Abstract
Symbiotic root micro-organisms such as arbuscular mycorrhizal fungi commonly change morphological, physiological and biochemical traits of their host plants and may thus influence the interaction of aboveground plant parts with herbivores and their natural enemies. While quite a few studies tested the effects of mycorrhiza on life history traits, such as growth, development and reproduction, of aboveground herbivores, information on possible effects of mycorrhiza on host plant choice of herbivores via constitutive and/or induced plant volatiles is lacking. Here we assessed whether symbiosis of the mycorrhizal fungus Glomus mosseae with common bean plants Phaseolus vulgaris influences the response of the two-spotted spider mite Tetranychus urticae to volatiles of plants that were clean or infested with spider mites. Mycorrhiza-naïve and -experienced spider mites, reared on mycorrhizal or non-mycorrhizal bean plants for several days before the experiments, were subjected to Y-tube olfactometer choice tests. Experienced but not naïve spider mites distinguished between constitutive volatiles of clean non-mycorrhizal and mycorrhizal plants, preferring the latter. Neither naïve nor experienced spider mites distinguished between spider mite-induced volatiles of mycorrhizal and non-mycorrhizal plants. Learning the odor of clean mycorrhizal plants, resulting in a subsequent preference for these odors, is adaptive because mycorrhizal plants are more favorable host plants for fitness of the spider mites than are non-mycorrhizal plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most plants can form symbiotic relations with soil-borne micro-organisms such as arbuscular mycorrhizal fungi (AMF) (Allen 1996). The symbiotic association between AMF and plant roots is commonly called arbuscular mycorrhiza (AM). Mycorrhizal symbiosis may be formed by ~150 different fungal species (Allen 1996; Koricheva et al. 2009) and is usually but not always mutualistic (Smith and Read 2008). The most important function of AM is improving the nutrient uptake by the plant, especially phosphorus (P), whereas the AMF obtains carbohydrates in return (Read 1998; van der Heijden and Sanders 2002; Smith and Read 2008; Koricheva et al. 2009). However, not only the plant and the AMF are affected by their symbiosis. Plant-associated organisms above- and belowground such as herbivores feeding on green plant parts (Koricheva et al. 2009) or roots (Gange 2001), 3rd trophic level organisms such as predators (Schausberger et al. 2012) and parasitoids (Guerrieri et al. 2004), or pollinators (Wolfe et al. 2005) are also commonly affected by AM.
Here we assessed the effects of the AMF Glomus mosseae Nicol. & Gerd. on long distance attraction of the herbivorous two-spotted spider mite Tetranychus urticae Koch (Acari, Tetranychidae) to their host plant, common bean Phaseolus vulgaris L. Glomus mosseae is a ubiquitous AMF, acting as root symbiont of numerous vascular plant species (Giovannetti et al. 1993). Tetranychus urticae is a globally distributed polyphagous herbivore, with over 1,000 host plant species recorded (Bolland et al. 1998), and major economic importance as crop pest (Helle and Sabelis 1985; Hoy 2011). In general, mycorrhiza-influenced nutrient uptake by the plant can increase or decrease herbivore performance, because of either rendering the plant a more favorable food source or enhancing the plant’s defensive system (e.g. Koricheva et al. 2009). For our study system, T. urticae achieves higher fitness on bean plants inoculated with the AMF G. mosseae than on non-mycorrhizal plants (Hoffmann et al. 2009; Hoffmann and Schausberger 2012). Since AM generally changes the chemical composition of plants, it may also affect plant-emitted volatiles, which are used for food location by many foraging plant-inhabiting arthropods (e.g. Bruce et al. 2005), incl. T. urticae (Dicke 2000). Plant volatiles may be constitutively emitted or induced by herbivore attack (HIPV), with the latter providing reliable information of prey or host presence on the plant for foraging carnivores (Dicke and van Loon 2000). HIPVs may mediate both direct and indirect defense, signal to nearby plants (Pichersky and Gershenzon 2002) and may also inform other herbivores in the surrounding about the presence of competitors and natural enemies on the plant (Dicke 2000).
