dionisi 1898), then from Pipistrellus pipistrellus, Myotis nattereri, M. daubentonii, M. mystacinus, N. noctula and Plecotus auritus in the Netherlands (Goedbloed et al. 1964). British studies found B. vesperuginis in the blood of Pi. pipistrellus and M. mystacinus (Gardner and Molyneux 1987; Concannon et al. 2005). Bats infected with this piroplasm naturally or experimentally showed reduced hemoglobin levels and splenomegaly, justifying the pathogenic nature of B. vesperuginis (Gardner and Molyneux 1987). In the latter surveys the only ectoparasites found on bats were larvae of Argas vespertilionis, therefore the vector role of this soft tick species was postulated in the transmission of B. vesperuginis.
Babesia vesperuginis has been recently identified in Central Europe (Hornok et al. 2016). Simultaneously with this species, DNA from a high diversity of piroplasms were identified molecularly in ixodid bat ticks (Hornok et al. 2016). In addition, a high number of argasid soft ticks (A. vespertilionis) have been collected in three European countries, and were used for taxonomic/phylogeographic comparison with specimens from Southeastern Asia (Vietnam) and Africa (Kenya) (Hornok et al. 2017). Therefore, the aim of the present study was to molecularly screen large numbers of soft tick larvae for piroplasms, in order to investigate if they carry a similar diversity of piroplasm DNA as do ixodid bat ticks. In addition, because A. vespertilionis larvae from Central Asia were not included in the above phylogeographical study, it was also within the scope of this study to molecularly analyze soft tick larvae from Northwestern China in the same context, i.e. to compare their two mitochondrial genetic markers with conspecific larvae from Europe and Vietnam.
Materials and methods
Sample collection
In this study 321 A. vespertilionis larvae were examined. The majority (310 larvae) were collected from 15 bat species in Hungary, Romania, Italy, Kenya and Vietnam as recently reported (Hornok et al. 2017). In addition, 11 A. vespertilionis larvae collected from Vespertilio murinus in Northwestern China (Xinjiang province) in 2016 were also included. Permission for bat capture was provided by the National Inspectorate for Environment, Nature and Water (Hungary), the Vietnamese Ministry of Agriculture and Rural Development (Vietnam Administration of Forestry), School of Medicine, Shihezi University (China) and the Underground Heritage Commission (Romania). Bat banding license numbers are 59/2003 (PE), 305/2015 (ADS), TMF-14/32/2010 (DK) and TMF-493/3/2005 (TG).
DNA extraction and molecular analyses
DNA was extracted from A. vespertilionis larvae individually or in small pools (i.e. two or three larvae collected from the same host individual) with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instruction, including an overnight digestion at 56 °C in tissue lysis buffer containing Proteinase-K. In order to monitor cross-contamination of samples, extraction controls (phosphate-buffered saline solution without larvae) were processed parallel with each set of samples.
The resultant 193 DNA extracts (Table 1) were first molecularly screened for the presence of piroplasm DNA with a conventional PCR (Hornok et al. 2016). In brief, this PCR amplifies an approx. 500 bp long part of the 18S rRNA gene of Babesia/Theileria spp., using the primers BJ1 (forward: 5′-GTC TTG TAA TTG GAA TGA TGG-3′) and BN2 (reverse: 5′-TAG TTT ATG GTT AGG ACT ACG-3′). In addition, two soft tick mitochondrial markers were amplified from the DNA of four A. vespertilionis larvae collected in China: a 710 bp long fragment of the cytochrome oxidase c subunit 1 (cox1) gene, and an approx. 460 bp part of the 16S rRNA gene (Hornok et al. 2017).
PCR products were visualized in 1.5% agarose gel. Purification and sequencing were done by Biomi Inc. (Gödöllő, Hungary). Obtained sequences were manually edited, then aligned and compared to reference GenBank sequences by nucleotide BLASTN program (https://blast.ncbi.nlm.nih.gov). Representative sequences were submitted to GenBank (accession numbers: KY657241-2 for B. vesperuginis from A. vespertilionis collected in Hungary and China, respectively; KY657239-40 for A. vespertilionis collected in China, cox 1 and 16S rRNA genes, respectively). Phylogenetic analyses of soft tick mitochondrial markers were conducted with the Maximum Likelihood method and Tamura-Nei model (cox1 gene) or Hasegawa-Kishino-Yano model (16S rRNA gene) by using MEGA version 6.0 (Hornok et al. 2017).
