Abstract
The coconut mite Aceria guerreronis (Eriophyidae) is considered the most important pest of coconut fruits in Africa; however, quantitative knowledge about its distribution and abundance is lacking. We conducted four diagnostic surveys—three in Southern Benin and one along the coast of Tanzania—to determine the distribution of A. guerreronis and the severity of its damage to coconut fruits, as well as the diversity and abundance of other associated mites and potential natural enemies. Aceria guerreronis was found in all visited plantations with the percentage of damaged fruits varying considerably among plantations—67–85% in Benin and 43–81% in Tanzania. Overall, 30–40% of the fruit surfaces were damaged by A. guerreronis. Damage severity increased with fruit age and negatively affected fruit weight of 7- to 12-months-old fruits. Aceria guerreronis was by far the most abundant mite on coconut fruits but its abundance depended on fruit age. The highest densities of A. guerreronis were observed on 3- to 4-months-old fruits. Neocypholaelaps sp. (Ameroseiidae) was the most abundant mite on inflorescences. Three species of predatory mites (Phytoseiidae)—Neoseiulus baraki, N. neobaraki and N. paspalivorus—were the most commonly found predatory mites beneath the coconut bracts in association with A. guerreronis. Neoseiulus neobaraki was the prevailing predator in Tanzania while N. paspalivorus was the most frequent predator in Benin. Other mites found beneath the bracts were the herbivore Steneotarsonemus furcatus (Tarsonemidae) and the detritivore and fungivore Tyrophagus putrescentiae (Acaridae).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coconut, Cocos nucifera L. (Arecaceae), is widespread throughout the tropics. Its geographical distribution has been favored by its adaptability to wide range of climatic and vegetational regions, floatability in seawater followed by germination on beaches once washed ashore, and its usefulness to humans (Foale 2003). With approximately 11 million ha harvested worldwide in 2006 (FAO 2010), coconut palm is considered one of the ten most important trees for humankind in the world, providing food and income for hundreds of millions of people (e.g. APCC 2010).
Aceria guerreronis Keifer (Eriophyidae), commonly called the coconut mite, presently is the most important pest of coconut fruits (Howard et al. 2001). This mite pest had been known for decades from the Americas and Africa (e.g. Mariau and Julia 1970; Howard et al. 1990), but it has been detected only recently in India and Sri Lanka, two major coconut producing countries (Fernando et al. 2002; Ramaraju et al. 2002). Aceria guerreronis, likely originating from South America (Navia et al. 2005), is a tiny worm-like organism that typically inhabits the area beneath the perianth (i.e. the floral bracts) of coconut fruits feeding on the tender meristematic tissue (Howard and Abreu-Rodriguez 1991; Aratchige 2007; Lawson-Balagbo et al. 2007a). Physical injuries resulting from feeding develop into necrotic and suberized tissues on the fruit surface. Infested fruits later become distorted and stunted due to uneven growth, leading to reductions in copra yield and premature fruit drop (e.g. Julia and Mariau 1979; Hall 1981). Yield losses attributable to damage by A. guerreronis range from 10 to 70% in many countries (e.g. Hernandez 1977; Moore et al. 1989). In the 1960s in Benin, copra losses due to A. guerreronis ranged from 6 to 18%, with an overall average of 10% (Mariau and Julia 1970). However, this loss was likely underestimated, as damage to coconut is presently widespread and more severe in major growing areas of Benin (K. Negloh, personal observations, years 2004–2010). Results of two surveys conducted in Tanzania in 1993 and 1996 revealed that 70–100% of sampled fruits were damaged by A. guerreronis, with an associated crop loss of 34% (Seguni 2002). These are substantial losses in a country (Tanzania) where coconut farming is one of the most important branches of agricultural production in the coastal region (Seguni 2002). In 2006, harvested coconut areas in Tanzania were estimated at 312,796 ha (FAO 2010).
Common control measures against A. guerreronis were traditionally based on repeated applications of pesticides. Although some chemicals such as monocrotophos and chinomethionate sprayed on fruit bunches significantly reduce mite damage (Hernandez 1977; Mariau 1977; Julia and Mariau 1979), the need of repeated applications renders chemical control economically and environmentally non-feasible in the long run. Several researchers therefore recommended biological control as an environment-friendly alternative to pesticides (e.g. Moore 2000; Moraes and Zacarias 2002; Perring 2002; Lawson-Balagbo et al. 2007a, b). Various predatory mites, particularly from the family Phytoseiidae, have been found associated with A. guerreronis (Moraes and Zacarias 2002; Moraes et al. 2004; Aratchige 2007; Lawson-Balagbo et al. 2007a, b, 2008) but their effect on A. guerreronis populations has not been extensively addressed (Reis et al. 2008; Negloh et al. 2010). Entomopathogenic fungi such as Hirsutella thompsonii Fisher have been found infecting A. guerreronis (Julia and Mariau 1979; Hall et al. 1980; Cabrera 2002; Moore 2000; Gopal and Gupta 2001; Gopal et al. 2002; Fernando et al. 2007). Recent efforts to develop H. thompsonii as a biopesticide against A. guerreronis show promising results but the duration of spore viability remains a major obstacle (Edgington et al. 2008).
