Abstract
Life cycle, fecundity and longevity of the avocado brown mite, Oligonychus punicae (Hirst), were studied on six grapevine cultivars (Tucupita, Villanueva, Red Globe, Sirah, Sauvignon and Chenin Blanc), under laboratory conditions at 27 ± 2°C, 80 ± 10% RH, and L12:D12 photoperiod. Mite-infested leaves were collected from vineyards, placed in paper bags and taken to the laboratory. A laboratory mite culture was established using the grape cultivar Criolla Negra as host plant. To elucidate potential effects on avocado brown mite parameters, we assessed levels of secondary metabolites, such as alkaloids, flavonoids, tannins and polyphenols, of leaves of the six grape cultivars, as well as the thickness of the adaxial cuticle-epidermis. The life cycle of O. punicae differed among cultivars with average values ranging between 8.2 days on Tucupita leaves and 9.1 days on Sirah. Relatively high fecundity was found on Tucupita leaves (2.8 eggs/female/day) during 11.4 oviposition days, while low fecundity values occurred on Sirah and Villanueva leaves, with 0.9 and 1.8 eggs/female/day during 7.9 and 6.7 days, respectively. Average longevity of O. punicae females ranged from 8.1 to 17.5 days on Sirah and Sauvignon leaves, respectively. Intrinsic rate of increase (r m) was highest on Sauvignon (0.292) and Tucupita (0.261), and lowest on Sirah (0.146) and Villanueva (0.135). Although significant differences in cuticle-epidermis thickness were detected among the six cultivars, it seemed not to affect mite parameters. Secondary metabolite content also varied between the cultivars. Generally, increasing flavonoid content coincided with decreasing reproductive parameters. The natural plant resistance observed in this study could be useful in the development of an integrated pest management program for mite pests in grape production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life-history parameters of tetranychid mite species are controlled by intrinsic and extrinsic factors, being host plant conditions among the most important (Huffaker et al. 1969; Wrensch 1985). Crooker (1985) emphasized that morphological and chemical plant features are thought to be associated with host plant resistance, inducing differences in mite development rate, fecundity, longevity and, consequently, in mite fitness. More recent research indicated that differential responses in phytophages can be raised by host plant quality, which also depends on quantity and nature of primary and secondary metabolites (Awmack and Leather 2002; van den Boom et al. 2003). These factors may vary among plant species and even among cultivars. As an example, fecundity of Tetranychus cinnabarinus (Boisduval) varied from 19 to 400 eggs in several Cucurbita and Lagenaria accessions (Edelstein et al. 2000), while Tetranychus urticae Koch showed greater population increases in strawberry ‘Hapil’ and ‘Pegasus’ cultivars than in others (Sonneveld et al. 1997). Furthermore, morpho-anatomical features such as trichomes and foliar glands, and epidermis-cuticle strata thickness can constitute physical barriers for tetranychid feeding (e.g. in cotton; Bailey et al. 1978).
The avocado brown mite, Oligonychus punicae (Hirst), is considered an important tetranychid pest in southern California (USA), as high population densities can cause severe defoliation on several avocado cultivars (McMurtry and Johnson 1966; McMurtry 1985). This tetranychid mite predominantly feeds on the upper leaf surface, although feeding could extend to the lower leaf surface at high population levels (Tomczyk and Kropczynska 1985). Damage in host plants is shown by a bronze tone on the leaves, which was shown to be associated with the rates of oviposition and female production (McMurtry 1970).
In tropical America, the avocado brown mite has been reported in more than 20 plant species such as Mangifera indica L., Musa sapientum L., Punica granatum L. and Vitis vinifera L. (Ochoa et al. 1994; Bolland et al. 1998). In Venezuela, it was recorded on Musa spp. from Sur del Lago, Zulia State (Freitez and Alvarado 1978; Quirós 1978). More recently it has been observed as an occasional pest on grape from Lara State where its feeding delays fruit ripening. This study was undertaken to investigate how feeding on leaves from six grape cultivars from Lara State influences life-history parameters of the avocado brown mite.
Material and methods
Mite collection and maintenance
Oligonychus punicae was collected on infested grape leaves in a vineyard of the Criolla Negra cultivar, located at Instituto de la Uva (IUVA) (9°48′ N, 69°47′ W), belonging to Universidad Centroccidentral ‘Lisandro Alvarado’, El Tocuyo, Morán Municipality, Lara State, Venezuela. In the laboratory, the mites were cultured on Criolla Negra vines for 6 months before the start of experiments. Experiments were conducted in a room at 27 ± 2°C, 80 ± 10% RH, and a photoperiod of 12:12 h.
