Abstract
The cold hardiness of the broad mite, Polyphagotarsonemus latus, a key pest in Rhododendron simsii hybrid production in northwestern Europe, was investigated in the laboratory. Survival of eggs, larvae and female adults and reproduction capacity of female P. latus were evaluated following cold exposure at 7 °C. Adult females were also exposed to temperatures of 2 and −3 °C. Further, the supercooling point and lower lethal times of adult females were determined. No eggs survived exposure to 7 °C for 17 or more days. Larval survival upon the cold treatment decreased from 53 to 13 % when exposed to 7 °C for 14 and 49 days, respectively. Two-day-old adult females exposed to 7 °C for up to 42 days did not suffer significant mortality, but when returned to 25 °C their oviposition rates were lower than those of mites maintained at 25 °C. Less than 40 % of females exposed for 13 days to 2 °C survived; only 20 % of these females was able to reproduce upon recovery. Subzero temperatures dramatically decreased survival and reproduction capacity of adult females. The supercooling point of female adults was −16.5 °C. Median lethal times averaged 61.2 h and 9.3 days at −3 and 2 °C, respectively. In conclusion, a long term exposure (up to 6 weeks) of R. simsii plants infested with P. latus to a temperature of 7 °C, which is required for breaking dormancy of the flowers, is not expected to have detrimental effects on the survival and reproductive performance of the female mites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae), is a pest of numerous crops in many parts of the world, including the tropical and subtropical climates of Australia, Asia, Africa, the Americas and the Pacific Islands (Gerson 1992). In temperate and subtropical areas the broad mite is a pest of greenhouse crops (Waterhouse and Norris 1987). According to Navajas et al. (2010), P. latus was first recorded in Europe (Italy) in 1961. Since then, the mite has also been found in a number of northwestern European countries, including Belgium, where it was reported for the first time on pot azalea, Rhododendron simsii, in the mid-1980s (Heungens 1986). The broad mite feeds on the youngest growth tips of R. simsii, causing malformation of the flower buds and the terminal leaves. The latter become severely stunted and hardened, curl down at the edges, and show brownish or reddish lower surfaces. Like in many other ornamental crops, the mite’s feeding behavior on R. simsii results in severe economic damage (Labanowski and Soika 2006).
Rhododendron simsii hybrids are the most important flowering pot plants produced in Belgium and are made available nearly year-round. Climatic conditions during the production process differ depending on the plant growth phase. The production process starts by rooting cuttings under a plastic cover during 2 months at a soil temperature of 22–25 °C. The plants are then grown vegetatively during several months in a greenhouse or in summer outside on container fields. Flower initiation takes place in June; at this stage temperature is less controlled. During winter, plants are protected from freezing temperatures. Exposure of the plants to the lower temperatures during winter is necessary to release the flowers from their dormant state. Unfulfilled chilling requirements lead to erratic bud break and low-quality flowering (Christiaens et al. 2014). Temperatures for dormancy breaking are generally between 2.5 and 9 °C (Richardson et al. 1974), and the optimum for R. simsii hybrids is 7 °C (Stuart 1965). In commercial plant production the material is placed in cold storage rooms at 7 °C during 4–6 weeks. The plants are then forced to flower in a heated greenhouse at a constant temperature of ca. 21 °C for 10–21 days. To speed up this process additional light (ca. 40 µmol m−2 s−1, day length 16–20 h) can be supplied during dark periods of the year.
Luypaert et al. (2014) estimated the relationship between temperature and developmental rate of P. latus on R. simsii ‘Nordlicht’ using a linear and two non-linear models. Thresholds for development of P. latus were estimated to be 10 and 36 °C for the lower and upper developmental threshold, respectively. Earlier, this relationship was studied based on a linear model using chili pepper (Li et al. 1985) and Vitis vinifera (Ferreira et al. 2006) as host plants. Besides these studies, there is very little information concerning the relationship between temperature and life history of P. latus, and in particular the cold hardiness of the pest has not been studied in detail. Karl (1965, cited in Gerson 1992) stated that P. latus could not survive outside of a tropical, subtropical or controlled greenhouse environment. More recently, Vincent et al. (2010) reconsidered the hypothesis that P. latus can also be a pest in subtropical climates or controlled environments, based on the fact that infestation on primocane-fruiting blackberry (Rubus rubus) continued during a 3-year period in the northern parts of Arkansas (USA).