In the present study, we report on diagnostic surveys conducted in coconut growing areas in southern Benin and along the coast of Tanzania to assess the current status of A. guerreronis. These surveys are essential components of a multi-institutional research project on the development of biological control strategies against the coconut mite in Africa and elsewhere in the world. Our objectives were to determine the occurrence and abundance of A. guerreronis, the incidence and severity of damage caused by this pest, and the diversity of mites associated with it on coconut fruits with emphasis on potential natural enemies.
Materials and methods
Three diagnostic surveys were conducted in 66 plantations of the three major growing areas of southern Benin,—Mono, Atlantique and Oueme provinces (located between 06°15′20″ N, 01°42′54″ W and 05°50′50″ N, 02°36′39″ W),—from July to August 2004, February to March 2005, and June to July 2005 (Fig. 1a). These periods corresponded respectively with the onset of the short dry season, the end of the long dry season and the long rainy season in all provinces. The Beninese plantations were selected randomly at distances of 2–10 km, depending on plantation frequency. In August 2005 one diagnostic survey was conducted in Tanzania, along the Indian Ocean coast from Manza (04°49′17″ S, 039°08′59″ E) (district of Tanga in the north) to Ziwani (10°20′90″ S, 040°14′99″ E) (district of Mtwara in the south) (Fig. 1b). This period coincided with the end of the rainy season in the north (from Manza to Saadani) and the dry season from Bagamoyo, at approximately mid-coastal zone, to Ziwani (Mtwara) in the south. The eighteen Tanzanian plantations were selected randomly at 10–45 km distance from each other. The longer distances between plantations in Tanzania were necessary due to the much bigger length of the coastal areas in Tanzania compared with Benin. In most surveyed plantations, minimal management was applied, mainly including weeding. In few plantations, farmers tethered cows to the palms in order to fertilize the soil with their excrements or they dug holes around the palm trees and dumped dead organic matter such as shed palm leaves and cut weeds in the hole. In all plantations, mature coconut fruits were harvested at intervals of 3–4 months.
Incidence and severity of damage caused by Aceria guerreronis
Incidence and severity of fruit damage caused by A. guerreronis were assessed in situ on 10 randomly selected palms per plantation by classifying all coconut fruits on each tree on the basis of the extent of characteristic A. guerreronis damage visible on fruit surfaces. Binoculars were used where trees were not reachable by a ladder. Coconut fruits were grouped into four grades—based on the percentage of fruit surface damaged by A. guerreronis (Moore et al. 1989): grade 1 (0%), grade 2 (1–10%), grade 3 (11–25%), grade 4 (26–50%), grade 5 (>50%).
Distribution and abundance of Aceria guerreronis and identification of associated mites
In each plantation, coconut fruits were sampled from 10 and 12 palms in Benin and Tanzania, respectively, to assess the distribution and abundance of A. guerreronis and identify other mites inhabiting coconut fruits and inflorescences. Fruit sampling was based on fruit bunch age classes (FBAS) defined as follow: FBA1 (fruit bunches 1–3); FBA2 (fruit bunches 4–6); FBA3 (fruit bunches 7–9) and FBA4 (fruit bunches 10–12) (Negloh et al. 2010). The number of a particular bunch corresponds approximately to its age in months. Fruit classification was based on the knowledge that a new inflorescence (the prospective bunch) is produced approximately every month (Moore and Alexander 1987; Foale 2003; K. Negloh personal observations). Each succeeding bunch, from the top of the palm, is therefore a month older than the previous one. Bunch 1 is that of just fertilized fruits (approximately 1 month). Inflorescences were not included in FBAs but sampled separately. Fruit samples were collected from FBA1–3 in Tanzania and from FBA1–4 in Benin. We considered only the first 3 FBAs in Tanzania because of the long travel distances, the limited time allotted to the survey and the fact that previous observations in Benin indicated that older fruits harbor very few mites and do not show significant damage variations (Negloh et al. 2010). One fruit was sampled from each FBA plus one branch of inflorescence per palm when available. Samples were taken only from palms bearing at least one bunch of each FBA.
