Abstract
The diversity and abundance of questing ticks and ticks parasitizing birds was assessed during 1 year in two recreational forests in western Portugal, a suburban forest and an enclosed game area. The aim of this study was to assess the distribution and seasonality of tick species and to understand the role of bird species as hosts for ticks. Ixodes ricinus was the most abundant questing tick collected in the enclosed game area, whereas in the suburban forest, only three ticks were collected by blanket dragging. Tick species parasitizing birds included I. ricinus, I. frontalis, I. arboricola, I. acuminatus, Haemaphysalis punctata, Hyalomma marginatum and H. lusitanicum. This is the first record of I. arboricola in Portugal. Tick prevalence and intensity of infestation differed between study areas and was higher in birds from the game area where a large population of deer and wild boar may support tick populations. Ground and shrub dwelling bird species such as Turdus merula, Erithacus rubecula and Sylvia melanocephala were the most heavily parasitized by ticks, but the importance of different bird species as hosts of larvae and nymphs of I. ricinus and I. frontalis differed. Therefore, different bird species may contribute differently for tick population maintenance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild birds are hosts for several species of ticks, contributing to the maintenance of their populations in a certain area. The presence of suitable hosts and other factors such as climatic change, environmental conditions and biotopes favour survival of different tick species, determining their geographic distribution (Barandika et al. 2011). This has implications for public health, since some tick species are competent vectors for emerging infectious pathogens which are being established in new endemic foci. Migratory birds may also contribute to tick dispersal (Olsen et al. 1995). Therefore, up to date knowledge on the distribution of ticks, their ecology and association with vertebrate hosts, namely birds, provides valuable information to define the risk area for different tick-borne diseases and better predict and eventually prevent disease outbreaks.
Some studies have been published on the infestation of birds by ticks in Europe, however, those were mainly restricted to central and northern Europe (Dubska et al. 2009; Papadopoulos et al. 2001; Olsen et al. 1995; Humair et al. 1993). Although a few studies have been published on the Portuguese tick fauna (Baptista et al. 2004; Estrada-Peña and Santos-Silva 2005), including a recent update on geographical distribution of Ixodidae ticks and their associations with hosts (Santos-Silva et al. 2011), little is known on tick infestation of common resident birds, many of which are adapted to urban environments and live in close contact with human populations (but see Santos-Silva et al. 2006, 2001). Also, though wild birds are considered to be important hosts for some tick species and life stages known to be present in Portugal, such as Ixodes ricinus and Haemaphysalis punctata, so far no association with birds as hosts has been established for I. ricinus immatures and H. punctata larvae.
The aim of this study was to assess the diversity of questing ticks and ticks parasitizing birds in two recreational mixed forest areas in Portugal, the most western country of continental Europe, where the climate varies from Mediterranean to Oceanic and Continental types. One of the study areas (Tapada de Mafra) is an enclosed game area, where the etiologic agent of Lyme borreliosis is known to be present (Baptista et al. 2004) but no information is available for the other area, a suburban forest often used for leisure activities (Mata do Choupal). Collections were performed monthly during 1 year, to assess seasonal variations in questing tick density and tick loads on birds. In addition, microclimatic data, such as temperature and humidity, were collected to help to explain spatial and temporal variation in tick distribution, abundance and infestation patterns. With this approach we also provide information about which bird species contribute the most to the maintenance of different tick species and, in consequence, to the pathogens they might carry.