The objective of the present laboratory study was to gain insight into the cold hardiness of P. latus, as a key pest of pot azalea in northwestern Europe. The egg hatchability, survival of larvae and female adults and reproduction capacity of females following a prolonged period of cold exposure was determined. The supercooling point (SCP) of female adult mites was measured and lower lethal times (LTime10,50,90) were estimated when the females were exposed to −3 or 2 °C.
Materials and methods
Plant material and Polyphagotarsonemus latus culture
Polyphagotarsonemus latus mites were taken from a colony started by placing naturally infested R. simsii hybrids in a greenhouse. The minimum temperature in the greenhouse was set at 21 °C, ventilation was activated starting at 26 °C. The photoperiod was 16:8 h (L:D). When light intensity was below 100 W m−2, additional assimilation light (50 µmol m−2 s−1) was given. When light exceeded 200 W m−2, shades were unrolled to reduce the light intensity below 200 W m−2.
Young leaves from newly emerging shoots of uninfested R. simsii ‘Nordlicht’ plants were used in the experiments to produce leaf discs. Clean plant material was kept in a separate greenhouse with a minimum temperature of 5 °C, ventilation temperature set at 20 °C and no supplementary assimilation light.
Survival and reproduction capacity following a cold exposure period
To ensure that all experimental mites developed under equal conditions, the following method was used. Using a fine brush, P. latus females were transferred from the stock colony in the greenhouse to the leaf discs, at a density of 1 female per leaf disc. Leaves were placed with the adaxial side on water-saturated tissue paper and bordered with water-saturated tissue paper inside 10-cm diameter insect breeding dishes (SPL Life Sciences, Pocheon, Korea) to produce leaf discs. The total leaf surface area available for the mites was 1.8 cm2 (1 × 1.8 cm). The water-saturated tissue paper prevented the mites from escaping. Adult females were given 24 h for oviposition and were subsequently removed from the leaf discs. Leaf discs containing eggs were transferred to an incubator (Vötsch-Industrietechnik Bio Line model VB 1014, Balingen-Frommern, Germany) at a constant temperature of 25 ± 1 °C, 80 ± 5 % relative humidity (RH), a 16 h photoperiod and a light intensity of 80–100 µmol m−2 s−1 (lamps: TL-D 36 W/33-640 1SL, Philips, Eindhoven, the Netherlands). Leaf discs were moistened daily to prevent the mites from escaping. Given the variation in oviposition and egg hatching success, 80 leaf discs were set up initially. Two-day-old adult females were transferred to new individual leaf discs at the beginning of the experiments. Experimental eggs and larvae were reared under the same conditions. Eggs and larvae <24 h old were collected from leaf discs infested with adult mites.
Polyphagotarsonemus latus mites were exposed to a 7 °C cold treatment, corresponding with the optimum temperature for dormancy breaking of pot azalea (Stuart 1965). The tested exposure periods for eggs were 1, 17, 31 and 52 days (corresponding with the 6–7 week timespan for cold storage of azalea plants). Larvae were exposed for periods of 14, 28 and 49 days, whereas 2-day-old adult females were exposed to 7 °C for 14, 28 and 42 days. Hatchability of eggs returned to rearing conditions [25 °C, 80 % RH and 16:8 h (L:D)] was monitored every 24 h for a 6 days period. The survivorship of exposed larvae was assessed 24 h and 7 days after the cold exposure. Likewise, survival of the exposed adults and the percentage of reproducing females was monitored 24 h and 7 days after the end of the cold period. The oviposition rate of the surviving females was calculated over a 2 day-period following cold exposure.