Results
Piroplasm DNA in Argas vespertilionis
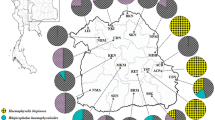
Based on the PCR amplifying part of the 18S rRNA gene, 12 samples contained the DNA of piroplasms: 10 from Hungary and two from China. In Hungary, PCR positive A. vespertilionis larvae originated from three locations, from three individuals of bats belonging to the following species: (1) E. serotinus (yielding 12 larvae, of which three pools of two larvae and two individual samples were positive, amounting to 42–67% prevalence among larvae from this single host); (2) Pl. austriacus (yielding six larvae, of which two pools of two larvae and two individual samples were positive, amounting to 67–100% prevalence among larvae from this single host); and (3) Pi. pipistrellus (yielding one larva, which was positive). In China, two individual DNA samples from A. vespertilionis (collected from two bat hosts, V. murinus) contained piroplasm DNA. Sequencing of all 12 PCR positive samples revealed the exclusive presence of B. vesperuginis, with 100% identity (448/448 bp) between samples from Hungary and China.
Mitochondrial marker analysis of Argas vespertilionis from Northwestern China (Xinjiang)
Argas vespertilionis cox1 sequences had 5–6 nucleotide (0.8–0.9%) differences, i.e. 99.1–99.2% (646–647/652 bp) similarity between isolates from Hungary and China. On the other hand, haplotypes from China had 45–48 nucleotide (6.9–7.4%) differences from A. vespertilionis larvae collected in Vietnam, meaning 92.6–93.1% (604–607/652 bp) similarity with the latter. The cox1 phylogenetic tree (Fig. 1) reflected these relationships, i.e. A. vespertilionis from Hungary and China clustered together, but separately (with a high, 99% bootstrap support) from those collected in Vietnam.
Phylogenetic tree of cytochrome oxidase c subunit 1 (cox1) gene of Argas vespertilionis based on reference sequences in GenBank. Accession number of the sequence from China (this study) is shown in inverse purple. Branch lengths represent the number of substitutions per site inferred according to the scale shown
Argas vespertilionis 16S rRNA gene sequences had 0–2 nucleotide (up to 0.5%) differences (438–440/440 bp = 99.5–100% similarity) between haplotypes from Hungary and China, whereas these had 23–24 nucleotide (5.2–5.4%) differences (418/441–442 bp = 94.6–94.8% similarity) from A. vespertilionis larvae collected in Vietnam. Based on the 16S rRNA phylogenetic tree (Fig. 2), A. vespertilionis from China belonged to the group formed by specimens reported from three European countries (Hungary, Romania and Italy), but the separation of A. vespertilionis collected in China versus Vietnam was highly supported (100%).
Discussion
To the best of the authors’ knowledge, this is the first molecular evidence on the occurrence of Babesia vesperuginis in Asia. In a previous study, ixodid ticks were collected from bats of three families, out of which B. vesperuginis DNA was only detected in hard ticks (Ixodes ariadnae and I. vespertilionis) from bats in Vespertilionidae, whereas DNA of other piroplasms (infecting ruminants and dogs) were shown to be present in ixodid ticks (I. vespertilionis and I. simplex) from Rhinolophidae and Miniopteridae (Hornok et al. 2016). This is in line with findings of the present study, because (1) up to now, despite extensive examinations of rhinolophid and miniopterid bats in Hungary (data not shown), A. vespertilionis was only found on members of Vespertilionidae (Table 1), and (2) in a high number of soft tick larvae from Vespertilionidae (investigated here for the presence of piroplasm DNA) only B. vesperuginis was detected. The most likely explanation for this phenomenon is (accepting that the most likely vector of B. vesperuginis is A. vespertilionis) that vespertilionid bats acquire B. vesperuginis from its biological soft tick vector, whereas Rhinolophidae/Miniopteridae species have access to a broader range of piroplasms or their DNA from relevant mechanical vectors in their food (Hornok et al. 2016).
Based on previous results (Hornok et al. 2016), B. vesperuginis 18S rRNA gene sequences were identical within Europe, i.e. between Hungary, Romania (KU958544) and the UK (AJ871610). Based on the sequence analysis performed here, the 18S rRNA gene of B. vesperuginis also appears to be highly conserved over much larger geographical distances (i.e. 5000 km between Hungary in Central Europe and Xinjiang in Central Asia). This is in contrast to several other Babesia spp., as exemplified by B. canis with different 18S rRNA genotypes within countries (e.g. KP835549-50 in Hungary: Hornok et al. 2015; KU958551-2 in Romania: Hornok et al. 2016).