Materials and methods
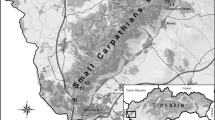
This study was performed in two recreational mixed forest areas in west Portugal located 160 km apart: Mata do Choupal (MC), Coimbra (40º13′N, 8º27′W) and Tapada de Mafra (TM), Mafra (38º57′N, 9º18′W). These areas are characterised by a climate predominantly oceanic with Atlantic front (Daveau 1980). MC is a suburban mixed deciduous forest with an area of 79 ha besides Mondego river, where several ditches with riparian vegetation are present. The flora includes ash (Fraxinus angustifolia), nettle-tree (Celtis australis), common alder (Alnus glutinosa), common sallow (Salix atrocinerea), white willow (Salix alba), black poplar (Populus nigra), English oak (Quercus robur), Eucalyptus sp., laurel (Laurus nobilis) and plane trees (Platanus sp.), among others. The herbaceous stratum is dominated by the exotic species Tradescantia fluminensis. TM is an enclosed game area of 819 ha with cork oak (Quercus suber), Portuguese oak (Quercus faginea), maritime pine (Pinus pinaster), poplar (Populus sp.), plane trees (Platanus sp.), ash (Fraxinus sp.) and wild olive tree (Olea europaea var. sylvestris). In the herbaceous stratum Brachypodium phoenicoides and the common bracken (Pterydium aquilinum) are found abundantly. The main difference between the two study areas in relation to fauna, which has implications for tick populations, is the presence of controlled hunting populations of fallow deer (Cervus dama), red deer (Cervus elaphus) and wildboar (Sus scrofa) in TM, which contribute to the successful adult tick engorgement and subsequent reproduction (Gray 1998) and allow the maintenance of tick populations.
Tick collections from birds and vegetation were performed monthly from March 2010 until March 2011. Periodicity of collections was 2 days, <7 days apart, per month. In MC, December captures were made in only 1 day due to adverse weather conditions, and in September collections were made 11 days apart. Birds were captured using mist nets. Each bird was ringed for identification and inspected visually for the presence of ticks, with special attention to the areas of the beak, eyes and ears. After examination and collection of ticks, birds were released as quickly as possible to minimize disturbance. Birds recaptured during the same month were removed from analyses. Questing ticks were collected by blanket (1 m2) dragging, between 360 and 408 m2 each month, at each of the study areas, along the sides of the mist nets. Ticks from birds and from the blanket were collected into tubes containing 70 % ethanol until identification. Questing larvae numbers were estimated visually. Ticks were separated by instars and gender, and identified to species by conventional keys and descriptions using a stereomicroscope (Pérez-Eid 2007; Estrada-Peña et al. 2004). One termohygrometer (HT 71-PCE Instruments) was placed in each study area to measure temperature and humidity every ½ h.
Tick prevalence and intensity of infestation on birds were compared between study areas using a χ 2 and a Mann-Whitney test, respectively. In order to assess the most parasitized bird species, we grouped species of birds by their foraging behaviour along the vegetation profile: low (mostly open ground), medium low (ground and shrubs), medium (shrubs or tree trunks) and high (mostly tree canopy), and compared tick prevalence (χ 2 test) and intensity of infestation (Kruskal–Wallis test) among these four groups. In order to assess if different bird species contributed differently as hosts to different tick species and life stages we used a χ 2 test to compare the number of I. ricinus and I. frontalis larvae and nymphs collected from different bird species (from which more than 5 individuals were sampled) from TM and MC, respectively. Density of questing I. ricinus nymphs and adults was correlated with mean temperature and relative humidity registered in the previous month (Spearman correlation).
Results
Questing ticks
At TM, 848 questing nymphal and adult ticks belonging to seven different species were collected by dragging a total surface of 5,160 m2. Identified tick species were, in decreasing order of abundance: I. ricinus, Hae. punctata, Hae. inermis, Dermacentor marginatus, Hyalomma lusitanicum, H. marginatum and Rhipicephalus sanguineus. Ixodes ricinus accounted for 78 % of the ticks collected (Table 1). At MC only 3 questing ticks were collected from the vegetation: two I. ricinus males and one H. punctata female. The seasonal questing dynamics of the most abundant tick, I. ricinus, revealed that the peak of nymph density was in late winter (February), and that the cycle of questing adults was bimodal with a peak in November–December and other smaller peak in March (Fig. 1). Larvae were present in May, June and July and by visual estimation they were found to peak in June (approximately 160 larvae/100 m2). Temperature and relative humidity at MC and TM are presented in Supplemental material 1. The observed number of questing I. ricinus nymphs at TM was correlated with the mean monthly ambient temperature and relative humidity registered during the previous month (r s = −0.89 and 0.78, respectively, P < 0.05, n = 7), using data from September 2010 until March 2011. Density of questing I. ricinus adults was not significantly correlated with temperature and humidity data.
