Abstract
Estimating the spatial and temporal variation in tick abundance is of great economical and ecological importance. Entire-blanket dragging is the most widely used method to sample free-living ixodid ticks. However, this technique is not equally efficient in different vegetation types. The height and structure of the vegetation under study will not only determine the likelihood of a tick-blanket contact, but will also determine the rate of dislodgement. The purpose of this study was therefore to determine whether the alternative strip-blanket is more effectively in picking up ticks than the standard entire-blanket. Sampling was carried out in four forest understory vegetation types that differed in height and structure on five collection dates between April and September 2008. A total of 8,068 Ixodes ricinus ticks was collected (778 adults, 1,920 nymphs, and 5,370 larvae). The highest numbers of ticks were collected along the forest trails, where the dominant vegetation consisted of short grasses. The lowest numbers of ticks were collected in bracken-fern-dominated sites, where the vegetation seriously hampered tick sampling. Surprisingly, in each vegetation type, significantly more nymphs and adults were collected using the entire-blanket. However, the strip-blanket was more effectively in collecting larvae, especially in dense and tall vegetation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hard ticks (Acari: Ixodidae) are ectoparasites of major importance due to their role as vector of various pathogenic organisms affecting humans, domesticated animals, and wildlife worldwide. In the Northern Hemisphere, Lyme borreliosis (Lyme disease) is by far the most prevalent vector-borne zoonotic infection (WHO 2004; Bacon et al. 2008). Lyme borreliosis is caused by the bacterial spirochete Borrelia burgdorferi sensu lato and is predominantly transmitted through the bite of infected Ixodes ricinus L. ticks in Europe and I. scapularis Say ticks in North America (Piesman and Gern 2004). The incidence of human infection with B. burgdorferi appears to be a function of tick population size and the proportion of ticks infected with the bacteria (Mather et al. 1996; Stafford et al. 1998; Falco et al. 1999). Consequently, accurate estimates of tick abundance are essential in order to evaluate the risk of Lyme disease transmission and for the development of successful prevention and control programs.
Drag sampling is the most commonly used method to estimate the abundance of free-living ticks because of its convenience and inexpensiveness. The technique involves dragging a blanket over the vegetation for a fixed time or distance and counting the number of ticks found clinging to the blanket (Gray and Lohan 1982; Ginsberg and Ewing 1989; Falco and Fish 1992). However, since drag sampling requires tick-blanket contact, the vertical distribution of ticks and the physical properties of the vegetation will influence sampling efficiency. Questing height, i.e. the height above the soil surface at which ticks wait for passing hosts, is species and life stage-dependent, which is probably related to differences in desiccation tolerance (Needham and Teel 1991; Randolph and Storey 1999) and host size (Mejlon and Jaenson 1997). Larvae of I. ricinus typically quest for small mammals in the leaf litter or near the base of the vegetation, nymphs readily ascend vegetation in search of medium-sized and large mammals, and adults usually climb even higher in search of large mammals (Tälleklint and Jaenson 1994; Mejlon and Jaenson 1997). Because larvae tend to quest lower in the vegetation than nymphs and adults, it is more difficult for them to seize the blanket. In dense vegetation, the blanket might not penetrate deep enough to reach the larvae, resulting in an underestimation of larval tick abundance (Gray 1985). Furthermore, adults, and to a lesser extent nymphs, are much larger than larvae and are more prone to dislodgement from the blanket. Rough vegetation surfaces especially will cause more ticks to get brushed off (Milne 1943; Sutherst et al. 1978; Li and Dunley 1998).
To date, we are aware of only one study that compared the entire-blanket and the strip-blanket, the latter being a slightly modified version of the commonly used entire-blanket, for collecting I. ricinus ticks. Gray and Lohan (1982) found the strip-blanket to be more efficient for capturing I. ricinus nymphs on a range of vegetation types (heather, bog, bracken, and short grass), which was attributed to the strips rotating considerably during dragging and protecting nymphs to some extent from being dislodged. In the present study, the efficiency of the strip-blanket and the entire-blanket was assessed for collecting all life stages of I. ricinus in four vegetation types differing in height and structure in a predominantly coniferous forest. We hypothesized that the strip-blanket would be superior compared to the entire-blanket in collecting nymphs, confirming the findings of Gray and Lohan (1982). Furthermore, we expected the strip-blanket to be a more efficient sampling method than the entire-blanket for collecting larvae, especially in dense vegetation, because the strip-blanket allows for a better penetration into the vegetation and is expected to result in a better contact with the larvae.