Sampled fruits were examined on-site with a 10× magnification head lens immediately after removal from the palm. Mites found on the fruit surface were collected with a brush and preserved in 75% ethanol. Each fruit was then labeled, placed in a paper bag and brought to the laboratory for further processing. In the laboratory, the bracts of each fruit were sequentially and carefully removed to uncover the meristematic zone. Mites other than A. guerreronis present beneath the bracts were counted and stored in 75% ethanol for further slide-mounting and species identification. Abundance of A. guerreronis was assessed using a methodology similar to that developed by Siriwardena et al. (2005). Bracts as well as the meristematic zone of the fruit and some distance away on the exocarp (2–5 cm depending on fruit size) were rinsed with 30 ml detergent solution into a small container. The solution was vigorously shaken to obtain a homogenous distribution immediately before 1.0 ml aliquot was drawn from the solution and placed in counting cells (Costar® Brand Cell Culture Clusters, 24 cells of 3.4 ml volume each). All individuals of A. guerreronis present in the 1.0 ml aliquot were counted using a stereomicroscope. Other mites in the solution were counted and added to those previously collected in alcohol. Abundance of A. guerreronis per fruit was then estimated by multiplying the obtained values by a factor of 30 (the total volume of the rinse solution). Inflorescences were dissected under a stereomicroscope and mites found were collected and preserved in 75% ethanol. For each sampled fruit, characteristics such as its age, FBA, damage grade, and weight were recorded.
Statistical analyses
All statistical analyses were performed using SAS 9.1 (SAS Institute 2005). Logistic regressions were used to assess variations in the incidence of damage among plantations, survey periods and growing areas. Based on grouping of fruits in damage grade classes, a Severity Index (SI) was calculated for each palm as \( {\text{SI}} = \frac{{\sum ({X_{i} *i}) }}{{\sum {X_{i} } }} \) where X i is the number of damaged fruits of grade i (i varies from 2 to 5; undamaged fruits were not included). SI values were used in general linear models (GLM) to compare damage severity among survey periods and coconut growing areas for each country separately. The same analysis was performed on arcsine-transformed proportions of damaged fruits per palm. Furthermore, based on season similarities (occurrence of the dry season) the results of the Tanzania survey, conducted in August, were compared to those of the second survey in Benin, which was conducted from February to March.
For each country, log-transformed densities of A. guerreronis (only mobile stages) were compared among fruit ages by analysis of variance (ANOVA). Mixed model analyses were used to assess the effect of survey period (random effects) and growing area (fixed effects) on A. guerreronis abundance and damage severity. The effect of damage severity on fruit weight within each FBA was assessed with general linear model (GLM) and subsequent Bonferroni multiple comparison tests on polled data for both countries.
Results
Incidence and severity of damage caused by Aceria guerreronis
In Benin coconut fruit damage by A. guerreronis was observed on 90–100% of palms across plantations and throughout the three surveys. Overall mean percentage of damaged fruits per palm was 73 ± 0.01% (Fig. 2a), but fruit damage incidence differed among provinces (mean ± SE: 67 ± 0.02, 70 ± 0.01 and 85 ± 0.01% damaged fruits per palm in Atlantique, Mono and Oueme, respectively) (logistic regression: χ 2 = 3,923.72, P < 0.0001). Of the coconut fruit showing visible mite damage, 39 ± 0.01% were severely damaged (≥25% of the fruit surface damaged) (Fig. 2a). Damage severity indices differed among survey periods (GLM: F 2,815 = 6.99, P < 0.0001) and growing areas (GLM: F 2,815 = 4.36, P = 0.0131). The interaction between survey period and growing area was also highly significant (F 4,815 = 18.90, P < 0.0001), indicating that the difference in the severity indices among survey periods varied with the growing area. Mean separation using Bonferroni showed no difference between Mono and Oueme and between the first and second survey (P > 0.05). Overall mean severity indices were 3.70 ± 0.05, 3.83 ± 0.04 and 3.82 ± 0.05, respectively, in Atlantique, Mono and Oueme.
Overall mean percentage of sampled fruits per damage severity grade (1  , 2
, 2  , 3
, 3  , 4
, 4  , 5
, 5  ; 4 + 5
; 4 + 5 ) in Benin (a) and Tanzania (b). Grades correspond to % fruit surface damaged (Moore et al. 1989) as follows: 1 (0%), 2 (1–10%), 3 (11–25%), 4 (26–50%), 5 (>50%)
) in Benin (a) and Tanzania (b). Grades correspond to % fruit surface damaged (Moore et al. 1989) as follows: 1 (0%), 2 (1–10%), 3 (11–25%), 4 (26–50%), 5 (>50%)
Incidence of A. guerreronis damage along the Tanzanian coast was 100%. All plantations surveyed and all palms in those plantations showed visible symptoms of A. guerreronis damage. All other plantations inspected informally midway between two surveyed sites were damaged also (K. Negloh, personal observations). Damage incidence assessed on-site varied among plantations from 43 ± 0.02 to 81 ± 0.01% of fruits damaged per palm. Overall, 63 ± 0.01% of coconut fruits per palm were damaged and 43 ± 0.01% of them were severely damaged, i.e. ≥25% of the fruit surface was damaged (Fig. 2b). Logistic regression revealed highly significant differences among plantations (Maximum Likelihood χ 2 = 184.36; P < 0.0001), with almost 40% difference between the maximum and minimum damage incidence. Damage severity indices ranged from 3.20 to 4.10 among plantations with an overall mean of 3.77 ± 0.05 (GLM: F 10,88 = 3.33, P = 0.001). Comparisons between the Tanzanian survey and the second Beninese survey showed similarities in damage incidence (73 vs. 63%) (GLM: F 1,347 = 0.14, P = 0.701) and severity indices (3.77 ± 0.05 vs. 3.88 ± 0.03) (GLM: F 1,347 = 3.22, P = 0.074).