Recent studies showed indeed that AM changes both constitutive volatiles and HIPVs emitted by aboveground plant parts (e.g. Bezemer and van Dam 2005). For example, aphid parasitoids were more strongly attracted to volatile blends released by plants inoculated with AMF than to those released by plants without AMF (Guerrieri et al. 2004). For our study system, Schausberger et al. (2012) showed that AM quantitatively and qualitatively changes the emission of constitutive volatiles and HIPVs of P. vulgaris, causing a stronger attraction of the predatory mite Phytoseiulus persimilis Athias-Henriot to the HIPVs of mycorrhizal plants than to those of non-mycorrhizal plants. However, it is unknown whether mycorrhiza-induced changes in plant volatiles also modulate attraction of host plant seeking herbivores. Regarding the spider mite T. urticae, preliminary tests did not indicate an innate ability to recognize the odor of mycorrhizal plants (Hoffmann and Schausberger unpublished) but spider mites are well able to learn discriminating different qualities of feeding and reproduction sites (Egas and Sabelis 2001). Accordingly, when having a choice, T. urticae experienced with various host plants should select those plants that offer the best prospects for their reproductive success. Experience-induced behavioral change enhancing fitness is generally dubbed adaptive learning (Papaj and Lewis 1993). Egas and Sabelis (2001) documented adaptive learning in T. urticae by showing that experience with cucumber resulted in a subsequent preference for cucumber in choice situations. Higher reproductive output of the mites on cucumber plants suggested that learning was adaptive.
Based on previous findings that the spider mites have well-developed learning abilities in foraging contexts (Egas and Sabelis 2001), the spider mites perform better on mycorrhizal bean plants (Hoffmann et al. 2009; Hoffmann and Schausberger 2012), mycorrhiza changes the volatiles of bean plants (Schausberger et al. 2012) and foraging spider mites orient themselves to plant volatiles (e.g. Dicke 1986), we hypothesized that mycorrhiza-experience modulates the host plant choice of T. urticae. Mycorrhiza-experienced spider mites should be more strongly attracted to the odors of mycorrhizal than non-mycorrhizal plants. In light of adaptive learning, learned host plant preference should only be displayed for host plants providing a fitness advantage to the alternative options. Thus, we predicted that, in choice situations, mycorrhiza-experience results in a matching preference for the odor of mycorrhizal plants, whereas mycorrhiza-naïve mites do not develop a matching preference for non-mycorrhizal plants. To test our hypothesis we performed two Y-tube olfactometer experiments assessing the choice of both mycorrhiza-naïve and -experienced spider mites between odors from clean (1) and spider mite-infested (2) non-mycorrhizal and mycorrhizal plants.
Materials and methods
Plants, mycorrhiza and mites
To generate mycorrhizal and non-mycorrhizal plants for experiments we followed the protocol described by Hoffmann et al. (2009). In brief, surface-sterilized seeds of P. vulgaris (var. Taylor’s Horticultural) were pre-germinated in perlite. After 8–10 days, two seedlings each were transferred into 1-l pots and grown in a 1:1:1 silicate sand/expanded clay/soil substrate mixture, which had been autoclaved before use. To generate mycorrhizal plants we added ~5 g per plant of G. mosseae inoculum (BEG 12; International Bank of Glomeromycota http://www.kent.ac.uk/bio/beg) to the substrate. For non-mycorrhizal plants a water filtrate of the inoculum was added. The only difference between mycorrhizal and non-mycorrhizal plants was thus the presence or absence of G. mosseae in the rhizosphere. All plants used in experiments were watered with a P-reduced nutrient solution once a week and with a NO3-fertilizer twice per week to avoid nodulation (Hoffmann et al. 2009 for details). After growing them for another 2–4 weeks at 60 ± 5 % RH and 16 (23 °C):8 (18 °C) h L:D the plants were ready for use in experiments.
After completing the olfactometer tests, the roots of all plants were checked for mycorrhizal colonization. To this end, the plants were removed from the pots exposing their roots and soil particles sticking to the roots rinsed off with cold tap water. The roots were first boiled in 10 % KOH for 10 min at 90 °C to clean them; after boiling with KOH the remaining liquid was removed and the roots stained by boiling them for 7–9 min at 90 °C in a 5 % ink (Schaeffer black ink), house-hold vinegar (equal to 5 % acetic acid) solution; after staining, the roots were again rinsed with cold tap water (Vierheilig et al. 1998). The percentage of root length colonized (RLC) by the AMF was estimated according to Newman (1966), using a modified gridline intersect method (Giovannetti and Mosse 1980).