Materials and methods
Study area
Fieldwork was conducted at Het Wik (50°58′N, 5°25′E), a 110 ha nature reserve situated in the Campine district in the northeast of Belgium, where forests largely consist of secondary pine plantations (mainly Pinus sylvestris L.) on nutrient poor and acid sandy soils. The mean (1971–2000) annual precipitation amounts to 820 mm and is evenly distributed throughout the year, with monthly precipitation ranging from 53 mm in February to 79 mm in November. The mean annual temperature is 10.1°C and the mean temperature of the coldest and warmest month is 3.1°C (January) and 17.7°C (July) (Royal Meteorological Institute of Belgium, www.kmi.be). We sampled ticks in four vegetation types less than 1 km apart, which were selected based on the dominant plant species: (a) European larch (Larix decidua Mill.) stands with a dense, homogeneous bracken fern (Pteridium aquilinum (L.) Kuhn) understory; (b) Scots pine (P. sylvestris) stands dominated by carpeting bilberry (Vaccinium myrtillus L.); (c) small forest gaps (<1 ha) dominated by purple moor-grass (Molinia caerulea (L.) Moench); and (d) forest trails with predominantly short grasses (generally 30–50 cm tall), such as Deschampsia flexuosa (L.) Trin. and Holcus lanatus L. Bracken is a perennial fern with triangular-shaped, stiff, upright fronds. New fronds emerge in early May, grow rapidly until August (approximately 180 cm tall), and start to wither in September. Bilberry, an ericaceous dwarf shrub with rigid stems, reaches a height of 30–50 cm and purple-moor grass, a tussock-forming perennial, reaches a height of 90–120 cm.
Study species
Tick surveillance since 1999 has shown I. ricinus to be consistently present in the study area (unpublished results of Cox 1999, Hoeyberghs 2000, and Tormans 2008). Common local hosts of adult and nymphal ticks include large and medium-sized mammals, mainly roe deer (Capreolus capreolus L.), red fox (Vulpes vulpes L.), red squirrel (Sciurus vulgaris L.), European hare (Lepus europaeus Pallas), and European rabbit (Oryctolagus cuniculus L.). Very common small mammalian hosts of larval ticks are wood mouse (Apodemus sylvaticus L.), bank vole (Myodes glareolus Schreber), and common shrew (Sorex araneus L.).
Tick sampling
Ticks were collected from the vegetation by drag sampling. Two blanket types were used: the standard entire-blanket and the strip-blanket. Both blankets had the same contact surface (1 × 1 m), but the latter was cut into ten strips, each 1 m long and 10 cm wide, to ease movement through the vegetation. The fabric (white flannel) was attached with Velcro tape to a wooden dowel and weighted at the opposite end using lead curtain weights (10 × 35 g) to aid contact between the blanket and the vegetation. Ticks were sampled during five visits to the study area between April and September 2008, between 07:00 am and 07:00 pm. Sampling was performed on dry and non-windy days. At each collection date and in each vegetation type, nine 1-minute drags were performed with each blanket (1 min ≈ 30 m distance). In total, 360 drags were performed (5 collection dates × 4 vegetation types × 2 blanket types × 9 drags). The two blankets were used side by side simultaneously and were exchanged between the two operators throughout the day to avoid operator bias. Before each pair of drags (one with each blanket), air temperature and relative humidity were recorded at a height of 1.25 m above the soil surface with a portable digital temp/RH meter (DM509, Eijkelkamp Agrisearch Equipment, Giesbeek, the Netherlands). To avoid time of day and changing meteorological conditions as a source of bias, the four vegetation types were alternately sampled throughout the day. After each drag, ticks adhering to the blankets were removed in the field with a pair of tweezers and stored in 70% ethanol for later identification and counting. Tick abundance was expressed as the number of ticks collected per drag.
Statistical analysis
As our abundance data showed a non-normal distribution and common transformation procedures failed, the assumptions for parametric methods could not be met. Instead, the generalized estimating equation (GEE) approach of Liang and Zeger (1986) was applied for each life stage (larvae, nymphs, and adults) separately. Each individual drag sample was used as a separate case. A negative binomial distribution and a log link function was applied to assess the influence of time (collection date), vegetation type, and blanket type on relative tick abundance, taking repeated sampling of the same dragging sites into account. All two-way interactions were included in the analysis. Post-hoc comparisons were done using the sequential Sidak multiple comparison test. The Spearman rank order correlation was applied to determine the correlation between the numbers of ticks gathered and the air temperature and relative humidity measured during tick collection. All statistical tests were performed with SPSS 15.0.