In both countries damage severity increased with fruit age (Fig. 3). Damage severity increased almost linearly from 1 to 5 months old, while it remained constant on older fruits (6–12 months old). Undamaged fruits were mostly younger fruits (1–3 months) with a rare occurrence of severe damage. The highest damage grades were observed on 4–12 months old fruits (Fig. 3).
Damage severity grade (1–5; mean ± SE) in relation to coconut fruit age. Grades correspond to % fruit surface damaged (Moore et al. 1989) as follows: 1 (0%), 2 (1–10%), 3 (11–25%), 4 (26–50%), 5 (>50%)
Damage grade affected fruit weight in FBA1, FBA3 and FBA4 (GLM: F 4,710 = 77.01 P < 0.0001, F 4,84 = 4.95, P = 0.0012 and F 4,75 = 5.45, P = 0.0007 for FBA1, FBA3 and FBA4, respectively) but not in FBA2 (4–6 months old fruits) (GLM: F 4,87 = 0.35, P = 0.85) (Fig. 4). Fruit weight differed between damage grades 2 and 5 in FBA3–4, between grades 4 and 5 in FBA3, and between grades 2 and 4 in FBA4 (Bonferroni, P < 0.05 for each pairwise comparison).
Weight of coconut fruits (mean ± SE) in relation to damage severity grade in four fruit bunch age classes (FBA1–4). FBA1 (bunches 1–3); FBA2 (bunches 4–6); FBA3 (bunches 7–9) and FBA4 (bunches 10–12). Grades correspond to % fruit surface damaged (Moore et al. 1989) as follows: 1 (0%), 2 (1–10%), 3 (11–25%), 4 (26–50%), 5 (>50%)
Abundance of Aceria guerreronis
In Benin, the abundance of A. guerreronis varied from 0 to a maximum of 46,200 individuals per fruit, the latter observed on a 3 month-old fruit. Average density per fruit varied from 430 to 2,900 individuals among Benin plantations (Fig. 5). In general, A. guerreronis population densities were higher in Benin than in Tanzania where densities varied from 97 to 1,266 mites per fruit among plantations (Fig. 5). In Tanzania, the minimum and maximum counts were 0 and 22,680, with the latter, as in Benin, observed on a 3 months old fruit.
Abundance of A. guerreronis varied greatly among fruit ages (Fig. 5) in both countries (ANOVA: F 11,734 = 13.32, P < 0.001 for Benin and F 8,615 = 13.95, P < 0.001 for Tanzania). There was an almost exponential increase in A. guerreronis densities from 1 to 3 or 4 months old fruits followed by a gradual decrease in abundance on 5–12 months old fruits (Fig. 5). The lowest average densities were observed on 1 month old fruits followed by 9 or 12 months old fruits in Tanzania and Benin, respectively. Variability in A. guerreronis densities was the highest in FBA1 (1–3 months old fruits) followed by FBA4 (9–12 months old fruits). Their respective coefficients of variation (CV) were 0.61 and 0.42, while they were 0.27 for FBA2 and 0.19 for FBA3. Total densities were highest in FBA2 and lowest in FBA4 (10–12 months old fruits) (Fig. 5).
Other mites found on coconut fruits and inflorescences
Mites other than A. guerreronis found on coconut fruits in Benin and Tanzania belonged to the families Phytoseiidae, Ascidae, Acaridae, Tarsonemidae, Bdellidae, Eupodidae, Tydeidae, Cunaxidae and Tenuipalpidae. They were encountered on the exocarp or beneath the floral bracts of the fruits, where A. guerreronis resides. Specimens of the family Ameroseiidae were found in very large numbers on inflorescences but not on fruits (Table 1).
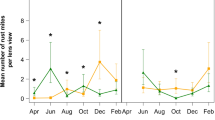
In Tanzania, four phytoseiid species were identified: Neoseiulus baraki Athias-Henriot, N. neobaraki Zannou, Moraes & Oliveira, N. paspalivorus De Leon, and Amblyseius largoensis (Muma) (Table 1). The first three species were found in the micro-habitat beneath coconut bracts, closely associated with their prey A. guerreronis, while A. largoensis specimens were collected mostly from the fruit surface outside the bracts. Neoseiulus neobaraki was the most abundant and most frequent predator on coconut fruits in Tanzania (Table 1). It was present in 17 of the 18 plantations surveyed, while only 42 and 62 specimens of N. paspalivorus and N. baraki, respectively, were collected from only four and five plantations (Table 1). In one location, Ras Matuso, N. neobaraki and N. paspalivorus occurred together on two palms and one fruit. Other mites often collected from beneath the bracts were the tarsonemid Steneotarsonemus furcatus De Leon and the acarid Tyrophagus putrescentiae Schrank. Only one specimen of Ascidae, Lasioseius sp., was found on the fruit surface. Furthermore, the bdellids Bdella distincta Baker & Balock and Spinibdella sp. Thor and the eupodid Eupodes sp. Thor were found on the fruit surface. Ameroseiid specimens collected in extremely large numbers from the inflorescences belonged to the genus Neocypholaelaps Vitzthum. They were only scarcely found on the fruit exocarp. Larvae of the family Tenuipalpidae were collected from several fruits but no adult specimen were found.