Estimation of O. punicae developmental time
Oligonychus punicae development was determined when feeding on leaves from Tucupita, Villanueva, Red Globe, Sirah, Sauvignon and Chenin Blanc grape cultivars, grown at El Tocuyo. Tucupita, Villanueva and Red Globe cultivars were sown under headlike arbor, while Sirah, Sauvignon and Chenin Blanc were conducted in trellis system. Studies on biological features were carried out on mature leaves sampled from the middle part of the stem of >5-year-old plants.
Ten adult females and three males were randomly selected from the laboratory culture and placed together on each of 10 rearing units containing grapevine leaf discs (3 cm diameter) from each cultivar, as described by Helle and Overmeer (1985), to lay eggs. Mites were removed 24 h later. The egg cohorts were observed at 12-h intervals. After that, a 2-day-old female and a male were both placed on a leaf disc from each of the six grape cultivars. Once one or three eggs were laid per leaf disc, the male and female mites were removed. Observations were made at 12-h intervals to determine egg hatching time and developmental time of larval, protonymphal and deutonymphal stages. Ten replications were run.
Estimation of O. punicae reproductive parameters and longevity
Pre-oviposition, oviposition and post-oviposition periods, total fecundity, daily oviposition rate and female longevity were determined on each of the six grape cultivars. A newly emerged female and one adult male mite were placed together on leaf discs (3 cm diameter) from each of the grape cultivars as described above. Pre-oviposition period was recorded at 6-h intervals, while other reproductive parameters and longevity were recorded every 12 h. Leaf discs were removed and replaced by new ones at 4-day intervals. Ten replications were run.
Life table parameters
Net reproduction rate (R 0), mean generation time (T), intrinsic rate of increase (r m) and finite rate of increase (λ) of O. punicae were calculated from data obtained for development time and reproductive parameters according to Birch (1948).
Estimation of chemical and anatomical features on grape cultivars
Two experiments were conducted to determine the content of secondary metabolites and some anatomy features of leaves of the six grape cultivars in order to elucidate their possible influence on O. punicae life-history parameters. In experiment 1, samples of grape leaves from each cultivar were collected at IUVA vineyards under the following two conditions: (a) mite-infested leaves (MIL), which were naturally infested with no chemical applications included, and (b) non-infested leaves (NIL) which were frequently sprayed with water to prevent mite population increase. Fully expanded leaves were taken from the middle third of the primary shoot, and in the case of MIL just leaves showing more than 50% of O. punicae damage symptoms were collected. Both MIL and NIL samples were dried at room conditions and then individually ground until obtaining a fine powder from each cultivar. Five hundred milligram of the leaf powder obtained from 25 to 30 leaf discs (3 cm diameter) from each cultivar were mixed with 96% alcohol during 24 h to obtain an ethanolic extract by using a rotoevaporator BrinkmamMR (Mod. RE111), at 100°C.
Secondary metabolites were determined in ethanolic extract as described by Marcano and Hasegawa (2002). Alkaloids, flavonoids, tannins and polyphenols were separated and quantified by thin-layer chromatography (Cromatoplates MerckMR to silica/gel 60 F254 TLC, 0.25 mm thin, 6.5 × 2.5 cm). One ethanolic extract drop (7 μl) from each cultivar was added to cromatoplates and treated with a 9:1 water/acetic acid solution, to separate tannins and polyphenols which were identified by adding 0.1% FeCl. Alkaloids and flavonoids were separated using hexane as a solvent and printings were observed under UV light as described by Marcano and Hasegawa (2002). Bands containing isolated secondary compounds were cut and weighed (silica/gel + secondary metabolite). A similar area was obtained from a clean silica/gel cromatoplate (silica/gel) and weighed for comparison purposes. The difference in weights indicates the amount of the particular secondary plant compound (in 7 μl extract). Based on these weights the contents (per g leaf material) could be calculated in a straightforward way.