The bat fauna of the Northern Palearctic is characterized by two isolated complexes, the European-Ural and the Siberian-Far Eastern (Orlova 2014). As already suggested, a similar pattern of spatial distribution also exists among ectoparasites found on relevant bat species in Northern Eurasia, and bat ectoparasites found on (common) hosts from these different faunistic complexes may elucidate the possibility of contacts between the European and Siberian parts (Orlova 2014). While studying this concept, it has to be taken into account that postglacial recolonization of Europe by several small Myotis spp. (including important hosts of A. vespertilionis, e.g. M. alcathoe, M. brandtii) occurred from the eastern direction, from the region of Caucasus (Dietz and Kiefer 2016), and historically this may have also contributed to the observed genetic homogeneity of bat ticks and a tick-borne pathogen between Central Europe and Central Asia. More recently, the West Siberian Plain acted as a barrier (segregating North Palearctic Chiroptera faunae), but during the past decades human settlements became more expanded into this area and non-migratory bat species became provided with increasing numbers of anthropogenic shelters, favoring the spread of bats between the West and East Palearctic (Orlova 2014). Some bat species that migrate middle range distances (e.g. M. daubentonii, a common host of B. vesperuginis), occur across the Northern Palearctic from Europe to the Far East (Bogdanowicz 1994; Dietz and Kiefer 2016). In addition, there are long-distance migratory (transpalearctic) species which may even cross this vast region, such as M. dasycneme, V. murinus and E. nilssonii (Orlova 2014)—the first two being important host species of A. vespertilionis (Hornok et al. 2017).
Vespertilio murinus, which carried genetically closely related A. vespertilionis larvae in Central Europe and Northwestern China as demonstrated here, has a broad Palearctic range (from Europe to Siberia and the Pacific coast). It shows relative genetic uniformity (below 1% cox1 sequence divergence) across this region (Kruskop et al. 2012), and has a parapatric distribution with its eastern congener V. sinensis. In addition, V. murinus colonies in Asia are frequently associated with human settlements (buildings), and their eastward expansion may have been linked historically with the spread of human-altered habitats (Kruskop et al. 2012). This bat species can migrate both in latitudinal and meridional directions (Orlova 2014). These background factors could thus allow gradual gene flow (mixing) between distant European and Central Asian populations of A. vespertilionis while associated with this bat host species, as suggested by the present results.
In contrast to this, both mitochondrial markers analyzed here (cox1 and 16S rRNA gene) indicate high genetic difference (i.e. reduced gene flow) between A. vespertilionis from Central Asia and Southeastern Asia (represented by Vietnam) over a shorter distance. This limited genetic exchange is most likely due to the presence of geographical barriers (high mountain ranges of the Himalayas and the Tibetan plateau), separating these regions and preventing overbridging of A. vespertilionis populations by bat hosts.
In summary, molecular analyses of A. vespertilionis suggest a genetic link of bat parasites (soft ticks and piroplasms) between Central Europe and Central Asia through the North Paleartic. Other flying vertebrates, i.e. birds had already been incriminated in spreading tick-borne pathogens in a similar geographical context, i.e. between the Far East, Siberia and Europe (Moskvitina et al. 2014; Ponomareva et al. 2015). Therefore, the present results draw the attention to the connectedness of these regions of Eurasia, which is relevant and should be taken into account whenever considering epidemiological scenarios associated with (not only tick-borne) pathogens of bats.