In Benin, N. paspalivorus was by far the most abundant non-eriophyid mite collected from coconut fruits (Table 1). This predator was present in all surveyed plantations. Neoseiulus baraki, the second most abundant predatory mite on coconut fruits, was collected from five plantations in Mono province. In the same province only a few specimens of N. neobaraki were collected in two plantations. Like in Tanzania the two Neoseiulus species along with T. putrescentiae and S. furcatus were collected from beneath coconut bracts. Steneotarsonemus furcatus was far less abundant in Benin than in Tanzania. Other Phytoseiidae encountered in Benin on the fruit surface outside the bracts were A. largoensis and Iphiseius degenerans (Berlese) (Table 1). Four genera of Ascidae were collected from the fruit surface: Asca sp. von Heyden, Gamasellodes sp. Athias-Henriot, Lasioseius sp. Berlese and Proctolaelaps bickleyi (Bram). Like in Tanzania thousands Neocypholaelaps sp. were collected from inflorescences (Table 1).
Discussion
Our surveys revealed that, on the basis of frequency and severity of coconut fruit infestations, the coconut mite A. guerreronis continues to be a serious pest of coconut in Benin and Tanzania. All surveyed coconut plantations in both countries were damaged by A. guerreronis and almost all palms in every plantation had damage symptoms. Such high levels of damage incidence and severity are probably due to the inability of existing natural enemies to sufficiently suppress A. guerreronis which inhabits the protected areas beneath the bracts. (Lawson-Balagbo et al. 2007a; Reis et al. 2008; Negloh et al. 2010).
The three predatory mites Neoseiulus baraki, N. neobaraki and N. paspalivorus were the three main predators found beneath the bracts but how many individuals and how early they reach the area beneath the bracts is of key importance to their effect on the pest (Lawson-Balagbo et al. 2007a; Negloh et al. 2010). Coconut mite damage levels recorded in our study were similar to the highest damage level reported from the Kalpitiya area in Sri Lanka (Fernando et al. 2002). In our study, at least 61% of fruits were damaged and more than 44% were at least severely damaged (>25% of surface damaged). In contrast, Ramaraju et al. (2002) observed only 5–48% damaged fruits in Pollachi and Udumaplet in India. In our studies, damage severity increased with fruit age probably as a consequence of the persistence of infestation by A. guerreronis. Indeed, A. guerreronis often colonizes the newly fertilized fruits (Moore et al. 1989; Fernando et al. 2003; Negloh et al. 2010) but visible injury to the fruits only appears a few weeks later. Population density of A. guerreronis increases progressively with fruit age, consequently increasing damage levels (Julia and Mariau 1979; Mariau 1986; Otterbein 1988).
Peak densities of A. guerreronis were observed on 3–4 months old fruits (see also Negloh et al. 2010). In Sri Lanka, Fernando et al. (2003) found that A. guerreronis densities increased within the first 5 months and declined thereafter. Declining densities on older fruits were likely caused by dispersal of A. guerreronis from these fruits to younger ones probably due to intensified intraspecific competition, reduced nutritional quality of feeding sites, and increased access beneath the bracts by natural enemies (Aratchige 2007; Lawson-Balagbo et al. 2007a).
Fruit weight loss increased with damage severity in maturing fruits of FBA3–4 (i.e. 7–12 months). Weight loss was not evident on fruits of FBA1 (i.e. 1–3 months old fruits) probably because development outweighed the negative effects of A. guerreronis feeding. However, the increase in fruit tissue suitable for feeding on 1–3 month-old fruits fostered population growth of the pest, resulting in the highest densities on 3 and 4 month-old fruits, which can explain the high damage severity level and associated weight loss in FBA3–4.
Various other mites, both herbivores and predators, were found on coconut fruits, some of which living in close association with A. guerreronis beneath the bracts. Steneotarsonemus furcatus was the second most frequently observed herbivorous mite, which was collected more frequently in Tanzania than in Benin (Table 1). Steneotarsonemus furcatus is, however, considerably less abundant in Benin and Tanzania than in Brazil (Navia et al. 2005; Lawson-Balagbo et al. 2008). Damage by S. furcatus was not observed in Benin and Tanzania, probably because of its very low density in these countries. Pollen feeding Neocypholaeplaps sp. (Ameroseiidae) were abundant on coconut inflorescences in Benin and Tanzania as in India (Haq 2001) and Sri Lanka (Ramaraju et al. 2002) but was completely absent in Brazil (Lawson-Balagbo et al. 2008).