The base population of two-spotted spider mite T. urticae used for this study was maintained on whole non-mycorrhizal common bean plants P. vulgaris, grown in a substrate mixture of commercial potting soil and sand, at room temperature (23 ± 3 °C, 60–80 % RH, 16:8 h L:D). Adult spider mite females to be used as mycorrhiza-naïve and -experienced individuals in the olfactometer tests were randomly withdrawn from the base population and reared on same-aged and -fertilized non-mycorrhizal or mycorrhizal bean plants for 6–10 days before the olfactometer experiment took place. Before being subjected to a choice test, each mite was starved for 16–20 h by placing it individually in an empty circular acrylic cage (1.5 × 0.3 cm) without any food (Schausberger 1997).
In the first experiment, we only used clean (non-infested) bean plants. For the second experiment, plants were infested by randomly adding 30 adult spider mite females to each plant, using a fine moistened camel’s hair brush, 5 days before the olfactometer tests took place. Eggs produced by the spider mite females did not hatch within the 5 days, keeping the density and damage caused by the spider mites similar between mycorrhizal and non-mycorrhizal plants.
Olfactometer
The Y-tube olfactometer used for the choice experiments was a modification of the olfactometer described by Sabelis and van de Baan (1983) and consisted of three glass tubes melted together in a Y-shape (Schausberger et al. 2012 for details). The two upper arms (called choice arms) ended in chambers containing the leaf (odor) samples; the lower base arm was connected to a mini-diaphragm-vacuum pump (Laboport® N86 KN.18; KNF Neuburger, Freiburg, Germany). A Y-shaped stainless steel wire placed inside the glass tube in equidistance to the inner walls served as walking path for the mites. During experiments, the olfactometer was placed on a black table and a cold-light lamp was centered above the intersection of the Y-tube to avoid any bias in lighting towards one or the other choice arm. The pump, connected to the bottom end of the base arm, sucked in air at both ends of the choice arms and then through the Y-tube with a flow rate of 2.5 l min−1 per arm, totaling 5.0 l min−1.
Choice experiments
In the first experiment, each adult spider mite female was given a choice between the odors from clean non-mycorrhizal and mycorrhizal bean leaflets. In the second experiment, the odors came from spider mite-infested non-mycorrhizal and mycorrhizal bean leaflets. Each leaf sample consisted of the youngest fully developed trifoliate leaf, detached from the plant immediately before the experiment. Detached leaves were preferred to whole plants as odor sources because they allowed more precise standardization of age, functional part and biomass, all of which may influence volatile emission (e.g. Choh and Takabayashi 2006), of the used plant material and excluded any influence of volatiles from other sources such as the growing substrate or roots (Schausberger et al. 2012 for details).
Each spider mite was individually released at the bottom end of the wire inside the base arm of the olfactometer, using a moistened camel’s hair brush. After release, each mite was observed for a maximum of 7 min. If the mite reached the end of a choice arm within 7 min, the chosen odor source (mycorrhizal or non-mycorrhizal) and response time were recorded. Mites not reaching the end of a choice arm within 7 min or falling down from the wire were discarded from choice analyses. After every mite tested, the wire, up to slightly after the intersection of the choice arms, was cleaned by an ethanol-moistened cotton swab. After every five mites, the wire was taken out from the Y-shaped glass tube and fully cleaned with an ethanol-moistened tissue, and the acrylic chambers containing the leaf samples were switched between sides of the olfactometer. After every 10 mites (five mycorrhiza-experienced and five mycorrhiza-naïve) a new pair of leaf samples was used.
Statistical analyses
SPSS 15.0 (SPSS, Chicago, IL, USA) was used for all statistical analyses. Data of the first and second experiment were analyzed separately. To analyze whether general responsiveness and choice of the spider mites for the odor of plants reared on before the olfactometer experiment (mycorrhizal or non-mycorrhizal) differed between mycorrhiza-naïve and -experienced spider mites, we performed generalized linear models (GLM; binomial distribution with logit link). To assess whether mycorrhiza-naïve and -experienced spider mites had a preference for the odors of clean mycorrhizal or non-mycorrhizal plants in the first experiment, or infested mycorrhizal or non-mycorrhizal plants in the second experiment, two-tailed binomial tests were performed within each choice situation (defined by the combination of mycorrhiza experience and plant infestation), assuming random choice. Within each experiment, a generalized linear model (GLM; gamma distribution with log link) was used to analyze the influence of odor source (mycorrhizal or not) and mycorrhiza-experience on the response time of the mites.