The cyclamen mite, Phytonemus pallidus (Banks) (Acari: Tarsonemidae), another plant feeding tarsonemid mite, has been noted to overwinter in the adult stage in temperate climates (Denmark 1988). Therefore, adult P. latus females were also exposed to temperatures of 2 or −3 °C. At 2 °C the time periods chosen were 6, 13 or 26 days, whereas at −3 °C the mites were exposed for 24 or 72 h, or 7 days. As before, survival of exposed adults and the percentage of reproducing females was monitored 24 h and 7 days after having been returned to rearing conditions. Oviposition rates of females surviving the −3 or 2 °C cold treatments were recorded as described above and compared with those of non-exposed females taken from the stock colony.
The mites were exposed to the cold temperatures (−3, 2 or 7 °C) in a cooled incubator (MIR-254, Panasonic Healthcare, Tokyo, Japan) without light. Humidity during cold exposure was not controlled, but given the fact that leaf discs were kept on water-saturated tissue paper relative humidity was assumed to be around 90 % throughout the trials (Luypaert et al. 2014). Mites were allowed to acclimatize for 20 min at different intermediate temperatures (18, 10 °C and, for exposure to −3, 2 °C) before and after exposure to the tested temperatures, in order to prevent cold and heat shock mortality, respectively. All observations were made using a stereo microscope (Leica MZ6, Leica, Wetzlar, Germany).
Lower lethal times
Lower lethal times (LTime10,50,90) were estimated for 2-day-old adult females by placing leaf discs with a single 2-day-old adult female at −3 or 2 °C for a varying amount of time. Calculations are based on the averages of two independent experiments with ten replicates. The cold exposure period varied between 0–96 h and 0–17 days for −3 and 2 °C, respectively. To cover survival ranging from 100 to 0 % at each temperature, the mites were exposed during 5 (0, 24, 48, 72 and 96 h) or 6 (0, 3, 7, 10, 14 and 17 days) different durations at temperatures of −3 or 2 °C, respectively. After the cold exposure period, the mites were returned to a temperature of 25 °C. Again, the mites were allowed to acclimatize by placing them at intermediate temperatures (18, 10 °C and, for exposure to −3, 2 °C) for 20 min. Mortality of the mites was assessed 24 h after recovery. Mites that were unable to move, upon prodding with a fine brush, were scored dead and the durations corresponding to 10, 50 or 90 % mortality (i.e., LTime10,50,90) were estimated.
Supercooling points
Individual 2-day-old P. latus females were attached to a type-T 30-gauge copper-constantan thermocouple after it was dipped in high vacuum grease (Silicone high vacuum grease medium, Merck, Darmstadt, Germany). The thermocouple was attached to a data logger (TC-08 Thermocouple Data Logger, Pico Technology, Eaton Socon, UK) which recorded the temperature of the probe at 100-ms intervals. The adults attached to the thermocouple were led through the small opening of a 1 mL pipet tip. A second pipet tip was placed over the lower end of the first pipet tip producing an experimental tube. These tubes were individually led in glass test tubes and placed in a Haake G50 alcohol bath (Thermo Fisher Scientific, Waltham, MA, USA), with a cooling rate of −0.5 °C min−1. The starting temperature was set at 20 °C. The SCP was defined as the lowest temperature recorded before the release of the latent heat of fusion (n = 30).
Data analysis
Survival rates and reproduction capacity after cold exposure were compared using a logistic regression in SPSS (Version 22.0, IBM, Armonk, NY, USA). In this regression a generalized linear model was constructed using a probit (log odds) link and a binomial error function (McCullagh and Nelder 1989). The oviposition rates after cold exposure were compared with a control group by way of independent samples Kruskal–Wallis tests in SPSS. LTime10,50,90-values were estimated in SPSS using probit analysis. Descriptive data analysis was conducted in STATISTICA (Version 12.0, StatSoft, Tulsa, OK, USA). Figures were prepared in SigmaPlot (Version 13.0, Systat Software, San Jose, CA, USA).