The most abundant predatory mite in Tanzania was N. neobaraki, consistently observed under the bracts in almost all plantations. Two closely related species, N. paspalivorus and N. baraki, were less abundant and found in only four plantations. In Benin, N. paspalivorus was the most frequent predator followed by N. baraki. The latter was found in only five plantations which were flooded during the rainy season or were bordering swamps, lakes or rivers. Neoseuilus paspalivorus and N. baraki also occur in Sri Lanka and Brazil (Fernando et al. 2003; Moraes et al. 2004; Lawson-Balagbo et al. 2008). Only a few specimens of N. neobaraki were found in two Beninese plantations together with N. baraki. The differing distribution of the three species in Tanzania and Benin seems to reflect species-specific humidity requirements. In Brazil, N. baraki was collected in regions characterized by elevated humidity whereas N. paspalivorus was more abundant in drier regions (Lawson-Balagbo et al. 2008). Similarly, climatic differences between Tanzania and Benin could explain the observed differences in the occurrence of the three Neoseiulus species. Rainfall in coastal Tanzania is almost everywhere above 1,000 mm and can exceed 1,500 mm per year in some locations, while in Benin most places on the coast experience rainfall below 1,000 mm (BBC 2010).
The three Neoseiulus species found on coconut fruits during this study are considered promising candidates for biological control of A. guerreronis (De Moraes and Zacarias 2002; Fernando et al. 2003; Lawson-Balagbo et al. 2007b; Negloh et al. 2008, 2010). All three species have elongated, flattened idiosomas and short legs, probably morphological adaptations to live in the tight areas similar to the areas beneath coconut bracts, and all three readily prey and reproduce on A. guerreronis (Lawson-Balagbo et al. 2007b; Negloh et al. 2008; Domingos et al. 2010; K. Negloh personal observations). Amlyseius largoensis was also frequently found on coconut palms, but unlike the three Neoseiulus species, it was mostly collected from the fruit surface outside the bracts. It has a larger idiosoma than the Neoseiulus spp., which hampers its ability to creep under the bracts. Other predatory mites encountered were P. bickleyi (uniquely from fallen fruits) and Lasioseius sp. of the family Ascidae, reported from Brazil (Lawson-Balagbo et al. 2008), and Lasioseius phytoseiodes Chan, reported from Columbia (Cardona and Potes 1971), and B. distincta reported from Brazil (Lawson-Balagbo et al. 2007a) and Benin (Mariau and Tchibozo 1973). The latter species preys on A. guerreronis but because of its large body size it rarely enters the area beneath the bracts as reported in this study and by Lawson-Balagbo et al. (2007a, 2008).
The present study focused on determining the diversity and distribution of the acarine fauna only on coconut fruits and inflorescences. Several additional studies are needed in order to obtain a comprehensive assessment of the diversity and distribution of the acarine fauna in the two targeted countries. First, similar future efforts should include other parts of the coconut palm and associated vegetation in order to get greater insight into the diversity and distribution of the acarine fauna. Of particular interest are the other host plants for the three Neoseiulus species found under coconut bracts, and if these phytoseiids move between the coconut fruits and the ground vegetation. Such findings can have significant implications for promoting phytoseiid abundance to enhance biological control of A. guerreronis. Second, for Tanzania, where only one survey was conducted, additional surveys should provide further insights into the diversity and abundance of the acarine fauna at different climatic conditions (e.g. rainy season, end of dry season, etc.) than those occurring during the survey of the present study. Third, we found that coconut mite damage is widespread in both countries despite the presence of adapted predators under the coconut bracts where the pest resides and thrive. This indicates the need to conduct further studies on biology, ecology and interactions among all mites found beneath the bracts in order to determine the efficiency of associated predators as natural and biological control agents. Studies conducted in Benin (Negloh et al. 2010) showed that A. guerreronis colonizes the young fruits almost 1 month earlier than the predators. This gives A. guerreronis a head-start in population build-up and results in a delayed impact of the predators on the pest. In addition the rate of colonization by the predators is relatively slow. Recent studies demonstrated that N. paspalivorus and N. baraki performed the best on A. guerreronis as prey compared to other food sources (Lawson-Balagbo et al. 2007b; Negloh et al. 2008). Moreover, a single female of both predator species could kill over 50 individuals of A. guerreronis per day (Negloh et al. unpublished). These results suggest that the predators would be effective against the pest when it is reachable for them. Lastly, the presence of numerous other predators outside the coconut fruit bracts suggests that they may also play an important role in suppressing dispersing individuals of A. guerreronis. Their presence on other parts of the coconut palm and heir role in the suppression of A. guerreronis merits consideration.