Results
Root length colonization (RLC)
Mycorrhizal plants used in the first (n = 18) and second (n = 19) experiment had 20.0 ± 2.0 (SE) and 17.0 ± 1.3 % RLC, respectively. All non-mycorrhizal plants had 0 % RLC.
Choice experiments
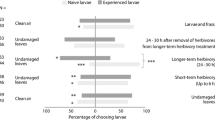
In experiment 1, 39 of 72 mycorrhiza-naïve mites and 38 of 75 mycorrhiza-experienced mites, respectively, responded to the odor of clean plants within 7 min (GLM: Wald \( \chi_{ 1}^{ 2} = 0.099 \); P = 0.75). Choice of the odor matching the odor of plants reared on before the olfactometer experiment (mycorrhizal or non-mycorrhizal) differed between mycorrhiza-naïve and -experienced mites (GLM: Wald \( \chi_{ 1}^{ 2} = 5.083 \); P = 0.02). Mycorrhiza-naïve mites did not distinguish between the odors of clean mycorrhizal and non-mycorrhizal plants but mycorrhiza-experienced mites were more strongly attracted to the odor of mycorrhizal than non-mycorrhizal plants (Fig. 1). The response times of the mites were not influenced by presence/absence of mycorrhiza (GLM: Wald \( \chi_{ 1}^{ 2} < 0.001 \); P = 0.99) or mycorrhiza-experience of the mites (Wald \( \chi_{ 1}^{ 2} = 1.156 \); P = 0.28) as main factors. However, their significant interaction (Wald \( \chi_{ 1}^{ 2} = 4.901 \); P = 0.03) indicates that mycorrhiza-experienced mites needed longer than mycorrhiza-naïve mites to respond to the odor of mycorrhizal plants, whereas the response times to the odor of non-mycorrhizal plants were similar (Fig. 2a).
Preference of mycorrhiza-naïve (Mna) and -experienced (Mex) spider mite females, Tetranychus urticae, given a choice between the odors of clean (cl) or infested (inf) mycorrhizal and non-mycorrhizal bean plants in a Y-tube olfactometer. One odor matched and the other did not match the odor of the pre-experimental host plant. Numbers in parenthesis after treatment acronyms are the numbers of mites making a choice within 7 min; P values refer to binomial tests within each situation, assuming random choice (indicated by broken vertical lines)
In experiment 2, 47 of 95 mycorrhiza-naïve mites and 48 of 95 mycorrhiza-experienced mites, respectively, responded to the odor of spider mite-infested plants (GLM: Wald \( \chi_{ 1}^{ 2} = 0.484 \); P = 0.49). Choice of the odor matching the odor of plants reared on before the olfactometer experiment (mycorrhizal or non-mycorrhizal) did not differ between mycorrhiza-naïve and -experienced spider mites (GLM: Wald \( \chi_{ 1}^{ 2} = 0.092 \); P = 0.76). Neither mycorrhiza-naïve nor -experienced mites had a preference for the odor of infested mycorrhizal or non-mycorrhizal plants (Fig. 1). The response times were neither influenced by the presence/absence of mycorrhiza (GLM: Wald \( \chi_{ 1}^{ 2} = 0.873 \), P = 0.35) nor by mycorrhiza-experience of the mites (Wald \( \chi_{ 1}^{ 2} = 0.456 \), P = 0.50) nor their interaction (Wald \( \chi_{ 1}^{ 2} = 0.029 \), P = 0.87) (Fig. 2b).