In experiment 2, three plants of each of the six cultivars were selected randomly from the vineyard to determine the cuticle-epidermis thickness and trichome density on the adaxial leaf surface. Sub-samples of three leaves per plant were randomly chosen and fixed in FAA medium (5% formaldehyde, 5% acetic acid, 90% alcohol). Subsequently, the sub-samples were divided in two groups. In group 1, leaves were subjected to mesophyll digestion with Jeffrey liquid (Johansen 1940) in order to detect leaf trichome presence and density. In group 2, cross-sections were obtained using a rotary microtome (Reichert-Jung) in order to measure cuticle-epidermis strata. To better show leaf anatomy, previous to microscope observation, leaves from both groups were stained with toluidine as described by Montenegro and Gómez (1997).
Statistical analysis
Biological parameters were determined in a completely randomized design with time-repeated measurements, analyzed through one-way ANOVA and Tukey tests, to separate treatment means. Secondary metabolite data were analyzed by one-way ANOVA and correlated to life-history parameters to determine potential association between them. Anatomy data were subjected to ANOVA and Tukey tests to determine differences between cultivars. All analyses were performed by using Statistix software version 8.0.
Results and discussion
Influence of grape cultivars on O. punicae developmental time
The total developmental time (egg-adult) of O. punicae showed significant differences among grape cultivars (P = 0.004, df = 5, F = 4.78). However, multiple linear regression analysis showed no relation between total developmental time and alkaloids, flavonoids, tannins or polyphenol content levels of the cultivars (P = 0.175, F = 1.86, R 2 = 0.12). On Tucupita and Sauvignon, mites spent 8.2 and 8.3 days to reach adulthood, while on Villanueva, Red Globe, Chenin Blanc and Sirah development was ca. 7–11% slower (Table 1). Previous studies have demonstrated cultivar effects on life cycle and population increase of a variety of tetranychid species. Opit et al. (2001), for example, observed that T. urticae populations increased faster on geranium cultivar ‘Amethyst’ than on ‘Sybil Holmes’ under glasshouse conditions. They hypothesized that possibly higher phosphorous content in ‘Amethyst’ caused the population increase since it has been demonstrated previously that phosphorous may enhance T. urticae fecundity. Another example is the developmental time of Amphitetranychus viennensis (Zacher), which was found to be lower when reared on apple cultivars ‘Starkrimson Delicious’ and ‘Golden Delicious’ (10.7 days) than on ‘Amasya’ and ‘Starking Delicious’ (11.7 days) (Kasap 2003).
Foliar anatomy of the grape cultivars seemed not to hinder O. punicae feeding, because mites reached adulthood also on cultivars Red Globe and Sauvignon, despite the greater cuticle-epidermis thickness (Table 2). Perhaps this need not come as a surprise—the length of the mites’ stylet, measured from basis to apex is approximately 43 ± 2.2, 74 ± 7.5 and 110 ± 4.0 μm in larvae (N = 15), males (N = 7) and females (N = 11), respectively, which is longer than even the thickest cuticle-epidermis measured, so probably mites are able to feed on the lower leaf strata on all cultivars (Table 2).
Studies about the influence of leaf anatomy on tetranychid life-history parameters are not conclusive. Skorupska (1998) showed that stoma count on the abaxial surface, spongy and palisade mesophyll and total leaf lamina width affected growth of A. viennensis in apple cultivars. Conversely, Nukenine et al. (2000) did not find any relation between anatomical features in cassava and resistance to the cassava green mite.
Reproductive parameters and longevity
Periods of pre-oviposition, oviposition and post-oviposition in O. punicae females varied among grape cultivars. Shorter pre- and post-ovipositional periods were found on Chenin Blanc leaves (1.2 and 0.9 days, respectively), while on others cultivars pre- and post-oviposition periods ranged from 1.6–2.0 to 1.2–2.5 days, respectively (Table 3). Oviposition periods lasted from 6.7 days (on Villanueva) to 16.1 days (on Sauvignon). In previous studies O. punicae had a pre-ovipositional period of 1.3 days and an oviposition period of 13.3 days (Tanigoshi and McMurtry 1977) or 2.0 days (McMurtry and Johnson 1966) on avocado trees.