Ticks parasitizing birds
A total of 935 birds from 37 different species were captured (including 16.6 % of recaptured birds in different months) and from those, 1,122 ticks were collected. At TM, ticks parasitizing birds belonged to 5 different species, in decreasing order of abundance: I. ricinus, Hae. punctata, I. frontalis, H. marginatum and H. lusitanicum. At MC, 4 species of ticks were collected from birds, in decreasing order of abundance: I. frontalis, I. ricinus, I. arboricola and I. acuminatus (Fig. 2; Supplemental material 2). Considering all bird species, birds were more affected by tick parasitism at TM than at MC (prevalence of tick infestation = 32.4 and 16.7 %, χ 2 = 26.6, P < 0.0001; mean no ticks/infested bird ± SE = 9.05 ± 1.73 and 2.72 ± 0.50, Z = 4.82, P < 0.0001, at TM and MC respectively).
Seasonal pattern of I. ricinus nymph infestation on birds at TM reflected the pattern of seasonal questing activity of nymphs at the same location, although the infestation on birds showed a peak slightly delayed in March/April (Fig. 3a). I. ricinus larvae on birds also peaked in June, as did questing larvae. However, at MC, inferring from I. ricinus parasitizing birds, it seems that the peak of I. ricinus larvae and nymphs was more synchronized than at TM, and occurred in March for both stages (Fig. 3b). The pattern of I. frontalis infestation on birds was similar between MC and TM. Larvae of I. frontalis presented two peaks, a larger peak in November and a smaller peak in February, whereas nymphs peaked in December, although at MC another small peak was apparent in February (Fig. 3c, d).
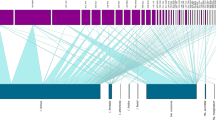
The bird species showing the higher prevalence of ticks were Turdus merula, Sturnus unicolor (but only 2 individuals were captured) and Erithacus rubecula. Highest infestation intensities were registered on T. merula, Sylvia melanocephala and E. rubecula (Table 2). When comparing groups of birds with different foraging behaviours, the bird species foraging at a medium–low level (ground and shrubs) had a significantly higher prevalence (χ 23 = 154.9, P < 0.0001) and intensity (H 3,198 = 27.37, P < 0.0001) of tick infestation than birds foraging at the other levels (Table 2). In particular, the number of infested birds that forage at the medium–low level was much higher than expected (152 observed vs. 76.4 expected). I. ricinus and I. frontalis were ubiquitous on birds and were collected from 15 and 13 different bird species, respectively. H. lusitanicum was found only on T. merula, and both H. marginatum and Hae. punctata were collected only from T. merula and Garrulus glandarius. One case of infestation by I. acuminatus was observed on T. merula. The relative importance of each bird species as host for different tick species and life stages differed, which was supported by the fact that I. arboricola was mostly associated with birds that forage in the tree canopy or roost/nest in tree holes such as S. unicolor, P. major, Cyanistes caeruleus, Certhia brachydactyla, although it was also present on T. merula (one larva). Also, considering bird species with more than 5 individuals captured in each area, we found that prevalence of both larvae and nymphs of I. ricinus, and of nymphs of I. frontalis, were higher on T. merula than on other bird species in both areas. T. merula had also a higher prevalence of co-infestation by larvae and nymphs than the other species. However, the prevalence of I. frontalis larvae was higher on E. rubecula in both areas (Table 3). This pattern of different life stages of different tick species parasitizing different bird species reflects a non-homogeneous distribution of I. ricinus and I. frontalis larvae and nymphs among bird species (Fig. 4). The most important host for I. ricinus larvae and nymphs, and I. frontalis nymphs, was T. merula. For I. frontalis larvae, the most important host was E. rubecula. Troglodytes troglodytes was an important host for larvae of I. ricinus and I. frontalis and nymphs of I. ricinus, at MC.