Discussion
Our study provides evidence that changes in the volatiles emitted by common bean plants, P. vulgaris, caused by symbiosis with the AMF G. mosseae (Schausberger et al. 2012), enhances the attraction of the spider mite T. urticae to its host plant. However, only mycorrhiza-experienced (i.e. those mites that experienced feeding and oviposition on mycorrhizal plants and the associated volatiles before the choice tests) but not mycorrhiza-naïve mites distinguished between the volatiles of clean mycorrhizal and non-mycorrhizal plants and displayed a preference for the former. In contrast, both mycorrhiza-naïve and -experienced mites were indiscriminate regarding mycorrhization in choice situations with odors of spider mite-infested bean plants.
The spider mite T. urticae and many other plant-inhabiting arthropods commonly use plant volatiles for host plant location and assessment (Dicke and van Loon 2000). AM commonly changes plant volatile emissions (Fontana et al. 2009; Leitner et al. 2010; Schausberger et al. 2012), which, in our system, signaled the mycorrhiza-experienced spider mites the presence of a higher-quality host plant. To the best of our knowledge, our study is the first showing that mycorrhiza-induced changes in plant volatiles may not only be perceived by foraging carnivorous parasitoids (Guerrieri et al. 2004) and predators (Schausberger et al. 2012) but also by foraging herbivores. In the second experiment, involving spider mite-infested plants, neither mycorrhiza-experienced nor -naïve mites showed a preference although G. mosseae changes the volatiles of both clean and spider mite-infested bean plants (Schausberger et al. 2012). HIPVs from mycorrhizal bean plants are more attractive to the prime natural enemy of the spider mites, the predatory mite P. persimilis (Schausberger et al. 2012). A likely reason for the lacking discrimination by the spider mites is that the main information conveyed by HIPVs, i.e. presence of a plant attacked by spider mites, overshadowed any signal indicating mycorrhiza-induced changes in host plant quality. In general, HIPVs may convey mixed information to other herbivores such as spider mites searching for suitable host plants. For example, HIPVs may indicate that the plant’s defensive system has been activated, repelling the spider mites (Dicke 1986). Alternatively, they may indicate that some herbivores have overcome the defensive system, weakening the plant and making it more susceptible to herbivore attack (Pallini et al. 1997). HIPVs could also indicate the presence of competitors reducing per capita resource availability, or indicate a higher risk of predation due to attraction of the predators by HIPVs (Pallini et al. 1997; Dicke and van Loon 2000).
In the first experiment, mycorrhiza-experienced spider mites took more time to assess and trace the preferred odors of mycorrhizal plants, probably reflecting the costs of information processing and decision-making (e.g. Dukas 2004; Ydenberg 2010). A common cost is the time needed to make a choice among alternative options. Mites perceiving a difference between the two odors inside the olfactometer had to decide which arm of the wire they should walk up to reach the preferred odor. In contrast, assuming that the mites choosing the odor of non-mycorrhizal plants were indiscriminative, these mites may have just walked up the wire without stopping and comparing the two odors.
Previous experience with mycorrhizal plants and their volatiles was a decisive factor for host plant choice. Experience allowed the mites to distinguish between the odors of mycorrhizal and non-mycorrhizal plants and to display a preference for the former. Mites kept on mycorrhizal plants for a couple of days apparently learned during this time to associate the odor of mycorrhizal plants with high-quality food, allowing faster development and higher reproduction than achieved on non-mycorrhizal plants (Hoffmann et al. 2009). Such a phenomenon is called adaptive learning (Papaj and Lewis 1993) and has been previously documented for spider mites in the context of host plant species discrimination (Egas and Sabelis 2001). Our study provides evidence that the spider mites can also adaptively learn qualitative differences within the same undamaged host plant species, i.e. recognize and prefer the odors of mycorrhizal bean plants to those of non-mycorrhizal counterparts.