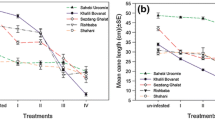
Average daily egg production was highest on Tucupita (2.8 eggs/female/day) and lowest on Sirah (0.9 eggs/female/day) (Table 3). Daily oviposition rate ranged from 2.0 to 6.1 eggs/female up to day 7 in females feeding on Tucupita leaves, while on Chenin Blanc, Red Globe, Sauvignon, Sirah and Villanueva leaves it varied from 1.1–6.1, 0.2–5.3, 0.3–5.0, 0.2–2.7 to 0.5–3.5 eggs/female, respectively (Fig. 1). Daily oviposition rates in this study were higher than reported by McMurtry and Johnson (1966) or Tanigoshi and McMurtry (1977) for O. punicae females reared on avocado leaves.
Also, female longevity of O. punicae was affected by grape cultivar: females lived longest on Sauvignon (17.5 days), and shortest on Villanueva (8.1 days) (Table 3). Similarly, Kerguelen and Hoddle (2000) found that mean female O. perseae longevity increased by 100% from 12 days in May to 24 days in July in the susceptible avocado cultivar Hass, while in more resistant cultivars, Pinkerton and Lamb Hass, longevity dropped by 30% over the same period.
An effect of host plant on reproduction has been established for several Tetranychid species (e.g., de Ponti 1977; Ribeiro et al. 1988; Hilker and Meiners 2002; Praslička and Huszár 2004). In our findings, reproductive parameters of O. punicae seemed to be associated with flavonoid content of grape cultivars: the higher the flavonoids content, the lower the mites’ fecundity (R 2 = 63.5, F = 23.48, P < 0.001) (Fig. 2). Previous studies have demonstrated that grape leaves and fruits synthesize phenolic compounds in response to fungal attacks or abiotic factors (Morrissey and Osbourn 1999). Furthermore, Harborne (1994) hypothesized that low molecular weight phenol compounds could act synergistically with tannins to provide plant resistance.
Life table parameters
The net reproductive rate (R 0) was highest on Sauvignon and lowest on Villanueva (Table 4). The intrinsic rate of increase (r m) ranged from 0.31 (on Chenin Blanc) to 0.14 (on Villanueva). Based on our life-history parameters, the cultivars Sirah and Villanueva proved to be the least suitable hosts for this pest mite, due to slower development and lower fecundity, longevity and r m-values. Sauvignon and Tucupita were much better hosts, based on the higher mite reproduction under laboratory conditions. Further field studies should be carried out to determine the interaction between plant, pest and environment in Lara state vineyards in order to establish more efficient pest management strategies.
References
Awmack C, Leather S (2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47:817–844
Bailey J, Furr R, Hanny B, Meredith W (1978) Field populations of two-spotted spider mites on sixteen genotypes at Stoneville. J Econ Entomol 71:911–912
Birch L (1948) The intrinsic rate of natural increases of an insect population. J Anim Ecol 17:15–26
Bolland HR, Gutierrez J, Fletchmann CHW (1998) World catalogue of the spider mite Family (Acari: Tetranychidae). Koninklijke Brill NV, Leiden, The Netherlands
Crooker A (1985) Embryonic and juvenile development. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier Science Publishers B. V, Amsterdam, The Netherlands, pp 149–163
de Ponti O (1977) Resistance in Cucumis sativus L. to Tetranychus urticae Koch. II. Designing a reliable laboratory test for resistance based on aspects of the host–parasite relationship. Euphytica 26:641–654
Edelstein M, Tadmor Y, Mansour F (2000) Resistance of Cucurbita and Lagenaria to the carmine spider mite, Tetranychus cinnabarinus (Acari: Tetranychidae). Acta Hortic 510:283–287
Freitez F, Alvarado G (1978) Contribución al conocimiento de ácaros en Musaceae de Venezuela (Acarina). Rev Fac Agron 26:197–207
Harborne J (1994) Do natural plant phenols play a role in ecology? Acta Hortic 381:36–43
Helle W, Overmeer W (1985) Rearing techniques. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier Science Publishers B. V, Amsterdam, The Netherlands, pp 331–335
Hilker M, Meiners T (2002) Induction of plant responses to oviposition and feeding by herbivorous arthropods: a comparison. Entomol Exp Appl 104:181–192
Huffaker CB, Van De Vrie M, McMurtry JA (1969) The ecology of tetranychid mites and their natural enemies. Annu Rev Entomol 14:125–174