References
APCC (2010) Webpage of Asian and Pacific coconut community. http://www.apccsec.org. Accessed on 30 Sept 2010
Aratchige AS (2007) Predators and the accessibility of herbivore refuges in plants. Ph.D. thesis, University of Amsterdam, The Netherlands
BBC (2010) Webpage of British Broadcasting Corporation. http://www.news.bbc.co.uk/weather/. Accessed 21 Oct 2010
Cabrera RIC (2002) Biological control of the coconut mite Aceria guerreronis (Acari: Eriophyidae) with the fungus Hirsutella thompsonii and its possible integration with other control methods. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 89–103
Cardona IZ, Potes SA (1971) Coconut scab or excoriation (Cocos nucifera L.) in Columbia. A. guerreronis. Acta Agronomica 21:133–139
De Moraes GJ, Zacarias MS (2002) Use of predatory mites for the control of eriophyid mites. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut Mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 78–88
Domingos CA, da S Melo JW, Gondim MGC Jr, de Moraes GJ, Hanna R, Lawson-Balagbo LM, Schausberger P (2010) Diet-dependent life history, feeding preference and thermal requirements of the predatory mite Neoseiulus baraki (Acari: Phytoseiidae). Exp Appl Acarol 50:201–215
Edgington S, Fernando LCP, Jones K (2008) Natural incidence and environmental profiling of the mite-pathogenic fungus Hirsutella thompsonii Fisher for control of the coconut mite in Sri Lanka. Int J Pest Manag 54:123–127
FAO (2010) Webpage FAOSTAT. http://faostat.fao.org. Accessed 10 Aug 2010
Fernando LCP, Wickramananda IR, Aratchige NS (2002) Status of coconut mite, Aceria guerreronis in Sri Lanka. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 1–8
Fernando LCP, Aratchige NS, Peiris TSG (2003) Distribution patterns of the coconut mite Aceria guerreronis K. and its predator Neoseiulus paspalivorus in coconut palms. Exp Appl Acarol 31:71–78
Fernando LCP, Manoj P, Hapuarachchi DCL, Edgington S (2007) Evaluation of four isolates of Hirsutella thompsonii against coconut mite (Aceria guerreronis) in Sri Lanka. Crop Prot 26:1062–1066
Foale M (2003) The coconut odyssey: the bounteous possibilities of the tree of life. Australian Centre for International Agricultural Research, vol 101
Gopal M, Gupta A (2001) Has Hirsutella thompsonii the wherewithal to counter coconut eriophyid mite scourge? Curr Sci 80:831–836
Gopal M, Saleena A, McCoy C (2002) Study of the microflora associated with coconut eriophyid mite as a preliminary step towards pathogen isolation. Curr Sci 82:22–24
Hall RA (1981) The coconut mite Eriophyes guerreronis, with a special reference to the problem in Mexico. In Proceedings of the Brazilian crop protection society, pp 113–119
Hall RA, Hussey NW, Mariau D (1980) Results of a survey of biological control agents of the coconut mite Eriophyes guerreronis Keifer. Oléagineux 35:395–400
Haq MA (2001) Life cycle and behavior of the coconut mite Neocypholaelaps stridulans (Evans) (Acari: Ameroseiidae) in India. In: Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff MJ (eds) Proceedings of the 10th international congress of acarology. CSIRO Publishing, Collingwood, pp 361–365
Hernandez RF (1977) Combate quimico del eriofido del cocotero, Aceria guerreronis K. en la costa de Guerrero. Agric en Mexico 4:23–38
Howard FW, Abreu-Rodriguez E (1991) Tightness of the perianth of coconuts in relation to infestation by coconut mites. Fla Entomol 74:358–362
Howard FW, Abreu-Rodriguez E, Denmark HA (1990) Geographical and seasonal distribution of the coconut mite, Aceria guerreronis (Acari: Eriophyidae), in Puerto Rico and Florida, USA. J Agric Univ PR 74:237–251
Howard FW, Moore D, Giblin-Davis RM, Abad RG (2001) Insects on palms. CABI Publishing, Wallingford, pp 251–257
Julia JF, Mariau D (1979) Nouvelles recherches en Côte d’Ivoire sur Eriophyes guerreronis Keifer, acarien ravageur des noix du cocotier. Oléagineux 34:181–189
Lawson-Balagbo LM, Gondim MGC Jr, de Moraes GJ, Hanna R, Schausberger P (2007a) Refuge use by the coconut mite Aceria guerreronis: fine scale distribution and association with other mites under the perianth. Biol Control 43:102–110
Lawson-Balagbo LM, Gondim MGC Jr, de Moraes GJ, Hanna R, Schausberger P (2007b) Life history of the predatory mites Neoseiulus paspalivorus and Proctolaelaps bickleyi, candidates for biological control of Aceria guerreronis. Exp Appl Acarol 43:49–61
Lawson-Balagbo LM, Gondim MGC Jr, de Moraes GJ, Hanna R, Schausberger P (2008) Exploration of the acarine fauna on coconut palm in Brazil with emphasis on Aceria guerreronis (Acari: Eriophyidae) and its natural enemies. Bull Entomol Res 98:83–96