Results
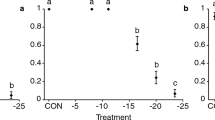
Eggs laid at 25 °C and then transferred to 7 °C did not survive an exposure period of either 17, 31 or 52 days (at each time period n = 20). When exposed to 7 °C for 24 h, all eggs (n = 23) hatched to larvae and successfully reached adulthood when returned to rearing conditions. Exposure of P. latus larvae to 7 °C during periods of 14 and 28 days resulted in similar survival rates ranging from 40 to 53 % 24 h after recovery (Fig. 1). When the exposure period was extended to 49 days, survival dropped to 13 %, which was significantly lower than after the 14-day exposure (Wald-χ2 = 5.26, df = 1, p = 0.022). The survival of adult P. latus females was not significantly altered when exposed to 7 °C for 14 or 42 days (Wald-χ2 = 0.23, df = 1, p = 0.63 and Wald-χ2 = 0.62, df = 1, p = 0.43, 24 h and 7 days after ending the treatment, respectively) (Fig. 2c). Rates of survival 24 h after exposure to 7 °C were 85, 100 and 90 % for exposure periods of 14, 28 and 42 days, respectively. Monitoring survival 24 h or 7 days after ending each specific exposure period (14, 28 or 42 days) yielded similar results. The proportion of ovipositing females was not significantly affected by the duration of the cold exposure at 7 °C and ranged from 15–30 % and 75–85 %, 24 h and 7 days after recovery, respectively (24 h after recovery: Wald-χ2 = 1.28, df = 1, p = 0.26; Wald-χ2 = 0.53, df = 1, p = 0.47 and Wald-χ2 = 0.17, df = 1, p = 0.68; 7 days after recovery: Wald-χ2 = 0.00; df = 1; p = 1, Wald-χ2 = 0.62; df = 1; p = 0.43 and Wald-χ2 = 0.62; df = 1; p = 0.43; for comparisons between periods of 14 and 28 days, 14 and 42 days and 28 and 42 days, respectively) (Fig. 2f). The oviposition rate of females exposed to a temperature of 7 °C for 14 days did not differ from that of control mites maintained at rearing conditions (H = 0.48, df = 1, p = 0.49) (Table 1). A prolonged exposure of females at 7 °C significantly decreased the oviposition rate as compared to control mites (H = 15.44, df = 1, p < 0.001 and H = 10.29, df = 1, p = 0.001, for a 28- and 42-day cold treatment, respectively) (Table 1).
Percentages of surviving (a, b, c) and ovipositing (d, e, f) adult female Polyphagotarsonemus latus mites after exposure to −3, 2 or 7 °C during different periods, monitored 24 h (black bars) and 7 days (gray bars) post treatment. Bars within each graph marked by the same letter are not significantly different (p > 0.05, Wald-χ2; n = 15 at −3 °C and n = 20 at 2 and 7 °C)
When exposed to 2 °C, survival rates of adult females monitored 24 h or 7 days after recovery were similar for each exposure period (6, 13 and 26 days) (Fig. 2b). Survival rates dropped significantly after an exposure period of 13 days as compared to a 6-day exposure (Wald-χ2 = 11.94, df = 1, p = 0.001 and Wald-χ2 = 8.42, df = 1, p = 0.004, 24 h and 7 days after ending the treatment, respectively). No survival was observed after a 26-day cold exposure period. Females were only able to oviposit within the first 24 h after a 6-day exposure at 2 °C (Fig. 2e); at that time point 20 % of the females had laid a single egg. One week after having been returned to rearing conditions, the percentage of ovipositing females increased to 50 and 15 % after a cold exposure period of 6 or 13 days, respectively. Ovipositing females from both groups had similar oviposition rates as control mites (H = 3.49, df = 1, p = 0.062 and H = 0.35, df = 1, p = 0.56, for a 6- and 13-day cold treatment, respectively) (Table 1). Whereas in this experiment hatching of the deposited eggs was not monitored, the appearance of juveniles and new adults in the arenas during the recovery period indicated that at least part of these eggs were viable.