Results
During tick collection, the ambient air temperature ranged from 3.5 to 31.4°C and the relative humidity ranged from 32 to 94%. A total of 8,068 ticks, all identified as I. ricinus, were collected, of which 778 were adults (422 males and 356 females), 1,920 were nymphs, and 5,370 were larvae. Ixodes ricinus is a non-nidiculous tick that awaits its host on the vegetation. Other tick species occurring in Belgium, such as I. hexagonus and I. arboricola, on the other hand, are nidiculous (nest dwelling) ticks and are rarely found outside of the nests and burrows of their hosts.
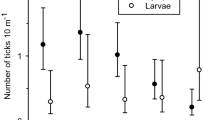
The abundance of questing ticks was significantly affected by time and vegetation type, with a significant interaction between both factors (Table 1; Fig. 1). Larvae were found in significantly lower numbers in spring (April and May) compared to the warmer summer months (June–September), which resulted in a positive correlation between larval abundance and air temperature (Rs = 0.598, P < 0.001, n = 180) and in a negative correlation with relative humidity (Rs = −0.304, P < 0.001, n = 180). The larval abundance was significantly highest along the grass-dominated forest trails (64% of total larval catch) and lowest in the bracken-fern-dominated larch stands (only 6%), but no significant difference was found between the forest gaps with purple-moor grass (14%) and the bilberry-dominated pine stands (16%). Nymphs and adults were present throughout the study period with a significantly higher abundance of nymphs in May and a significantly lower abundance of both nymphs and adults in September. Neither the nymphal nor the adult abundance was significantly correlated with temperature or humidity. The abundance of nymphs and adults was significantly lowest in the bracken-fern-dominated larch stands, which was especially noticeable in June and July when the bracken understory was very tall and dense. Furthermore, the abundance of nymphs was significantly higher along the grass-dominated trails compared to the bracken sites and the forest gaps dominated by purple-moor grass. Additionally, a significant effect of blanket type on the abundance of all life stages and a significant vegetation × blanket type interaction term for the larvae was discernible (Table 1; Fig. 2). For both nymphs and adults, the entire-blanket appeared to be more efficient than the strip-blanket in each vegetation type. Overall, the entire-blanket sampled 12% more nymphs and 14% more adult ticks than the strip-blanket. The strip-blanket, on the other hand, was more efficient for collecting larvae. This was especially apparent in the bracken-fern-dominated larch stands, where twice as many larvae were collected with the strip-blanket.
Abundance of Ixodes ricinus larvae, nymphs, and adults collected in four vegetation types. The results from the strip-blanket and entire-blanket were pooled. Bars represent the mean number of ticks collected per drag (n = 18), error bars indicate the standard error of the mean. Note the scale difference on the y-axes
Comparison of the efficiency of the strip-blanket and the entire-blanket for sampling Ixodes ricinus larvae, nymphs, and adults in four vegetation types. The results from the different collection dates were pooled. Bars represent the mean number of ticks collected per drag (n = 45), error bars indicate the standard error of the mean. Note the scale difference on the y-axes
Discussion
Entire-blanket sampling is an established method for tick collection (Milne 1943; Falco and Fish 1992). However, adult ticks are easily dislodged from the blanket through contact with vegetation during dragging (Milne 1943; Sutherst et al. 1978) and larvae are often missed in dense and tall vegetation because the blanket inadequately contacts the lowest parts of the vegetation and the litter layer (Gray 1985). In the present study, the strip-blanket was proposed as an alternative for the conventional entire-blanket to overcome these problems.
Surprisingly, we showed that significantly more nymphs and adults were caught by the entire-blanket instead of the strip-blanket. Rotation of the strips did not seem to protect ticks from being dislodged at all. On the contrary, the strips often got stuck in the vegetation, which caused undesirable abrupt movements of the blanket and hence, very probably, lead to a higher drop-off. Tick sampling was particularly difficult in the forest stands with a dense bracken fern understory. In spring, when new fronds had just emerged and started to grow, the sampled number of ticks differed little between vegetation types. In summer, however, the abundant bracken foliage strongly interfered with tick sampling and ticks were easily knocked off of the blanket, which resulted in significantly lower counts of nymphs and adults.
In line with our expectations, the strip-blanket was more efficient for sampling larvae in dense vegetation. The bracken understory made it very difficult to reach the questing larvae with the blankets, which resulted in overall low larval counts compared to the other vegetation types. However, the strip-blanket did make it easier to move through the ferns and picked up significantly more larvae than the entire-blanket. In the other vegetation types, where the vegetation was less tall, the strip-blanket did not seem to offer an advantage over the entire-blanket.