Discussion
Seasonal and geographic patterns of tick abundance
This study revealed a large contrast in terms of abundance and diversity of ticks, between two spatially close study areas, which share a similar climate, supporting the knowledge that biotic differences such as type of vegetation and host community have a large impact on tick populations (Barandika et al. 2006; Estrada-Peña et al. 1992). The fact that at MC, only three ticks were collected from vegetation through blanket dragging suggests that this technique is not always efficient to assess tick abundance (Daniels et al. 2000). Also, the type of vegetation at MC, dominated in the herbaceous stratum by the exotic species Tradescantia fluminensis, probably explains the unsuccessful collection of questing ticks. This plant species usually presents high levels of humidity (due to condensation), which results in a low collection of ticks, known to be associated with wet vegetation (Barandika et al. 2006). Exophilic ticks, such as I. ricinus, which were known to be present at MC through collection on birds, might also be heterogeneously distributed in this forest and our standardized approach failed to collect them. However, as we sampled ticks along walking paths this may be a proxy of human tick bite risk.
Ixodes ricinus was especially abundant at TM, which has a mild climate with high relative humidity (see Supplemental material 1), and this is in accordance with this species preference for humidity and fresh environments in forested areas of temperate Europe (Barandika et al. 2011; Pérez-Eid 2007). The second most abundant tick species at TM, Hae. punctata, is also considered a forest tick and shares the same preferences in terms of environmental conditions as I. ricinus (Barandika et al. 2011; Uspensky 2002). On the other hand, Hyalomma, a genus usually associated with open areas, and both Dermacentor and Rhipicephalus, tick genera more resistant to desiccation (Uspensky 2002), were present in lower numbers at TM. The dependence of I. ricinus on meteorological conditions was further supported by the fact that the abundance of I. ricinus questing nymphs was correlated with ambient temperature and relative humidity. This is in accordance with the observed decline in density of questing nymphs due to an increase in saturation deficit at field sites (Perret et al. 2000). No significant correlation was found for adults, probably due to their higher resistance, associated with a larger size and higher energetic and water reserves (Perret et al. 2004; Herrmann and Gern 2010).
The seasonal abundance pattern of I. ricinus described in this study is based on the collection of questing ticks during only 1 year in one location, and should not be generalised. However, our results were similar to those presented by Baptista et al. (2004) and Baptista (2006) for the same study area: both studies reported an unimodal pattern of questing larvae density, peaking during the summer months, and questing nymph density, with a peak extended for several months, from late winter to early spring. We found that the abundance of questing adult I. ricinus presented a smaller peak in spring (March) and a larger peak in autumn (November/December), and therefore we considered it as a bimodal cycle. Baptista et al. (2006), used data from several years and described it as an unimodal cycle with a decrease during December and January.
The seasonal pattern of tick infestation on birds was similar to that of questing ticks, considering data from I. ricinus larvae and nymphs at TM, and could be used as an alternative method to estimate the active population of certain tick species and life stages that are associated with birds. This use of sentinel animals has been successfully applied previously using small mammals (Nilsson 1988). The fact that the peak of I. ricinus larvae and nymphs feeding on birds was synchronous at MC suggests a rapid spring warming, which should decrease the time gap between the threshold temperatures necessary for nymphs and larvae to quest, and allowed simultaneous questing activity of these stages (Sumilo et al. 2007). The seasonal pattern of I. frontalis activity observed in our study areas, recorded only from September to March, with a peak in November/December, is in accordance with the pattern of activity of this tick species described in Great Britain (Martyn 1988).