Survival of adult P. latus females monitored 24 h or 7 days after recovery from cold exposure was not affected by the duration of the exposure period (24, 72 or 168 h) to −3 °C (Fig. 2a). A significantly lower survival was noted after an exposure period of 72 h as compared to a 24 h exposure (Wald-χ2 = 8.35, df = 1, p = 0.004 and Wald-χ2 = 12.61, df = 1, p < 0.001, 24 h and 7 days after ending the treatment, respectively). Continuous exposure for 7 days to −3 °C resulted in no surviving females. None of the females was capable to oviposit within the first 24 h after exposure to −3 °C during 24 or 72 h (Fig. 2d). After 7 days of recovery, however, 53 and 20 % of the females exposed during 24 and 72 h, respectively, oviposited. Their oviposition rates were significantly lower than those of non-exposed females (H = 17.85, df = 1, p < 0.001 and H = 4.88, df = 1, p = 0.027, for a cold exposure period of 24 and 72 h, respectively) (Table 1).
Lethal time values for adult females exposed to temperatures of −3 or 2 °C are shown in Fig. 3. The LTime50-values were estimated to be 61.2 h and 9.3 days at −3 and 2 °C, respectively. The SCP for 2-day-old adult females averaged −16.5 ± 0.2 °C (mean ± SE).
Discussion
In the production of pot azalea, P. latus mites are exposed to temperatures below the lower developmental threshold of 10 °C (Luypaert et al. 2014) during part of the growing cycle. Temperatures in the range of 2–7 °C are common when R. simsii hybrids are placed in cold storage rooms to break dormancy of flower buds. At an optimum temperature of 7 °C (Stuart 1965) or even at temperatures of 2–3 °C plants can be stored for 4–6 weeks. Chilling of R. simsii plants is necessary to avoid erratic bud break and low-quality flowering (Christiaens et al. 2014). Plant damage caused by P. latus is frequently recorded at the end of the production cycle when flowers are forced in heated greenhouses (ca. 21 °C), where also additional light can be supplied. It has been shown that these conditions are favorable for P. latus development on R. simsii (Luypaert et al. 2014), as well as on other crops (Li et al. 1985; Ferreira et al. 2006). Whereas several studies have focused on the relation of temperature and development of P. latus (e.g. Jones and Brown 1983; Li et al. 1985; Ferreira et al. 2006; Luypaert et al. 2014) to our knowledge no workers have exposed the mite to temperatures below the lower developmental threshold. Furthermore, experimental evidence on the cold hardiness or overwintering capacity of P. latus is limited in the literature. Two studies discussed the climatic regions where P. latus might able to survive being the tropical, subtropical [Karl 1965 (cited by Gerson 1992)] and moderate temperate climatic regions (Vincent et al. 2010). Overwintering and cold tolerance is also poorly studied in other plant dwelling tarsonemid mites. Tarsonemid mites are generally believed to overwinter in the adult stage (e.g. Smith and Goldsmith 1936; Wiesmann 1941; Jeppson et al. 1975; Denmark 1988). Jeppson et al. (1975) stated that the adult stage of tarsonemid mites is known to survive through prolonged exposure of freezing temperatures. Further, there are no reports of the existence of diapause within the family of Tarsonemidae (Hoy 2011).
The present study shows that egg hatchability was zero after an exposure of 17 days to a temperature of 7 °C. This indicates a detrimental effect on embryogenesis of temperatures below the lower developmental threshold for eggs of P. latus, which was estimated to be 10.2 °C (Luypaert et al. 2014). However, eggs are capable to withstand a temperature of 7 °C during a period of 24 h, without any effects on hatching or offspring performance. Further, our data also indicate that a substantial proportion of larvae was able to withstand exposure to 7 °C for much longer periods: about half of the larvae were still alive after an exposure period of 14 days, and survival only dropped below 20 % after 49 days of cold exposure. The adult females were clearly more robust, showing limited detrimental effects from a 7 °C cold exposure up to 6 weeks, both in terms of survival rates and reproduction capability: nearly all females surviving an exposure to 7 °C were able to resume egg laying when returned to favorable conditions. However, their oviposition rates were affected by the duration of the cold treatment: whereas oviposition rates of females exposed to 7 °C for 14 days were similar to those of control females, they were 2.9 and 2.4 times lower after a 28- and 42-day exposure, respectively.