The lower abundance of larvae, nymphs, and adults in the bracken-dominated larch stands was clearly caused by the height and the structure of the understory, which impeded tick sampling. The higher abundance of larvae and nymphs along the forest trails can be explained partly by the fact that the short grasses did not obstruct the blankets from making close contact with questing ticks and did not cause the blankets to get stuck, but may also be down to other factors, such as differences in roe deer habitat selection. Because adult I. ricinus ticks predominantly feed on deer, the distribution of deer will determine the location where engorged females drop off and lay eggs (Ruiz-Fons and Gilbert 2010). Roe deer are probably highly attracted to the palatable grass and herbaceous cover along the trails, resulting in a higher import of eggs.
In summary, our results show that the strip-blanket did not appear to provide an advantage over the standard entire-blanket for sampling nymphs and adult ixodid ticks. However, the alternative strip-blanket method may have its utility in collecting larval ticks, especially in dense and tall vegetation where the penetration capacity of the entire-blanket is limited. Moreover, it is important to note that the vegetation structure strongly influences the efficiency of drag sampling, which must be taken into consideration when tick densities between different sites and vegetation types are to be compared.
References
Bacon RM, Kugeler KJ, Mead PS (2008) Surveillance for Lyme disease: United States, 1992–2006. MMWR Morb Mortal Wkly Rep 57:1–9
Falco RC, Fish D (1992) A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp Appl Acarol 14:165–173
Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Wormser GP (1999) Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am J Epidemiol 149:771–776
Ginsberg HS, Ewing CP (1989) Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari: Ixodidae). Exp Appl Acarol 7:313–322
Gray JS (1985) A carbon dioxide trap for prolonged sampling of Ixodes ricinus L. populations. Exp Appl Acarol 1:35–44
Gray JS, Lohan G (1982) The development of a sampling method for the tick Ixodes ricinus and its use in a redwater fever area. Ann Appl Biol 101:421–427
Li X, Dunley JE (1998) Optimal sampling and spatial distribution of Ixodes pacificus, Dermacentor occidentalis and Dermacentor variabilis ticks (Acari: Ixodidae). Exp Appl Acarol 22:233–248
Liang K-Y, Zeger SL (1986) Longitudinal data analysis using generalized linear models. Biometrika 73:13–22
Mather TN, Nicholson MC, Donnelly EF, Matyas BT (1996) Entomologic index for human risk of Lyme disease. Am J Epidemiol 144:1066–1069
Mejlon HA, Jaenson TGT (1997) Questing behaviour of Ixodes ricinus ticks (Acari: Ixodidae). Exp Appl Acarol 21:747–754
Milne A (1943) The comparison of sheep-tick populations (Ixodes ricinus L.). Ann Appl Biol 30:240–250
Needham GR, Teel PD (1991) Off-host physiological ecology of Ixodid ticks. Annu Rev Entomol 36:659–681
Piesman J, Gern L (2004) Lyme borreliosis in Europe and North America. Parasitology 129:S191–S220
Randolph SE, Storey K (1999) Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): implications for parasite transmission. J Med Entomol 36:741–748
Ruiz-Fons F, Gilbert L (2010) The role of deer as vehicles to move ticks, Ixodes ricinus, between contrasting habitats. Int J Parasitol 40:1013–1020
Stafford KC III, Cartter ML, Magnarelli LA, Ertel S-H, Mshar PA (1998) Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. J Clin Microbiol 36:1240–1244
Sutherst RW, Wharton RH, Utech KBW (1978) Guide to studies on tick ecology. Division of Entomology, Commonwealth Scientific and Industrial Research Organization, Technical Paper No. 14, 59 pp
Tälleklint L, Jaenson TGT (1994) Transmission of Borrelia burgdorferi s.l. from mammal reservoirs to the primary vector of Lyme borreliosis, Ixodes ricinus (Acari: Ixodidae), in Sweden. J Med Entomol 31:880–886
WHO (2004) The vector-borne human infections of Europe: their distribution and burden on public health. World Health Organization Regional Office for Europe, Copenhagen, 144 pp
Acknowledgments
The authors thank Marijn Tormans, Kris Ceunen, Karen Wuyts, and Miguel Lyssens-Danneboom for assistance with fieldwork. We are also grateful to Natuurpunt vzw for the permission to work in the nature reserve. This research was funded by IWT-Flanders, the Institute for the Promotion of Innovation by Science and Technology in Flanders.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tack, W., Madder, M., De Frenne, P. et al. The effects of sampling method and vegetation type on the estimated abundance of Ixodes ricinus ticks in forests. Exp Appl Acarol 54, 285–292 (2011). https://doi.org/10.1007/s10493-011-9444-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-011-9444-6