Birds as hosts for ticks
Concerning ticks parasitizing birds, at TM there was a higher prevalence and intensity of infestation than at MC, which is related with high abundance of I. ricinus at TM, probably due to the abundance of suitable reproduction hosts. All stages of I. ricinus were observed on birds, with 15 species belonging to different foraging niches parasitized. These results bridge the gap in information concerning birds as hosts for I. ricinus in Portugal (Santos-Silva et al. 2011), because this species was not reported parasitizing birds previously.
In both areas, I. frontalis was ubiquitous on birds, parasitizing a total of 13 species and all stages were observed. This species is considered a bird specific parasite, feeding mainly on passerines, and known to be present in a wide range of habitats (Pérez-Eid 2007), including those with riparian vegetation (Estrada-Peña et al. 1992), such as MC, where it was specially abundant. The finding of only one I. acuminatus individual parasitizing birds, in this case a T. merula from MC, is in agreement with the reports that this species is mostly associated with mammals and only occasionally found parasitizing birds (Pérez-Eid 2007).
This study presents the first report of I. arboricola in Portugal, a species reported for northern and central Europe, Italy (Manilla 1991), and the Mediterranean coast of Egypt (Hoogstraal et al. 1967) and Spain (Palomar et al. 2011). I. arboricola is an endophilic species and was found parasitizing cavity nesting bird species (P. major, C. caeruleus, S. unicolor and C. brachydactyla), which agrees with data from Literak et al. (2007) and Pérez-Eid (2007).
Immature stages (both larvae and nymphs) of Hae. punctata were collected from T. merula and G. glandarius. This is the first report of birds as hosts for Hae. punctata larvae in Portugal, since Santos-Silva et al. (2011) in their recent survey reported only the presence of nymphs of this species on birds. Immatures of Hyalomma spp. have been previously reported feeding on birds (Santos-Silva et al. 2011).
The most parasitized bird species were similar between the two areas, and were those that forage mostly on the ground and/or low shrub vegetation, such as T. merula and E. rubecula. This suggests that their level of parasitism is a consequence of their behaviour, which brings them often into contact with subadult ticks. Similar results on prevalence and tick infestation intensity of birds exploring different ecological niches were found in other studies (Movila et al. 2008; Dubska et al. 2009; Taragel’ová et al. 2008; Papadopoulos et al. 2001; Humair et al. 1993; Poupon et al. 2006; James et al. 2011). However, the importance of E. rubecula as host for ticks varied between studies (Dubska et al. 2009), which may be related with the diversity and dominant tick species at each study area. We also registered an high infestation intensity on S. melanocephala, which, to our knowledge has not been reported before. This is a species common in Southern Europe, nesting in areas with dense low shrub vegetation (Schaefer and Barkow 2004), where it probably comes into contact with ticks. In fact, it was during the breeding season that the infestation was higher on this species (pers. obs.). A detailed analyses of our most sampled bird species, revealed that T. merula was an important host for nymphs and larvae of I. ricinus, and nymphs of I. frontalis, whereas I. frontalis larvae were more associated with E. rubecula. This difference on host importance for I. frontalis larvae and nymphs suggests that this tick might switch hosts between stages. This is in accordance with the increasing evidence that this species is not strictly endophilic: it has been detected on the undergrowth beneath trees used by nesting birds and on the vegetation (Pérez-Eid 2007; Martyn 1988). A different role of bird species as hosts for different tick life stages has also been found by Hanincová et al. (2003) and Dubska et al. (2009), where E. rubecula and T. troglodytes were parasitized mostly by I. ricinus larvae, and Turdus sp. were more heavily infested by I. ricinus nymphs.
This study provided important information on tick species diversity, especially those parasitizing birds, in western Portugal. We confirmed that common resident birds are often parasitized by tick species that are competent vectors of pathogens transmitted to man, such as B. burgdorferi s.l. and Rickettsia spp. and may have an important role on disease epidemiology.