For temperatures far below the developmental threshold of 10 °C, i.e. 2 and −3 °C, the focus of the study was placed on the adult phase of P. latus, as this is reportedly the stage in which tarsonemid mites overwinter in temperate areas (e.g. Smith and Goldsmith 1936; Wiesmann 1941; Jeppson et al. 1975; Denmark 1988). Our findings indicate the limited ability of P. latus to survive a prolonged exposure to subzero temperatures. None of the females survived an exposure of 26 days at 2 °C and of 7 days at −3 °C. Reproduction of females surviving shorter exposure periods at such low temperatures was also affected. Most of the females exposed to 2 or −3 °C required a recovery period longer than 24 h to resume egg laying. Whereas the oviposition rates of females exposed for either 6 or 13 days to 2 °C were not different from those of control females, all females surviving exposure to −3 °C had lower oviposition rates than in the control.
Whereas data in the literature on cold hardiness parameters of members of the Tarsonemidae family are scarce, more information is available on the overwintering capacity of phytoseiid mites, some of which are used as biological control agents against P. latus. These include the economically important species Amblyseius swirskii (van Maanen et al. 2010; Onzo et al. 2012; Abou-Awad et al. 2014), Neoseiulus cucumeris (Li et al. 2003; Weintraub et al. 2003) and Neoseiulus californicus (Peña and Osborne 1996; Jovicich et al. 2008). The average SCP value of P. latus females (−16.5 °C) is somewhat higher than that reported for non-acclimated A. swirskii mites (−18.3 °C; Allen 2010). Neoseiulus cucumeris and N. californicus showed to have a much lower SCP, with values of −20.7 (Morewood 1992) and −21.6 °C (Hart et al. 2002), respectively. SCP data alone are not believed to be sufficiently reliable indicators of cold tolerance (Bale 1987; Bale et al. 1996) because the vast majority of species are freeze avoiding and SCP temperatures are rarely experienced by individuals in their natural habitats. Lethal time data best represent naturally occurring cold stress because they not only test temperature but also exposure time (Allen 2010). The lethal time experiments done in our study are the most closely related laboratory measurements to field survival because they take into account mortality over time at temperatures likely to be encountered by P. latus during the production process of R. simsii hybrids, with the exception of the −3 °C cold treatment. In our experiments, the microclimate and shelter provided by foliar trichomes and veins mimicking the natural environment was simulated using leaf discs. However, these leaf discs remain an artificial environment differing from real on-plant conditions where besides foliar trichomes and veins, shelters such as leaf axils, buds or flowers might provide a more favorable microclimate for the mite. The lethal times for 50 % mortality in P. latus females estimated here (61.2 h and 9.3 days for −3 and 2 °C, respectively) are higher than the values of 9.2 min, 1.6 days and 2.7 days for non-acclimated A. swirskii adults at −5, 0 and 5 °C, respectively (Allen 2010), indicating better level of cold tolerance in P. latus than in A. swirskii. On the other hand, N. californicus appears to be more cold hardy, with much higher LTime90-values of 8 and 15 days for −5 and 0 °C, respectively (Hart et al. 2002), versus 76.9 h and 12.1 days for −3 and 2 °C, respectively, for P. latus. Information on the thermal responses of P. latus and its phytoseiid predators may be relevant for biological control programs during cooler parts of the growing season.