Mariau D (1977) Aceria (Eriophyes) guerreronis Keifer: an important pest of African and American coconut groves. Oléagineux 32:109–111
Mariau D (1986) Comportement de Eriophyes guerreronis Keifer à l’égard de différentes variétés de cocotiers. Oléagineux 41:499–503
Mariau D, Julia JF (1970) L’acariose à Aceria guerreronis Keifer, ravageur du cocotier. Oléagineux 25:459–464
Mariau D, Tchibozo HM (1973) Trials of chemical control of A. guerreronis (Keifer). (Coconuts). Oléagineux 28:133–135
Moore D (2000) Non-chemical control of Aceria guerreronis on coconuts. Biocontrol News Info 21:83–88
Moore D, Alexander L (1987) Aspects of migration and colonization of the coconut palm by the coconut mite, Eriophyes guerrreronis (Keifer) (Acari: Eriophyidae). Bull Entomol Res 77:641–650
Moore D, Alexander L, Hall RA (1989) The coconut mite Eriophyes guerreronis Keifer in St Lucia: yield losses and attempts to control it with polybutene and Hirsutella fungus. Trop Pest Manage 35:83–89
Moraes GJ, Zacarias MS (2002) Use of predatory mites for the control of eriophyid mites. In: Fernando LCP, Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 78–88
Moraes GJ, Lopes PC, Fernando LCP (2004) Phytoseiid mites (Acari: Phytoseiidae) of coconut growing areas in Sri Lanka, with description of three new species. J Acarol Soc Jpn 13(2):141–160
Navia D, de Moraes GJ, Roderick G, Navajas M (2005) The invasive coconut mite Aceria guerreronis (Acari: Eriophyidae): origin and invasion sources inferred from mitochondrial (16S) and nuclear (ITS) sequences. Bull Entomol Res 95:505–516
Negloh K, Hanna R, Schausberger P (2008) Comparative demography and diet breadth of Brazilian and African populations of the predatory mite Neoseiulus baraki, a candidate for biological control of coconut mite. Biol Control 46:523–531
Negloh K, Hanna R, Schausberger P (2010) Season- and fruit age-dependent population dynamics of Aceria guerreronis and its associated predatory mite Neoseiulus paspalivorus on coconut in Benin. Biol Control 54:349–358
Otterbein HD (1988) Studies on the biology, importance and control of E. guerreronis on Cocos nucifera in Costa Rica. Dissertation, University of Kiel, Germany
Perring TM (2002) Eriophyoid mites: special considerations in applied ecological research. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 71–77
Ramaraju K, Natarajan K, Sundara Babu PC, Palalnisamy S, Rabindra RJ (2002) Studies on coconut eriophyid mite, Aceria guerreronis Keifer in Tamil Nadu, India. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 13–31
Reis AC, Gondim MGC Jr, de Moraes GJ, Hanna R, Schausberger P, Lawson-Balagbo LM, Barros R (2008) Population dynamics of Aceria guerreronis Keifer (Acari: Eriophyidae) and associated predators on coconut fruit in Northeastern Brazil. Neotrop Entomol 37:452–467
SAS Institute (2005) SAS/SAT user’s guide, release v9.1. SAS Institute, Cary
Seguni Z (2002) Incidence, distribution and economic importance of the coconut eriophyid mite, Aceria gurreronis K. in Tanzanian coconut based cropping systems. In: Fernando LCP, de Moraes GJ, Wickramananda IR (eds) Proceedings of the international workshop on the coconut mite (Aceria guerreronis). Coconut Research Institute, Sri Lanka, pp 54–57
Siriwardena PHAP, Fernando LCP, Peiris TSG (2005) A new method to estimate the population size of coconut mite, Aceria guerreronis, on a coconut. Exp Appl Acarol 37:123–129
Acknowledgments
The authors are grateful to G. J. De Moraes and Ignace Dossa Zannou for their assistance in identifying some of the mites collected during this study. They also thank Beatrice Pallangyo and her team in Kibaha, Tanzania for their support during the survey in Tanzania. We acknowledge coconut growers in Benin and Tanzania and the staff at IITA, Benin for their valuable collaboration and assistance. The research reported in this manuscript was supported by a grant to the International Institute of Tropical Agriculture (IITA) from the Austrian government. K. Negloh was additionally supported by a grant to the University of Amsterdam and IITA from Netherlands Foundation for the Advancement of Tropical Research (WOTRO). The manuscript is part of K. Negloh’s PhD thesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Negloh, K., Hanna, R. & Schausberger, P. The coconut mite, Aceria guerreronis, in Benin and Tanzania: occurrence, damage and associated acarine fauna. Exp Appl Acarol 55, 361–374 (2011). https://doi.org/10.1007/s10493-011-9474-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-011-9474-0