References
Baptista SSSG (2006) Lyme borreliosis in Portugal. Study on vector(s), agent(s) and risk factors. PhD Thesis, Universidade Nova de Lisboa, Lisboa
Baptista S, Quaresma A, Aires T, Kurtenbach K, Santos-Reis M, Nicholson M, Collares-Pereira M (2004) Lyme borreliosis spirochetes in questing ticks from mainland Portugal. Int J Med Microbiol 293:109–116
Barandika JF, Berriatua E, Barral M, Juste RA, Anda P, García-Pérez AL (2006) Risk factors associated with ixodid tick species distributions in the Basque region in Spain. Med Vet Entomol 20:177–188
Barandika JF, Olmeda SA, Casado-Nistal MA, Hurtado A, Juste RA, Valcárcel F, Anda P, García-Pérez AL (2011) Differences in questing tick species distribution between Atlantic and continental climate regions in Spain. J Med Entomol 48:13–19
Daniels TJ, Falco RC, Fish D (2000) Estimating population size and drag sampling efficiency for the blacklegged tick (Acari: Ixodidae). J Med Entomol 37:357–363
Daveau S (1980) Dois Mapas Climáticos de Portugal. Nevoeiro e Nebulosidade. Contrastes Térmicos. Linha de Acção de Geografia Física, Centro de Estudos Geográficos
Dubska L, Literak I, Kocianova E, Taragelova V, Sychra O (2009) Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in central Europe. Appl Environ Microbiol 75:596–602
Estrada-Peña A, Santos-Silva MM (2005) The distribution of ticks (Acari : Ixodidae) of domestic livestock in Portugal. Exp Appl Acarol 36:233–246
Estrada-Peña A, Osacar JJ, Gortazar C, Calvete C, Lucientes J (1992) An account of the ticks of the northeastern of Spain (Acarina, Ixodidae). Ann Parasit Hum Comp 67:42–49
Estrada-Peña A, Bouattour A, Camicas J-L, Walker AR (2004) Ticks of domestic animals in the Mediterranean Region—a guide to identification of species. University of Zaragoza, Zaragoza
Gray JS (1998) The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol 22:249–258
Hanincová K, Taragelova V, Koci J, Schafer SM, Hails R, Ullmann AJ, Piesman J, Labuda M, Kurtenbach K (2003) Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol 69:2825–2830
Herrmann C, Gern L (2010) Survival of Ixodes ricinus (Acari: Ixodidae) under challenging conditions of temperature and humidity is influenced by Borrelia burgdorferi sensu lato infection. J Med Entomol 47:1196–1204
Hoogstraal H, Kaiser MN, Seymour C, Gaber S (1967) Noteworthy recent tick records from Egypt. 1. Ixodes arboricola Schulze and Schlottke infesting resident, migrant, and wintering birds in the western coastal desert. J Egypt Public Health Assoc 42:223–229
Humair PF, Turrian N, Aeschlimann A, Gern L (1993) Ixodes ricinus immatures on birds in a focus of Lyme borreliosis. Folia Parasitol 40:237–242
James MC, Furness RW, Bowman AS, Forbes KJ, Gilbert L (2011) The importance of passerine birds as tick hosts and in the transmission of Borrelia burgdorferi, the agent of Lyme disease: a case study from Scotland. Ibis 153:293–302
Literak I, Kocianova E, Dusbabek F, Martinu J, Podzemny P, Sychra O (2007) Winter infestation of wild birds by ticks and chiggers (Acari: Ixodidae, Trombiculidae) in the Czech Republic. Parasitol Res 101:1709–1711
Manilla G (1991) A new species of the Italian tick fauna. II. Ixodes arboricola Schulze and Schlottke, 1929. Parassitologia 33:175–181
Martyn KP (1988) Provisional atlas of the ticks (Ixodoidea) of the British Isles. Natural Environment Research Center, Dorset
Movila A, Gatewood A, Toderas I, Duca A, Papero M, Uspenskaia I, Conovalov J, Fish D (2008) Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus and I. lividus ticks collected from wild birds in the Republic of Moldova. Int J Med Microbiol 298:149–153