In conclusion, the findings of our study suggest that P. latus is a freeze intolerant, chill susceptible species (Bale 2002). However, females of the pest are expected to be able to withstand all temperatures encountered during the R. simsii hybrid production process in northwestern Europe. Problems caused by this pest during the forcing process are likely due to female mites easily surviving cold storage needed to break bud dormancy. Further, data from the present study suggest that under open field conditions P. latus is insufficiently cold tolerant to withstand winters generally occurring in the temperate climate of northwestern Europe, as subzero temperatures dramatically decrease survival and reproduction capacity of the mite. More in-depth studies on the indoor overwintering of P. latus or other members of the tarsonemid family may help optimizing the integrated management of this pest in the production of R. simsii hybrids or other ornamentals and vegetable crops grown in the greenhouses of temperate climatic zones.
References
Abou-Awad BA, Hafez SM, Farhat BM (2014) Biological studies of the predacious mite Amblyseius swirskii, a predator of the broad mite Polyphagotarsonemus latus on pepper plants (Acari: Phytoseiidae: Tarsonemidae). Arch Plant Pathol Plant Protec 47(3):349–354
Allen CM (2010) Thermal biology and behaviour of two predatory Phytoseiid mites: Amblyseius swirskii (Athias-Henriot) (Acari:Phytoseiidae) and Phytoseiulus longipes (Evans) (Acari:Phytoseiidae). Ph.D. thesis, University of Birmingham
Bale JS (1987) Insect cold hardiness—freezing and supercooling—an ecophysiological perspective. J Insect Physiol 33(12):899–908
Bale JS (2002) Insects and low temperatures: from molecular biology to distribution and abundance. Philos Trans R Soc Lond B Biol Sci 357(1423):849–862
Bale JS, Jones TH, Gibbons D (1996) Impacts of climate change. J Zool 240:593–597
Christiaens A, De Keyser E, Lootens P, Pauwels E, Roldán-Ruiz I, De Riek J, Gobin B, Van Labeke M-C (2014) Cold storage to overcome dormancy affects the carbohydrate status and photosynthetic capacity of Rhododendron simsii. Plant Biology. doi:10.1111/pbl.12195
Denmark HA (1988) The cyclamen mite, Phytodromus pallidus (Banks) (Acari: Tarsonemidae). Ent Circ 306
Ferreira RCF, de Oliveira JV, Haji FNP, Gondim MG Jr (2006) Biologia, exigências térmicas e tabela de vida de fertilidade do ácaro-branco Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae) em videira (Vitis vinifera L.) cv. Itália. Neotrop Entomol 35:126–132
Gerson U (1992) Biology and control of the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Exp Appl Acarol 13:163–178
Hart AJ, Bale JS, Tullet AG, Worland MR, Walters KFA (2002) Effects of temperature on the establishment potential of the predatory mite Amblyseius californicus McGregor (Acari: Phyoseiidae) in the UK. J Insect Physiol 48:593–599
Heungens A (1986) Soft-skinned mites in azalea culture and comparable control results on other host plants. Verbondsnieuws voor de Belgische Sierteelt 30:257–269 (in Dutch)
Hoy M (2011) Agricultural acarology: introduction to integrated mite management. Chapter 7. The tarsonemidae. CRC Press, Boca Raton, pp 103–108
Jeppson LR, Keifer HH, Baker EW (1975) Mites injurious to economic plants. University of California Press, Berkeley, xxiv + 614 pp
Jones VP, Brown RD (1983) Reproductive responses of the broad mite Polyphagotarsonemus latus (Acari: Tarsonemidae), to constant temperature–humidity regimes. Ann Entomol Soc Am 76:466–469
Jovicich E, Cantliffe DJ, Osbore LS, Stoffella PJ, Simonne EH (2008) Release of Neoseiulus californicus on pepper transplants to protect greenhouse-grown crops from early broad mite (Polyphagotarsonemus latus) infestations. In: Mason PG, Gillespie DR, Vincent C (eds) Proceedings of the third international symposium on biological control of arthropods, Christchurch, New Zealand, pp 347–353