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York, p 503
Kasap I (2003) Life history of hawthorn spider mite Amphitetranychus viennensis (Acarina: Tetranychidae) on various apple cultivars and at different temperatures. Exp Appl Acarol 31:79–91
Kerguelen V, Hoddle MS (2000) Comparison of the susceptibility of several cultivars of avocado to the persea mite, Oligonychus persea (Acari: Tetranychidae). Sci Hortic 84:101–114
Marcano D, Hasegawa M (2002) Fitoquímica orgánica. UCV-CDCHT, Caracas
McMurtry JA (1970) Some factors of foliage condition limiting populations growth of Oligonychus punicae (Acarina: Tetranychidae). Ann Entomol Soc Am 63:406–412
McMurtry JA (1985) Avocado. In: Helle W, Sabelis M (eds) Spider mites: their biology, natural enemies and control, vol 1B. Elsevier Science Publishers B. V, Amsterdam, The Netherlands, pp 327–338
McMurtry JA, Johnson HG (1966) An ecological study of the spider mite Oligonychus punicae (Hirst) and its natural enemies. Hilgardia 37(11):363–402
Montenegro G, Gómez M (1997) Anatomía y evolución del cuerpo vegetativo de las plantas vasculares. curso de la red latinoamericana de botánica. La Habana, Cuba, p 91
Morrissey J, Osbourn AE (1999) Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev 63:708–724
Nukenine EN, Hassan AT, Dixon GO (2000) Influence of variety on the within-plant distribution of cassava green spider mite (Acari: Tetranychidae), and leaf anatomical characteristics and chemical components in relation to varietal resistance. Int J Pest Manag 46:177–186
Ochoa R, Aguilar H, Vargas C (1994) Phytophagous mites of Central America: an illustrated guide. CATIE, Turrialba, Costa Rica, p 234
Opit GP, Jonas VM, Williams KA, Margolies DC (2001) Effects of cultivar and irrigation management on population growth of the twospotted spider mite Tetranychus urticae on greenhouse ivy geranium. Exp Appl Acarol 25:849–857
Praslička J, Huszár J (2004) Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Prot Sci 40(4):141–144
Quirós M (1978) Presencia del ‘ácaro marrón del aguacate’, Oligonychus punicae (Hirst) en hojas de plátano del Distrito Baralt, estado Zulia. Rev Fac Agron (LUZ) 5(1):422–424
Ribeiro LG, Villacorta A, Foerster LA (1988) Life cycle of Panonychus ulmi (Koch, 1836) (Acari: Tetranychidae) in apple trees, cultivar Gala and Golden Delicious. Acta Hortic 232:228
Skorupska A (1998) Morphologic-anatomical structure of leaves and demographic parameters of the hawthorn spider mite, Tetranychus viennensis Zacher and the two-spotted spider mite, Tetranychus urticae (Koch) (Acarina: Tetranychidae) on selected scab-resistance apple varieties. J Appl Entomol 122:493–496
Sonneveld T, Wainwright H, Labuschagne L (1997) Two methods for determining the resistance of strawberry cultivars to two-spotted mite (Acari: Tetranychidae). Acta Hortic 439:199–204
Tanigoshi LK, McMurtry JA (1977) The dynamics of predation of Stethorus picipes (Coleoptera: Coccinellidae) and Typhlodromus floridanus on the prey Oligonychus punicae (Acarina: Phytoseiidae, Tetranychidae). Part I. Comparative life history and life table studies. Hilgardia 45(8):237–261
Tomczyk A, Kropczynska D (1985) Effects of the plants. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier Science Publishers B. V, Amsterdam, The Netherlands, pp 317–329
van den Boom EM, van Beek TA, Dicke M (2003) Differences among plant species in acceptance by the spider mite Tetranychus urticae Koch. J Appl Entomol 127:177–183
Wrensch DL (1985) Reproductive parameters. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier Science Publishers BV, Amsterdam, The Netherlands, pp 165–170
Acknowledgements
We wish to thank Noel Troxclair for his critical revision of the manuscript, Lisbeth Díaz for her help in statistical analysis. This work was funded by Consejo de Desarrollo Científico, Humanístico y Tecnológico (CDCHT-UCLA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vásquez, C., Aponte, O., Morales, J. et al. Biological studies of Oligonychus punicae (Acari: Tetranychidae) on grapevine cultivars. Exp Appl Acarol 45, 59–69 (2008). https://doi.org/10.1007/s10493-008-9154-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-008-9154-x