Nilsson A (1988) Seasonal occurrence of Ixodes ricinus (Acari) in vegetation and on small mammals in southern Sweden. Ecography 11:161–165
Olsen B, Jaenson TGT, Bergstrom S (1995) Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl Environ Microbiol 61:3082–3087
Palomar AM, Santibáñez P, Mazuelas D, Estrada-Peña A, Gutiérrez Ó, Santibáñez S, Portillo A, Oteo JA (2011) Genetic characterization of Ixodes arboricola using 16S rRNA e 12S rRNA as PCR targets. In: Sánchez N, Estrada-Penã A (eds) TTP7, Zaragoza
Papadopoulos B, Humair PF, Aeschlimann A, Vaucher C, Buttiker W (2001) Ticks on birds in Switzerland. Acarologia 42:3–19
Pérez-Eid C (2007) Les tiques: identification, biologie, importance médicale et veterinaire. Monographies de microbiologie, TEC & DOC EMinter, Lavoisier
Perret J-L, Guigoz E, Rais O, Gern L (2000) Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol Res 86:554–557
Perret J-L, Rais O, Gern L (2004) Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J Med Entomol 41:361–365
Poupon MA, Lommano E, Humair PF, Douet W, Rais O, Schaad M, Jenni L, Gern L (2006) Prevalence of Borrelia burgdorferi sensu lato in ticks collected from migratory birds in Switzerland. Appl Environ Microbiol 72:976–979
Santos-Silva MM, Formosinho P, Melo P, Santos A, Filipe AR (2001) Ixodídeos (Acari: Ixodidae) parasitas de aves silváticas em Portugal. Rev Port Ciências Vet 96:197–199
Santos-Silva M, Sousa R, Santos A, Melo P, Encarnação V, Bacellar F (2006) Ticks parasitizing wild birds in Portugal: detection of Rickettsia aeschlimannii, R. helvetica and R. massiliae. Exp Appl Acarol 39:331–338
Santos-Silva M, Beati L, Santos A, De Sousa R, Núncio M, Melo P, Santos-Reis M, Fonseca C, Formosinho P, Vilela C, Bacellar F (2011) The hard-tick fauna of mainland Portugal (Acari: Ixodidae): an update on geographical distribution and known associations with hosts and pathogens. Exp Appl Acarol 55:85–121
Schaefer T, Barkow A (2004) Habitat and nest site preferences of Sylvia atricapilla and S. melanocephala in Majorca. Ardeola 51:445–450
Sumilo D, Asokliene L, Bormane A, Vasilenko V, Golovljova I, Randolph SE (2007) Climate change cannot explain the upsurge of tick-borne encephalitis in the baltics. PLoS ONE 2(6):e500
Taragel’ová V, Koci J, Hanincová K, Kurtenbach K, Derdaková M, Ogden NH, Literák I, Kocianová E, Labuda M (2008) Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of Borreliosis in central Europe. Appl Environ Microbiol 74:1289–1293
Uspensky I (2002) Preliminary observations on specific adaptations of exophilic Ixodid ticks to forests or open country habitats. Exp Appl Acarol 28:147–154
Acknowledgments
We thank Tapada Nacional de Mafra and Mata Nacional do Choupal, ICNB for logistic support, Agustín Estrada-Peña and Carlos Bernardes for help with tick identification, Luís Silva, Miguel Araújo and Pedro Lopes for help with fieldwork and Paulo Sousa for laboratory facilities. This study was financially supported by a post-doctoral grant to Ana Cláudia Norte from the Portuguese Foundation for Science and Technology SFRH/BPD/62898/2009.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Norte, A.C., de Carvalho, I.L., Ramos, J.A. et al. Diversity and seasonal patterns of ticks parasitizing wild birds in western Portugal. Exp Appl Acarol 58, 327–339 (2012). https://doi.org/10.1007/s10493-012-9583-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9583-4