Karl E (1965) Untersuhungen zur Morphologie und Ökologie von Tarsonemiden gärtnerischer Kulturpflanzen. II Hemitarsonemus latus (Banks), Tarsonemus confusus Ewing, T. talpae Schaarschmidt, T. setifer Ewing, T. smithi Ewing und Tarsonemoides belemnitoides. Weis-Fogh Biol Zentralbl 84:331–357 (in German)
Labanowski G, Soika G (2006) Tarsonemid mites on ornamental plants in Poland: new data and an overview. Biol Lett 43:341–346
Li J, Yanyun Y, Yunfang Q, Qianhong W (2003) Experimental population life table of Amblyseius cucumeris Polyphagotarsonemus latus as prey. Acta Phytophylacica Sin 30(4):389–395 (in Chinese)
Li LS, Li YR, Bu GS (1985) The effect of temperature and humidity on the growth and development of the broad mite, Polyphagotarsonemus latus. Acta Entomol Sin 28:181–187 (in Chinese)
Luypaert G, Witters J, Van Huylenbroeck J, Maes M, De Riek J, De Clercq P (2014) Temperature-dependent development of the broad mite Polyphagotarsonemus latus (Acari: Tarsonemidae) on Rhododendron simsii. Exp Appl Acarol 6(3):389–400
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman and Hall, New York, p 511
Morewood WD (1992) Cold hardiness of Phyoseiulus perisimilis Athias-Henriot and Amblyseius cucumeris (Oudemans) (Acarina: Phytoseiidae). Can Entomol 124(6):1015–1025
Navajas M, Migeon A, Estrada-Pena A, Mailleux AC, Servigne P, Petanovic R (2010) Chapter 7.4: The Acari. In: Roques A et al. (ed) Invasive terrestrial invertebrates in Europe. BioRisk (special issue) pp 149–192
Onzo A, Houedokoho AF, Hanna R (2012) Potential of the predatory mite, Amblyseius swirskii to suppress the broad mite, Polyphagotarsonemus latus, on the gboma eggplant, Solanum macrocarpon. J Insect Sci 12:7
Peña JE, Osborne L (1996) Biological control of Polyphagotarsonemus latus (Acarina: Tarsonemidae) in greenhouse and field trials using introductions of predacious mites (Acarina: Phytoseiidae). Entomophaga 41(2):279–285
Richardson E, Seeley S, Walker D (1974) A model for estimating the completion of rest for Redhaven Elberta peach trees. HortScience 9:331–332
Smith LM, Goldsmith EV (1936) The cyclamen mite, Tarsonemus pallidus, and its control on field strawberries. Hilgardia 10(3):53–94
Stuart NW (1965) Groth retardants, storage temperature and length of storage for controlling the flowering of greenhouse azaleas. Flor Rev 136:14–15
van Maanen R, Vila E, Sabelis MW, Janssen A (2010) Biological control of broad mites (Polyphagotarsonemus latus) with the generalist predator Amblyseius swirskii. Exp Appl Acarol 52:29–34
Vincent CI, García ME, Johnson DT, Rom CR (2010) Broad mite on primocane-fruiting blackberry in organic production in Arkansas. HortTechnol 20(4):718–723
Waterhouse DF, Norris KR (1987) Biological control: Pacific prospects. Inkata Press, Melbourne
Weintraub PG, Kleitman S, Mori R, Shapira N, Palevsky E (2003) Control of the broad mite (Polyphagotarsonemus latus (Banks)) on organic greenhouse sweet peppers (Capsicum annuum L.) with the predatory mite, Neoseiulus cucumeris (Oudemans). Biol Control 27:300–309
Wiesmann R (1941) Investigations on the biology and control of the strawberry mite, Tarsonemus pallidus (fragariae Z.) Banks. Landwirtschaftliches Jahrbuch der Schweiz 55(3):259–329
Acknowledgments
The authors wish to thank the ILVO greenhouse technicians for excellent plant cultivation and Magali Losschaert for outstanding technical assistance during the experiments. This research was funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen Grant No. 100859).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luypaert, G., Witters, J., Berkvens, N. et al. Cold hardiness of the broad mite Polyphagotarsonemus latus (Acari: Tarsonemidae). Exp Appl Acarol 66, 29–39 (2015). https://doi.org/10.1007/s10493-015-9894-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9894-3