References
Altringham JD (1996) Bats: biology and behaviour. Oxford University, Oxford, p 272
Bogdanowicz W (1994) Myotis daubentonii. Mammalian Species 475:1–9
Concannon R, Wynn-Owen K, Simpson VR, Birtles RJ (2005) Molecular characterization of haemoparasites infecting bats (Microchiroptera) in Cornwall, UK. Parasitology 131:489–496
Dietz C, Kiefer A (2016) Bats of Britain and Europe. Bloomsbury Natural History, London, p 400
Dionisi A (1898) Les parasites endoglobulaires des chauves-souris. Atti della Reale Academia dei Lincei 7:153–156
Gardner RA, Molyneux DH (1987) Babesia vesperuginis: natural and experimental infections in British bats (Microchiroptera). Parasitology 95:461–469
Gardner RA, Molyneux DH, Stebbings RE (1987) Studies on the prevalence of haematozoa of British bats. Mammal Rev 17:75–80
Goedbloed E, Cremers-Hoyer L, Perié NM (1964) Blood parasites of bats in the Netherlands. Ann Trop Med Parasitol 58:257–260
Hoogstraal H (1956) Argas vespertilionis. In: African Ixodoidea I. Ticks of the Sudan. Department of the Navy, Bureau of Medicine and Surgery, Washington DC, pp 104–111
Hornok S, Estók P, Kováts D, Flaisz B, Takács N, Szőke K, Krawczyk A, Kontschán J, Gyuranecz M, Fedák A, Farkas R, Haarsma A-J, Sprong H (2015) Screening of bat faeces for arthropod-borne apicomplexan protozoa: Babesia canis and Besnoitia besnoiti-like sequences from Chiroptera. Parasites Vectors 8:441
Hornok S, Szőke K, Kováts D, Estók P, Görföl T, Boldogh SA, Takács N, Kontschán J, Földvári G, Barti L, Corduneanu A, Sándor AD (2016) DNA of Piroplasms of ruminants and dogs in Ixodid bat ticks. PLoS ONE 11(12):e0167735
Hornok S, Szőke K, Tu TV, Kontschán J, Takács N, Sándor DA, Halajian A, Földvári G, Estók P, Plantard O, Epis S, Görföl T (2017) Mitochondrial gene heterogeneity of the bat soft tick Argas vespertilionis (Ixodida: Argasidae) in the Palaearctic. Parasites Vectors 10:109
Hunfeld KP, Hildebrandt A, Gray JS (2008) Babesiosis: recent insights into an ancient disease. Int J Parasitol 38(11):1219–1237
Jaenson TG, Tälleklint L, Lundqvist L, Olsen B, Chirico J, Mejlon H (1994) Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol 31:240–256
Klimpel S, Mehlhorn H (2014) Bats (Chiroptera) as vectors of diseases and parasites: facts and myths. In: Parasitology research monographs, vol 5. Springer, Berlin, p 187
Kruskop VS, Borisenko VA, Ivanova VN, Lim KB, Eger LJ (2012) Genetic diversity of northeastern Palaearctic bats as revealed by DNA barcodes. Acta Chiropterol 14(1):1–14
Manzano-Román R, Díaz-Martín V, de la Fuente J, Pérez-Sánchez R (2012) Soft ticks as pathogen vectors: distribution, surveillance and control. In: Manjur Shah M (ed) Parasitology. http://www.intechopen.com/books/parasitology/soft-ticks-as-pathogen-vectors-distribution-surveillance-and-control. Accessed 10 March 2017
Moskvitina SN, Korobitsyn GI, Tyutenkov OY, Gashkov IS, Kononova VY, Moskvitin SS, Romanenko NV, Mikryukova PT, Protopopova VE, Kartashov YM, Chausov VE, Konovalova NS, Tupota LN, Sementsova OA, Ternovoy AV, Loktev BV (2014) The potential role of migratory birds in the spread of Tick-borne infections in Siberia and the Russian Far East. Achiev Life Sci 8(2):118–120
Orlova M (2014) Invasion of specific ectoparasites of Siberian-Far Eastern bat species to the Urals. Russ J Biol Invasions 5(1):29–31
Ponomareva PE, Mikryukova PT, Gori VA, Kartashov YM, Protopopova VE, Chausov VE, Konovalova NS, Tupota LN, Gheorghita DS, Burlacu IV, Ternovoi AV, Loctev BV (2015) Detection of Far-Eastern subtype of tick-borne enchephalitis viral RNA in ticks collected in Republic of Moldova. J Vector Borne Dis 52:334–336
Wilson DE, Reeder DM (2005) Mammal species of the world: a taxonomic and geographic reference, vol 2, 3rd edn. Johns Hopkins University Press, Baltimore, p 2142
Acknowledgements
The study was organized in the frame of EurNegVec COST Action TD1303. Financial support was provided by OTKA K115854, OTKA K112440 and Domus Hungarica. G. F. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hornok, S., Szőke, K., Görföl, T. et al. Molecular investigations of the bat tick Argas vespertilionis (Ixodida: Argasidae) and Babesia vesperuginis (Apicomplexa: Piroplasmida) reflect “bat connection” between Central Europe and Central Asia. Exp Appl Acarol 72, 69–77 (2017). https://doi.org/10.1007/s10493-017-0140-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0140-z