References
Allen MF (1996) The ecology of arbuscular mycorrhizas: a look back into the 20th century and a peek into the 21st. Mycol Res 100:769–782
Bezemer TM, van Dam NM (2005) Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol 20:617–624
Bolland HR, Gutierrez J, Flechtmann CHW (1998) World catalogue of the spider mite family (Acari, Tetranychidae). Brill, Leiden
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Choh Y, Takabayashi J (2006) Intact lima bean plants exposed to herbivore-induced plant volatiles attract predatory mites and spider mites at different levels according to plant parts. Appl Entomol Zool 42:537–543
Dicke M (1986) Volatile spider-mite pheromone and host-plant kairomone, involved in spaced-out gregariousness in the spider mite Tetranychus urticae. Physiol Entomol 11:251–262
Dicke M (2000) Chemical ecology of host-plant selection by herbivorous arthropods: a multitrophic perspective. Biochem Syst Ecol 28:601–617
Dicke M, van Loon JJA (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Dukas R (2004) Evolutionary biology of animal cognition. Annu Rev Ecol Evol Syst 35:347–374
Egas M, Sabelis MW (2001) Adaptive learning of host preference in a herbivorous arthropod. Ecol Lett 4:190–195
Fontana A, Reichelt M, Hempel S, Gershenzon J, Unsicker SB (2009) The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J Chem Ecol 35:833–843
Gange AC (2001) Species-specific responses of a root- and shoot-feeding insect to arbuscular mycorrhizal colonization of its host plant. New Phytol 150:611–618
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular infection in roots. New Phytol 84:489–500
Giovannetti M, Avio L, Sbrana C, Citernesi A (1993) Factors affecting appressorium development in the vesicular-arbuscular mycorrhizal fungus Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol 123:115–122
Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G (2004) Do interactions between plant roots and the rhizosphere affect parasitoid behavior? Ecol Entomol 29:753–756
Helle W, Sabelis MW (eds) (1985) Spider mites: their biology, natural enemies and control. Elsevier, Amsterdam
Hoffmann D, Schausberger P (2012) Plant-mediated aboveground-belowground interactions: the spider mite perspective. Acarologia 52:17–27
Hoffmann D, Vierheilig H, Riegler P, Schausberger P (2009) Arbuscular mycorrhizal symbiosis increases host plant acceptance and population growth rates of the two-spotted spider mite Tetranychus urticae. Oecologia 158:663–671
Hoffmann D, Vierheilig H, Schausberger P (2011) Mycorrhiza-induced trophic cascade enhances fitness and population growth of an acarine predator. Oecologia 166:141–149
Hoy MA (2011) Agricultural Acarology: Introduction to Integrated Mite Management. CRC Press, Boca Raton
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097
Leitner M, Kaiser R, Hause B, Boland W, Mithöfer A (2010) Does mycorrhization influence herbivore-induced volatile emission in Medicago truncatula? Mycorrhiza 20:89–101
Newman EI (1966) A method of estimating total length of root in a sample. J Appl Ecol 3:139–140
Pallini A, Janssen A, Sabelis MW (1997) Odour-mediated responses of phytophagous mites to conspecific and heterospecific competitors. Oecologia 110:179–185
Papaj DR, Lewis AC (eds) (1993) Insect learning: ecology and evolutionary perspectives. Chapman & Hall, London
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Read DJ (1998) Mycorrhiza–state of the art. In: Varma A, Hock B (eds) Mycorrhiza. Springer, Berlin, pp 3–34
Sabelis MW, van de Baan HE (1983) Location of distant spider mite colonies by phytoseiid predators: demonstration of specific kairomones emitted by Tetranychus urticae and Panonychus ulmi. Entomol Exp Appl 33:303–314
Schausberger P (1997) Inter- and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 21:131–150
Schausberger P, Peneder S, Jürschik S, Hoffmann D (2012) Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct Ecol 26:441–449
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Elsevier, Amsterdam
Van der Heijden MGA, Sanders I (eds) (2002) Mycorrhizal Ecology. Springer, Heidelberg
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Wolfe BE, Husband BC, Klironomos JN (2005) Effects of a belowground mutualism on an aboveground mutualism. Ecol Lett 8:218–223
Ydenberg R (2010) Decision Theory. In: Westneat DE, Fox CW (eds) Evolutionary Behavioral Ecology. Oxford University Press, New York, pp 131–147
Acknowledgments
We thank D. Hoffmann for advice during the experiments and A. Walzer, J. M. Gratzer and T. Pina Desfilis for comments on an earlier version of the manuscript. J. D. Patiño-Ruiz was partially funded by an Erasmus-Mundus stipend.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patiño-Ruiz, J.D., Schausberger, P. Spider mites adaptively learn recognizing mycorrhiza-induced changes in host plant volatiles. Exp Appl Acarol 64, 455–463 (2014). https://doi.org/10.1007/s10493-014-9845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9845-4